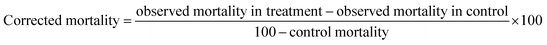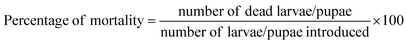Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Beauveria bassiana (Clavicipitaceae): a potent fungal agent for controlling mosquito vectors of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti (Diptera: Culicidae)
Chinnasamy Ragavendrana,
Nawal Kishore Dubeyb and
Devarajan Natarajan *a
*a
aNatural Drug Research Laboratory, Department of Biotechnology, School of Biosciences, Periyar University, Salem – 636 011, Tamil Nadu, India. E-mail: mdnataraj@rediffmail.com; natarajpu@gmail.com
bCentre of Advanced Study in Botany, Banaras Hindu University, Varanasi-221005, India
First published on 13th January 2017
Abstract
Mosquitoes are the carriers of severe and well-known illnesses such as malaria, arboviral encephalitis, dengue, chikungunya and yellow fever, which cause significant morbidity and mortality in humans and domestic animals around the world. Entomopathogenic fungal metabolites act as a mosquito control agent and are potential alternatives to chemical control because they can be innovative and more selective than chemical insecticides. The main aim of the present study was to perform experiments on the larvicidal and pupicidal effects of the entomopathogenic fungus Beauveria bassiana (isolated from infected grasshopper) against the first to fourth instar larvae of Anopheles stephensi, Culex quinquefasciatus and Aedes aegypti. The larval and pupal mortality were observed after 24 h of exposure. The efficacy of an ethyl acetate mycelium extract at all the tested concentrations (50, 100, 150, 200, 250 and 300 μg mL−1) exhibited better activity against the 1st to 4th instar larvae of An. stephensi (LC50 = 42.82, 39.45, 25.72, and 32.66; LC90 = 254.67, 367.11, 182.27, and 199.20 μg mL−1), Cx. quinquefasciatus (LC50 = 72.38, 68.11, 27.06, and 35.495; LC90 = 481.68, 254.69, 129.83, and 146.24 μg mL−1) and Ae. aegypti (LC50 = 62.50, 52.89, 58.60, and 47.12; LC90 = 314.82, 236.18, 247.53, and 278.52 μg mL−1), respectively. The pupicidal activity of the fungal mycelium extracts was tested against An. stephensi, Cx. quinquefasciatus and Ae. Aegypti, where the ethyl acetate extracts had different LC50 values (LC50 = 40.66, 54.06, 44.26, and LC90 = 184.02, 225.61, and 263.02 μg mL−1). Based on Fourier transform infrared spectroscopy (FTIR) analysis and gas chromatography-mass spectrometry (GC-MS) analyses, the ethyl acetate mycelium extract contained six major chemical compounds identified as 9,12-octadecadienoic acid (ZZ)– (63.16%), n-hexadecanoic acid (21.28%), octadecanoic acid, phenyl methyl ester (10.45%), dehydroegosterol 3,5-dinitrobenzoate (1.86%), squalene (1.66%) and bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate (1.56%). The n-hexadecanoic acid standard was found to be better larvicidal against An. stephensi, Cx. quinquefasciatus, followed by Ae. aegypti. The HPLC analysis of the ethyl acetate mycelium extract was compared with that of the n-hexadecanoic acid standard and it was found to show a similar chromatographic peak (at a retention time of 3.383 and 3.378 min). The outcome of the present study identifies the bioactive compounds obtained from B. bassiana that can be used as effective and alternate larvicidal and pupicidal agents against the An. stephensi Cx. quinquefasciatus and Ae. aegypti mosquito vectors.
Introduction
Vector-borne diseases are illnesses caused by pathogens and parasites in human populations. Globally, every year there are about more than 1 billion cases and over 1 million deaths due to vector-borne diseases, such as malaria, dengue, schistosomiasis, human African trypanosomiasis, leishmaniasis, chagas disease, yellow fever, Japanese encephalitis and onchocerciasis. Vector-borne diseases account for over 17% of all infectious diseases. Malaria is a parasitic disease spread by infected Anopheles mosquitoes, which is caused by parasite species namely Plasmodium falciparum, P. vivax, P. malariae and P. ovale.1 Malaria causes symptoms that typically include fever and headache, which in severe cases can lead to coma or death. A recent survey released in December 2014 reported about 198 million cases of malaria in 2013 with an uncertainty range from 124 million to 283 million and an estimated 584000 deaths (with an uncertainty range of 367![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 755
000 to 755![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Malarial mortality rates have fallen globally by 47% since 2000 and 54% reported in the African regions.2
000). Malarial mortality rates have fallen globally by 47% since 2000 and 54% reported in the African regions.2
Culex mosquitoes are painful and persistent biters and are responsible for filariasis. Lymphatic filariasis is a neglected tropical disease. Lymphatic filariasis is commonly known as elephantiasis and infection occurs when filarial parasites are transmitted to humans through mosquitoes.3 When a mosquito with infective stage larvae bites a person, the parasites are deposited on the person's skin from where they enter into the body. The larvae then migrate to the lymphatic vessels where they develop into adult worms in the lymphatic system. Worldwide, more than 1.3 billion people from 72 countries are threatened by lymphatic filariasis, commonly known as elephantiasis.4 Chikungunya is a viral tropical disease transmitted by Aedes mosquitoes. The disease is prevalent in Africa, Asia, the islands in the Caribbean, India and Pacific oceans. Typical symptoms are an acute illness with fever, skin rash and incapacitating joint pain that can last for weeks.5 The latter distinguishes chikungunya virus from dengue, which otherwise shares the same vectors, symptoms and geographical distribution. There is no cure or commercial vaccine for the disease. Most patients recover fully; however, in some cases, joint pain may persist for several months or even years. As with dengue, the only method to reduce the transmission of the chikungunya virus is to control vector mosquitoes and protect against mosquito bites. Yellow fever is an acute viral hemorrhagic disease transmitted by Aedes mosquitoes. The “yellow” in the name refers to the jaundice that affects some patients. There are an estimated 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cases of yellow fever, which cause 30
000 cases of yellow fever, which cause 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths worldwide per year. The virus that causes yellow fever is endemic in densely populated countries, viz., Tropical Africa and Latin America. Small numbers of imported cases occur in countries free of yellow fever.6
000 deaths worldwide per year. The virus that causes yellow fever is endemic in densely populated countries, viz., Tropical Africa and Latin America. Small numbers of imported cases occur in countries free of yellow fever.6
The common control agents for mosquito larvicides are mainly dependent on chemical methods using synthetic insecticides that are likely to include, organophosphates such as temephos, fenthion, phytochemicals and insect growth regulators such as diflubenzuron, and methoprene.7 However, most of these synthetics have adverse effects on the environment. Due to their residual nature there are reports on the development of pesticide resistance in mosquitoes8 rendering them ineffective for further applications. These problems encourage the search for safer and better alternative bioactive larvicidal agents. Although various biocontrol measures are in vogue, to date, their effective control of larval mosquitoes has not been practically highlighted. Microbial control is recommended as an alternative way, and microbial based larvicides are employed for minimizing the mosquito population, which provides an effective, environmentally friendly and sociable approach to bring the mosquito population to the lowest level.9,10
Beauveria bassiana (Clavicipitaceae) is a soil borne fungus that feeds on insects and can be used effectively to control thrips, aphids, whitefly, caterpillars, beetles, and subterranean insects like ants and termites. B. bassiana is non-toxic to mammals, birds and plants, and its use is not expected to have any deleterious effects on human health or the environment.11 Conidia of B. bassiana has been reported to be effective in killing mosquito larvae when applied as conidia dust in the breeding sites. Besides infecting larvae, the fungus has also proven to be virulent to adult mosquitoes.12 B. bassiana is applied to the target pest as a spore, which is the reproductive and dispersal structure of the fungus. Once the spores have made contact with the insect exoskeleton, they grow hyphae (long, branching vegetative appendages) that secrete enzymes, which in turn dissolve the cuticle (outermost layer of the skeleton). These fungal hyphae grow into the insect, feed on its body tissue, produce toxins, and reproduce. It takes up to seven days for the insect to die. During favorable (moist) conditions (92% humidity or greater), B. bassiana will “bloom” and release more spores into the environment to repeat the cycle on other pest insects.13 The species of Beauveria has been reported to produce secondary metabolites, including bassianin, bassiacridin, beauvericin, bassianolide, beauverolides, tenellin and oosporein.14–16 It also produces proteases, chitinases and lipases, which can degrade the insect cuticle.17 In this regard, the entomopathogenic fungi, viz., Aspergillus flavus, A. parasiticus, Penicillium falicum, Fusarium vasinfectum and Trichoderma viride and soil bacteria, Bacillus thuringiensis and B. sphaericus have been reported to be effective against Cx. quinquefasciatus.18 Hence, the present study was focused on the insecticidal potential of Beauveria bassiana mycelial extracts against target mosquitoes.
Materials and methods
Isolation and identification of entomopathogenic fungus
The entomopathogenic fungus B. bassiana was isolated from an infected grasshopper (Melanoplus sanguinipes) collected from an agricultural field (latitude 11.6500° N, longitude 78.1600° E) in the Salem District, Tamilnadu, India. The cadaver was placed on potato dextrose agar (PDA (Hi-Media)) supplemented with streptomycin (1 mg/100 mL) and incubated for 7 days at 27 °C ± 2 °C.19 After 7 days of incubation, the pure culture of B. bassiana was subcultured into PDA using the streak plate method. The isolated culture was identified using the slide culture method subjected to lactophenol cotton blue staining and observed under a light microscope (Labomed). Mycotaxonomic keys followed by Samson20 and Samson et al.21 were used to identify the fungus.Morphological identification of B. bassiana
The fungus was primarily identified based on its morphological features, descriptions of species, keys to taxa and additional information from ‘‘Studies in Mycology’’.22 Colonies of B. bassiana fungus were cultivated on Sabouraud's dextrose agar at 25 °C for 7 days. The following morphological characteristics were assessed: colony growth (length and width), the presence or absence of aerial mycelium, colony color, presence of wrinkles, furrows and pigment production.21Preparation of Sabouraud's dextrose broth and mass culture of B. bassiana
The broths were prepared for the culture of fungus as per the modified method of Gardner and Pillai.23 B. bassiana was grown in Sabouraud's Dextrose Broth (SDB). Ten 250 mL conical flasks, each containing 100 mL of SDB (dextrose 40 g, peptone 10 g, deionized water 1000 mL), autoclaved at 20 psi for 20 min. The broths were supplemented with 50 μg mL−1 chloramphenicol, which acted as a bacteriostatic agent. The B. bassiana colonies grown on the Sabouraud's dextrose agar plates were transferred to each flask (using an inoculation needle). The conical flasks inoculated with B. bassiana were incubated at 25 °C for 25 days.Secondary metabolite extraction from B. bassiana
Mass cultivation of the fungus was carried out in a 250 mL Erlenmeyer flask containing 100 mL of Sabouraud's dextrose broth medium. The culture flasks were incubated under the optimized culture conditions (pH 7.0, temperature 27 °C) for 25 days. For the liquid culture, the fungal mycelium mat was washed three times with sterile distilled water to remove adherent filtrate and subjected to an extraction of biologically active components using ethyl acetate and methanol solvents. The solvents were mixed to the mycelia mat for cold extraction for 7 days at room temperature. After thorough mixing, the immiscible portion of ethyl acetate (pale yellow colored) was separated from the mycelium. The mycelium was filtered through Whatmann no. 1 filter paper. The separated portions of ethyl acetate and methanol extracts were finally dried using a rotary vacuum evaporator at 45 °C as per the modified method of Belofsky et al. (2000).24Larvae collection and rearing
For the laboratory trial, the different (1st to 4th) instar larvae stages of An. stephensi, Cx. quinquefasciatus and Ae. aegypti were obtained from the Institute of Vector Control and Zoonoses, (IVCZ), (latitude 12.7200° N, longitude 77.8200° E), Hosur, Tamilnadu, India. The larvae were kept in plastic enamel trays containing dechlorinated tap water. They were maintained as per the previous report of Patil et al.25 The larvae were fed on dog biscuits and yeast powder in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Adults were fed with blood through a paraffin membrane and provided with 10% sucrose solution. Mosquitoes were kept at 28 °C ± 2 °C and 70–85% relative humidity with a photoperiod of 12 h light/12 h dark.
1 ratio. Adults were fed with blood through a paraffin membrane and provided with 10% sucrose solution. Mosquitoes were kept at 28 °C ± 2 °C and 70–85% relative humidity with a photoperiod of 12 h light/12 h dark.
Larvicidal bioassay
The larval mortality bioassays were carried out according to the method suggested by the World Health Organization26 with slight modifications.27 Sufficient amounts of ethyl acetate and methanol extracts were transferred to a vial, and the residual solvent was removed under high vacuum. Stock solutions of each test mycelium extract in dimethyl sulfoxide (DMSO) were prepared with a concentration of 10% w/v (1 mg of extracts in 1000 μL of DMSO) prepared into five different concentrations viz. 50, 100, 150, 250 and 300 μg mL−1 with distilled water at pH 7.0. Twenty numbers of late first to early fourth-instar mosquito larvae were placed in a 2% v/v aqueous solution of DMSO (99 mL of distilled water plus 1 mL of DMSO), followed by the addition of the test solutions. Five replicates per dose were maintained, and a treatment with 99 mL of tap water and 1 mL of DMSO was added to each bioassay as the control at pH 7.0. During this experiment, no food was provided to the larvae. The larval mortality was calculated after 24 h of exposure.Pupal toxicity tests
The laboratory colony of mosquito pupae was used to test the pupicidal activity of the B. bassiana extracts. Twenty freshly emerged pupae were kept in a 100 mL glass beaker containing 99 mL of dechlorinated water and a different concentrations of mycelium extracts (50, 100, 150, 200, 250 and 300 μg mL−1). The experiment consists of five replicates; the control containing 1 mL of DMSO in 99 mL of dechlorinated water at pH 7.0. The mortality in the treatments and control was corrected using Abbott's formula.28 The LC50 and LC90 were calculated from toxicity data using probit analysis.29Dose response bioassay
The stock solutions obtained from the mycelia extract at different concentrations (ranging from 50 to 300 μg mL−1) were prepared as per the method of Rahuman et al.30 Based on the preliminary screening results, the mycelium extracts of B. bassiana were subjected to a dose-response bioassay for larvicidal and pupicidal activity against first to fourth instar larvae and pupae of An. stephensi, Cx. quinquefasciatus and Ae. aegypti. The number of dead larvae were counted after 24 h of exposure, and the percentage mortality was reported from the average of five replicates.Preparation of the standard
n-Hexadecanoic acid was procured from Sigma, USA and DMSO was used as the solvent to prepare the stock solution. The stock solution was diluted further to produce the required concentrations to perform the bioassay tests.31Control experiment (Acremonium sp. non-pathogenic fungi)
The larval and pupal mortality bioassays of Acremonium sp. were carried out according to the method suggested by the World Health Organization with slight modifications.26,27 A sufficient amount of the Acremonium sp. ethyl acetate extracts was transferred to a vial, and the residual solvent was removed under high vacuum. The stock solution of Acremonium mycelial ethyl acetate extract was prepared using dimethyl sulfoxide (DMSO) with a concentration of 10% w/v (1 mg of extracts in 1000 μL of DMSO). Then, it was diluted into five different concentrations, viz., 50, 100, 150, 250 and 300 μg mL−1, and used for bioassay.Fourier transformed infrared spectroscopy (FTIR)
1.0 mg of sample was mixed with 100 mg of KBr (binding agent) using a clean mortar and a pestle to produce a powder. The powder was made into pellets using a hydraulic press. The pellets were then subjected to FTIR analysis on a BRUKER α-T FTIR spectrometer. The precision of the FTIR spectra was better than 0.09 cm−1 and the scanning range was from 4000 to 500 cm−1.32 FTIR analysis was carried out in the Department of Physics, Periyar University, Salem, Tamilnadu, India.Gas chromatography-mass spectrophotometry (GC-MS) analysis
GC-MS analysis of the samples was carried out on a Perkin Elmer (clarus 680) series GC-MS (Marathon, USA) system equipped with clarus 600 (EI) auto-sampler coupled with an Elite-5 MS capillary column (30 m × 0.25 mm i.d., and 0.250 μm) (PerkinElmer, Inc, made in USA). Helium was used as the carrier gas at a flow rate of 1 mL min−1; split ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; mass scan 50–600 Da; ionization energy, 70 eV; ion source temperature, 240 °C; injector temperature, 250 °C. The oven temperature was programmed as follows: initially at 60 °C for 2 min, rising at 10 °C min−1 to 300 °C and then held isothermally (6 min) at 300 °C with a total run time of 32 min. The percentage composition of the crude extract constituents was expressed as a percentage of the peak area. The chemical compounds were identified and characterized based on their retention time (RT). The obtained mass spectral data (GC-MS) was matched with those of standards available in the existing computer library (NIST) data base.33 The GC-MS analysis was carried out in the Sophisticated Instrument Facility, (SAIF). Vellore Institute of Technology (VIT), Vellore, Tamilnadu, India.
1; mass scan 50–600 Da; ionization energy, 70 eV; ion source temperature, 240 °C; injector temperature, 250 °C. The oven temperature was programmed as follows: initially at 60 °C for 2 min, rising at 10 °C min−1 to 300 °C and then held isothermally (6 min) at 300 °C with a total run time of 32 min. The percentage composition of the crude extract constituents was expressed as a percentage of the peak area. The chemical compounds were identified and characterized based on their retention time (RT). The obtained mass spectral data (GC-MS) was matched with those of standards available in the existing computer library (NIST) data base.33 The GC-MS analysis was carried out in the Sophisticated Instrument Facility, (SAIF). Vellore Institute of Technology (VIT), Vellore, Tamilnadu, India.
High performance liquid chromatography (HPLC) analysis
The B. bassiana mycelium ethyl acetate extract and pure n-hexadecanoic acid were diluted and subjected to high performance liquid chromatography (HPLC) analysis. For the chromatographic analysis of ethyl acetate extract and pure n-hexadecanoic acid, the samples were detected using an LC-20AD HPLC system (Shimadzu Chromatographic Instruments, Japan) equipped with a C18 reverse phase column (particle size: 5 μm; length: 4.6 × 250 mm) and a SPD-20A UV/Vis detector at 242 nm absorbance with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) at a flow rate of 1 mL min−1 and head pressure of 300 kgf cm−2. The entire instrument room setup was maintained at room temperature (23 °C) following the method of Junaid Khan et al.34 n-Hexadecanoic acid was used as the standard. The amount of specific compounds that resembles the standard was expressed as micrograms per gram.
50) at a flow rate of 1 mL min−1 and head pressure of 300 kgf cm−2. The entire instrument room setup was maintained at room temperature (23 °C) following the method of Junaid Khan et al.34 n-Hexadecanoic acid was used as the standard. The amount of specific compounds that resembles the standard was expressed as micrograms per gram.
Statistical analysis
The percentage of larval mortality was calculated using the Abbott formula.28 The dose-response data were subjected to probit regression analysis29 for calculating the LC50, LC90, 95% fiducial limits of upper confidence limit and lower confidence limit, and the chi-square values were calculated using the IBM SPSS (Statistical Package of Social Sciences) software version 20.0 developed by Reddy et al.35 Results with P < 0.05 were considered to be statistically significant.Results
The fungal strain was isolated from an infected grasshopper, Melanoplus sanguinipes. The SDA plates showed (after incubation) a fungus with white fluffy cottony growth with pale yellow edges. The piece of mycelium was stained with lactophenol cotton blue and observed under a microscope (Lobomed, 40×) showing abundant conidiospores arising from the vegetative hyphae, bearing groups of clustered conidiogenous cells with the apical zig-zag appearance, branched to give rise to further conidiogenous cells; globose to flask-shaped, one-celled spherical conidia were recorded. Previously Hermanides36 and Seyed Ali Safari37 identified B. bassiana using fungal key manual ‘Studies in Mycology’.The larvicidal activity of mycelium ethyl acetate and methanol extracts obtained from B. bassiana was investigated. The ethyl acetate mycelium extract had a promising larvicidal activity (Table 1) against the 1st to 4th instar larvae (after 24 h of exposure period) on An. stephensi (LC50 = 42.82, 39.45, 25.72, and 32.66; LC90 = 254.67, 367.11, 182.27 and 199.20 μg mL−1) Cx. quinquefasciatus (LC50 = 72.38, 68.11, 27.06, and 35.495; LC90 = 481.68, 254.69, 129.83, and 146.24 μg mL−1) and Ae. aegypti (LC50 = 62.50, 52.89, 58.60, and 47.12; LC90 = 314.82, 236.18, 247.53, and 278.52 μg mL−1). The methanol mycelium extract (Table 2) showed considerable mortality against the vector mosquitoes i.e. An. stephensi, which had the better LC50 and LC90 values (LC50 = 65.22, 68.96, 67.64 and 52.95; LC90 = 317.77, 431.59, 345.35 and 687.70 μg mL−1) followed by Cx. quinquefasciatus (LC50 = 98.56, 80.85, 61.72 and 41.16; LC90 = 678.66, 399.97, 336.85 and 470.47 μg mL−1) and Ae. aegypti (LC50 = 64.94, 72.61, 61.90 and 57.65; LC90 = 961.97, 901.21, 439.32 and 916.04 μg mL−1). At a concentration of less than 50 μg mL−1 from B. bassiana, the mortality rates were slower, but the larvae became very slow-moving when compared with the control. The sub-lethal effects on the first and second larval instars were correlated with the minimum survival of the third and fourth instar larvae. The third and fourth instars larvae were also susceptible in the bioassay at the lowest lethal concentration. The dose dependent assay results showed that maximum (100%) mortality was obtained at a higher concentration (300 μg mL−1) against the different stages of instar larvae of the An. stephensi, Cx. quinquefasciatus and Ae. aegypti mosquitoes. At a higher concentration of extracts, the mortality rate was exhibited within 5 h of exposure. More than 50% mortality was observed within the first 10 h. The control showed a nil mortality in the concurrent assay. The χ2 value was significant at the P < 0.05 level.
| Mosquito species | Larvae stage | Concentration (μg mL−1) | Percentageb mortality ± SE | LC50 (LCL–UCL) (μg mL−1) | LC90 (LCL–UCL) (μg mL−1) | χ2 (df = 3) |
|---|---|---|---|---|---|---|
| a Control (deionized water) – nil mortality. LC50 – lethal concentration that kills 50% of the exposed larvae, LC90 – lethal concentration that kills 90% of the exposed larvae, LCL = lower confidence limit, UCL = upper confidence limit, df degree of freedom, * χ2 – chi-square values are significant at P < 0.05 levels.b The mean value of five replicates (±SE). | ||||||
| An. stephensi | I | 50 | 62.66 ± 2.5 | 42.826 (22.661–59.994) | 254.679 (196.072–400.697) | 14.266 |
| 100 | 68.00 ± 1.0 | |||||
| 150 | 71.66 ± 2.0 | |||||
| 200 | 81.66 ± 1.5 | |||||
| 250 | 93.66 ± 5.1 | |||||
| 300 | 99.33 ± 1.1 | |||||
| II | 50 | 63.33 ± 3.5 | 39.459 (15.560–60.018) | 367.114 (253.777–811.269) | 14.442 | |
| 100 | 66.66 ± 1.5 | |||||
| 150 | 68.66 ± 1.5 | |||||
| 200 | 74.66 ± 2.0 | |||||
| 250 | 87.33 ± 2.0 | |||||
| 300 | 98.33 ± 2.0 | |||||
| III | 50 | 71.00 ± 1.0 | 25.727 (8.271–42.558) | 182.275 (140.331–278.069) | 9.289 | |
| 100 | 79.00 ± 5.5 | |||||
| 150 | 83.33 ± 3.0 | |||||
| 200 | 86.33 ± 3.0 | |||||
| 250 | 93.66 ± 5.0 | |||||
| 300 | 100 ± 0.0 | |||||
| IV | 50 | 67.66 ± 3.2 | 32.664 (14.232–49.187) | 199.206 (155.735–297.324) | 8.545 | |
| 100 | 73.00 ± 2.0 | |||||
| 150 | 84.00 ± 1.0 | |||||
| 200 | 85.33 ± 1.5 | |||||
| 250 | 93.00 ± 3.6 | |||||
| 300 | 100 ± 0.0 | |||||
| Cx. quinquefasciatus | I | 50 | 48.33 ± 1.5 | 72.385 (47.687–92.674) | 481.686 (334.801–960.417) | 17.270 |
| 100 | 54.00 ± 2.0 | |||||
| 150 | 62.33 ± 1.5 | |||||
| 200 | 65.00 ± 1.7 | |||||
| 250 | 77.33 ± 1.1 | |||||
| 300 | 98.33 ± 0.5 | |||||
| II | 50 | 48.33 ± 3.2 | 68.117 (51.556–82.429) | 254.698 (208.256–343.894) | 13.911 | |
| 100 | 52.00 ± 2.6 | |||||
| 150 | 73.33 ± 3.0 | |||||
| 200 | 83.00 ± 3.0 | |||||
| 250 | 90.00 ± 3.6 | |||||
| 300 | 100 ± 0.0 | |||||
| III | 50 | 74.66 ± 1.5 | 27.063 (11.301–41.487) | 129.836 (103.262–172.038) | 8.658* | |
| 100 | 81.66 ± 1.5 | |||||
| 150 | 85.33 ± 2.0 | |||||
| 200 | 95.33 ± 2.5 | |||||
| 250 | 98.66 ± 1.5 | |||||
| 300 | 100 ± 0.0 | |||||
| IV | 50 | 70.33 ± 1.5 | 35.495 (19.588–49.247) | 146.249 (119.821–190.880) | 15.145 | |
| 100 | 75.00 ± 2.0 | |||||
| 150 | 82.33 ± 3.2 | |||||
| 200 | 95.00 ± 5.5 | |||||
| 250 | 99.66 ± 0.5 | |||||
| 300 | 100 ± 0.0 | |||||
| Ae. aegypti | I | 50 | 53.00 ± 1.0 | 62.506 (42.337–79.404) | 314.823 (242.389–487.932) | 14.334 |
| 100 | 55.33 ± 2.0 | |||||
| 150 | 66.66 ± 1.5 | |||||
| 200 | 80.00 ± 2.0 | |||||
| 250 | 85.66 ± 2.5 | |||||
| 300 | 99.33 ± 1.1 | |||||
| II | 50 | 56.00 ± 3.0 | 52.896 (34.846–68.158) | 236.183 (189.696–332.423) | 13.939 | |
| 100 | 65.00 ± 3.0 | |||||
| 150 | 73.66 ± 4.1 | |||||
| 200 | 82.33 ± 5.8 | |||||
| 250 | 93.66 ± 4.0 | |||||
| 300 | 100 ± 0.0 | |||||
| III | 50 | 52.00 ± 2.0 | 58.603 (40.851–73.647) | 247.535 (199.550–345.351) | 16.537 | |
| 100 | 65.33 ± 1.5 | |||||
| 150 | 68.33 ± 2.0 | |||||
| 200 | 81.00 ± 7.0 | |||||
| 250 | 94.00 ± 4.5 | |||||
| 300 | 100 ± 0.0 | |||||
| IV | 50 | 57.00 ± 1.0 | 47.125 (26.419–64.574) | 278.528 (212.833–445.541) | 15.999 | |
| 100 | 69.00 ± 1.3 | |||||
| 150 | 74.00 ± 1.0 | |||||
| 200 | 78.66 ± 0.5 | |||||
| 250 | 87.66 ± 1.5 | |||||
| 300 | 100 ± 0.0 | |||||
| Mosquito species | Larvae stage | Concentration (μg mL−1) | Percentageb mortality ± SE | LC50 (LCL–UCL) (μg mL−1) | LC90 (LCL–UCL) (μg mL−1) | χ2 (df = 3) |
|---|---|---|---|---|---|---|
| a Control (deionized water) – nil mortality. LC50 – lethal concentration that kills 50% of the exposed larvae, LC90 – lethal concentration that kills 90% of the exposed larvae, LCL = lower confidence limit, UCL = upper confidence limit, df degree of freedom, χ2 – chi-square values are significant at P < 0.05 levels.b The mean value of five replicates (±SE). | ||||||
| An. stephensi | I | 50 | 44.33 ± 1.5 | 65.224 (45.224–82.072) | 317.772 (246.041–484.853) | 5.135 |
| 100 | 64.33 ± 3.0 | |||||
| 150 | 69.00 ± 1.0 | |||||
| 200 | 79.00 ± 1.0 | |||||
| 250 | 83.33 ± 4.1 | |||||
| 300 | 96.00 ± 5.2 | |||||
| II | 50 | 43.33 ± 2.8 | 68.964 (45.281–88.493) | 431.598 (308.932–799.046) | 7.405 | |
| 100 | 64.00 ± 2.0 | |||||
| 150 | 66.00 ± 0.5 | |||||
| 200 | 68.33 ± 1.5 | |||||
| 250 | 81.00 ± 1.0 | |||||
| 300 | 92.66 ± 2.5 | |||||
| III | 50 | 49.00 ± 4.3 | 67.647 (46.999–85.017) | 345.357 (262.909–547.261) | 8.495 | |
| 100 | 52.66 ± 4.1 | |||||
| 150 | 68.00 ± 1.0 | |||||
| 200 | 73.00 ± 1.0 | |||||
| 250 | 85.00 ± 2.0 | |||||
| 300 | 92.33 ± 6.6 | |||||
| IV | 50 | 51.66 ± 2.5 | 52.954 (21.812–77.823) | 687.709 (398.781–2673.123) | 3.488 | |
| 100 | 62.66 ± 2.5 | |||||
| 150 | 67.66 ± 0.5 | |||||
| 200 | 71.66 ± 2.0 | |||||
| 250 | 75.33 ± 1.1 | |||||
| 300 | 88.33 ± 1.5 | |||||
| Cx. quinquefasciatus | I | 50 | 40.00 ± 7.2 | 98.565 (72.255–121.752) | 678.665 (441.025–1565.144) | 16.361 |
| 100 | 45.00 ± 2.0 | |||||
| 150 | 54.33 ± 1.5 | |||||
| 200 | 60.00 ± 3.6 | |||||
| 250 | 67.00 ± 2.6 | |||||
| 300 | 94.00 ± 6.9 | |||||
| II | 50 | 45.00 ± 5.5 | 80.851 (59.967–98.793) | 399.970 (300.347–648.342) | 7.986 | |
| 100 | 45.6 ± 3.0 | |||||
| 150 | 66.00 ± 1.0 | |||||
| 200 | 73.33 ± 1.5 | |||||
| 250 | 81.33 ± 1.5 | |||||
| 300 | 92.33 ± 6.8 | |||||
| III | 50 | 48.33 ± 3.2 | 61.721 (40.554–79.435) | 336.852 (255.152–542.614) | 1.783 | |
| 100 | 62.66 ± 2.5 | |||||
| 150 | 69.00 ± 1.0 | |||||
| 200 | 84.33 ± 1.5 | |||||
| 250 | 88.33 ± 1.5 | |||||
| 300 | 88.33 ± 1.5 | |||||
| IV | 50 | 59.66 ± 4.7 | 41.165 (14.889–63.584) | 470.474 (302.367–1328.572) | 3.475 | |
| 100 | 66.66 ± 2.0 | |||||
| 150 | 72.33 ± 2.5 | |||||
| 200 | 74.33 ± 1.5 | |||||
| 250 | 83.66 ± 4.0 | |||||
| 300 | 90.00 ± 1.0 | |||||
| Ae. aegypti | I | 50 | 50.66 ± 4.1 | 64.944 (29.362–92.251) | 961.973 (501.659–5352.134) | 4.495 |
| 100 | 51.66 ± 1.5 | |||||
| 150 | 60.33 ± 1.5 | |||||
| 200 | 67.33 ± 2.5 | |||||
| 250 | 72.00 ± 1.7 | |||||
| 300 | 85.00 ± 4.5 | |||||
| II | 50 | 43.33 ± 0.5 | 72.613 (38.530–99.148) | 901.215 (494.937–3877.842) | 0.352 | |
| 100 | 55.00 ± 1.0 | |||||
| 150 | 62.66 ± 4.9 | |||||
| 200 | 72.00 ± 2.6 | |||||
| 250 | 74.33 ± 1.5 | |||||
| 300 | 76.33 ± 2.8 | |||||
| III | 50 | 46.33 ± 6.6 | 61.909 (37.187–82.136) | 439.325 (307.906–869.960) | 2.492 | |
| 100 | 63.00 ± 2.0 | |||||
| 150 | 66.66 ± 3.5 | |||||
| 200 | 71.00 ± 1.0 | |||||
| 250 | 84.00 ± 1.0 | |||||
| 300 | 85.00 ± 1.7 | |||||
| IV | 50 | 51.00 ± 3.6 | 57.651 (22.651–84.807) | 916.043 (478.320–5338.628) | 0.937 | |
| 100 | 55.00 ± 2.6 | |||||
| 150 | 67.60 ± 2.0 | |||||
| 200 | 71.00 ± 1.0 | |||||
| 250 | 76.66 ± 3.0 | |||||
| 300 | 79.66 ± 4.7 | |||||
The results of the pupal mortality of mosquitoes (Table 3) were tested with six different concentrations (50 to 300 μg mL−1) of the fungus extracts. The fungal ethyl acetate mycelium extracts show better results against An. stephensi (LC50 = 40.66; LC90 = 184.02 μg mL−1) followed by Cx. quinquefasciatus (LC50 = 54.06; LC90 = 225.61 μg mL−1) and Ae. aegypti (LC50 = 44.26; LC90 = 263.02 μg mL−1) (Fig. 1), whereas the methanol extract revealed moderate pupicidal effects against An. stephensi (LC50 = 51.92; LC90 = 1196 μg mL−1), Cx. quinquefasciatus (LC50 = 69.29; LC90 = 862.25 μg mL−1) and Ae. aegypti (LC50 = 76.34; 1178.15 μg mL−1), (Table 4). At the concentrations of 300 μg mL−1 for the B. bassiana ethyl acetate constituents, about 90% of the mortality was observed within 18 h for An. stephensi and Cx. quinquefasciatus, followed by Ae. Aegypti, and a 100% pupal mortality was observed at the higher concentration of the extracts. The pupal toxicity revealed a dose-dependent mortality in the treatment against the An. stephensi, Cx. quinquefasciatus and Ae. aegypti. Based on the results, the ethyl acetate extract obtained from the fungal species was found to be an excellent pupicidal agent against the targeted mosquitoes An. stephensi, Cx. quinquefasciatus and Ae. aegypti.
| Mosquito species | Concentration (μg mL−1) | Percentageb mortality ± SE | LC50 (LCL–UCL) (μg mL−1) | LC90 (LCL–UCL) (μg mL−1) | χ2 (df = 3) |
|---|---|---|---|---|---|
| a Control (deionized water) – nil mortality. LC50 – lethal concentration that kills 50% of the exposed larvae, LC90 – lethal concentration that kills 90% of the exposed larvae, LCL = lower confidence limit, UCL = upper confidence limit, df degree of freedom, * χ2 – chi-square values are significant at P < 0.05 levels.b The mean value of five replicates (±SE). | |||||
| An. stephensi | 50 | 64.66 ± 1.0 | 40.661 (23.465–55.408) | 184.022 (149.315–250.834) | 14.510 |
| 100 | 73.66 ± 1.1 | ||||
| 150 | 74.33 ± 2.5 | ||||
| 200 | 91.33 ± 1.5 | ||||
| 250 | 97.33 ± 1.5 | ||||
| 300 | 100 ± 0.0 | ||||
| Cx. quinquefasciatus | 50 | 54.33 ± 1.5 | 54.064 (36.734–68.769) | 225.619 (183.306–309.150) | 10.558 |
| 100 | 66.66 ± 1.5 | ||||
| 150 | 73.00 ± 1.0 | ||||
| 200 | 85.00 ± 1.0 | ||||
| 250 | 93.66 ± 3.2 | ||||
| 300 | 100 ± 0.0 | ||||
| Ae. aegypti | 50 | 62.00 ± 2.6 | 44.263 (23.883–61.530) | 263.002 (201.843–417.120) | 14.921 |
| 100 | 67.00 ± 1.0 | ||||
| 150 | 71.00 ± 2.6 | ||||
| 200 | 81.33 ± 4.1 | ||||
| 250 | 91.66 ± 3.7 | ||||
| 300 | 100 ± 0.0 | ||||
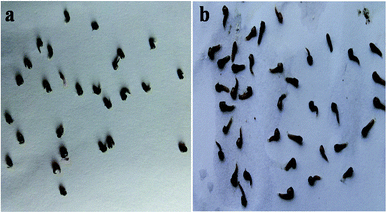 | ||
| Fig. 1 The pupicidal efficacy of the ethyl acetate extracts of B. bassiana against Cx. quinquefasciatus after 24 h of exposure: (a) control pupa, (b) pupa treated at a concentration of 300 μg mL−1. | ||
| Mosquito species | Concentration (μg mL−1) | Percentageb mortality ± SE | LC50 (LCL–UCL) (μg mL−1) | LC90 (LCL–UCL) (μg mL−1) | χ2 (df = 3) |
|---|---|---|---|---|---|
| a Control (deionized water) – nil mortality. LC50 – lethal concentration that kills 50% of the exposed larvae, LC90 – lethal concentration that kills 90% of the exposed larvae, LCL = lower confidence limit, UCL = upper confidence limit, df degree of freedom, χ2 – chi-square values are significant at P < 0.05 levels.b The mean value of five replicates (±SE). | |||||
| An. stephensi | 50 | 51.00 ± 3.6 | 51.925 (14.109–81.604) | 1196.224 (541.648–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 498.889) 498.889) |
1.285 |
| 100 | 61.33 ± 1.5 | ||||
| 150 | 65.00 ± 1.0 | ||||
| 200 | 68.33 ± 1.5 | ||||
| 250 | 71.33 ± 2.0 | ||||
| 300 | 81.33 ± 1.5 | ||||
| Cx. quinquefasciatus | 50 | 48.66 ± 3.7 | 69.299 (35.648–95.455) | 862.253 (477.816–3641.619) | 1.827 |
| 100 | 54.33 ± 1.5 | ||||
| 150 | 62.00 ± 2.0 | ||||
| 200 | 68.33 ± 1.5 | ||||
| 250 | 77.66 ± 2.5 | ||||
| 300 | 80.33 ± 1.5 | ||||
| Ae. aegypti | 50 | 48.66 ± 3.2 | 76.346 (38.351–105.396) | 1178.151 (578.043–7953.579) | 4.314 |
| 100 | 51.33 ± 2.5 | ||||
| 150 | 58.33 ± 1.5 | ||||
| 200 | 62.00 ± 4.3 | ||||
| 250 | 70.66 ± 2.0 | ||||
| 300 | 82.66 ± 2.5 | ||||
In addition, the toxicity of the n-hexadecanoic acid standard was tested against An. stephensi, Cx. quinquefasciatus and Ae. aegypti. The LC50 values of n-hexadecanoic acid against the first, second, third and fourth instar larvae of An. stephensi (LC50 = 50.22, 58.72, 2.27 and 38.61; LC90 = 105.09, 148.19, 15.910 and 81.98) and Cx. quinquefasciatus (LC50 = 10.64, 23.23, 12.75 and 0.72; 39.82, 55.53, 38.47 and 5.18) followed by Ae. aegypti (LC50 = 5.53, 12.46, 8.13 and 9.41; 21.25, 33.75, 30.57 and 27.36 μg mL−1) were recorded from present investigation. Similar observations were made for the pupicidal activity against An. stephensi, Cx. quinquefasciatus and Ae. aegypti; the LC50 and LC90 values were represented as follows: 8.66, 0.69, 3.05; 28.86, 4.38 and 11.43 μg mL−1, respectively. n-Hexadecanoic acid was found to show effective insecticidal activity against An. stephensi and Cx. quinquefasciatus, followed by Ae. aegypti.
Simultaneously, the Acremonium mycelium ethyl acetate extract showed larvicidal effects after 24 h of exposure. Considerable mortality was evident after the treatment of Acremonium for 1–4th instar larvae of three important mosquitoes. The LC50 and LC90 values of the first, second, third and fourth instars of An. stephensi (LC50 = 11.38, 8.18, 8.56 and 5.30; LC90 = 22.42, 17.19, 17.23 and 11.84 μg mL−1); Cx. quinquefasciatus (LC50 = 10.11, 13.35, 4.01 and 8.06; LC90 = 20.23, 25.13, 9.83 and 17.83 μg mL−1) and Ae. aegypti (LC50 = 8.50, 9.58, 15.26 and 10.35; LC90 = 18.02, 20.00, 28.88 and 21.51 μg mL−1) and the LC50 and LC90 values of the pupae (LC50 = 5.48, 9.60 and 3.99; LC90 = 14.46, 20.56 and 11.10 μg mL−1) were obtained from the present study.
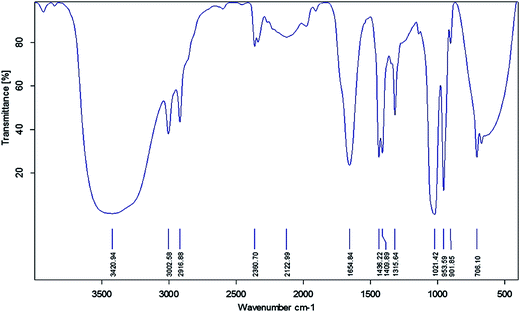
FTIR spectroscopy was used to identify the functional groups of the active compounds based on the peak value in the infra-red region. FTIR analysis of the ethyl acetate mycelium extract showed the presence of prominent bands due to the O–H group of hydrogen-bonded alcohols or phenols (3420.94), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H aromatics (3002.58), C–H alkanes (2916.88), –C
C–H aromatics (3002.58), C–H alkanes (2916.88), –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– nitriles (2122.99), –C
C– nitriles (2122.99), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– alkanes (1654.84), C–H alkanes (1436.22), C–C aromatics (1409.89), C–O carboxylic acids (1315.64), C–N aliphatic amines (1021.42),
C– alkanes (1654.84), C–H alkanes (1436.22), C–C aromatics (1409.89), C–O carboxylic acids (1315.64), C–N aliphatic amines (1021.42), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H alkenes (953.59), N–H primary amines (901.85) and C
C–H alkenes (953.59), N–H primary amines (901.85) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketones (706.10) cm−1 (Fig. 2 and Table 5).
O ketones (706.10) cm−1 (Fig. 2 and Table 5).
| Observed wavenumber (cm−1) | Functional group | Bonding pattern |
|---|---|---|
| 3420.94 | O–H stretch alcohols or phenols | Strong, broad |
| 3002.58 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H stretch aromatics C–H stretch aromatics |
Sharp |
| 2916.88 | C–H alkanes | Medium |
| 2122.99 | –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– stretch nitriles C– stretch nitriles |
|
| 1654.84 | –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– stretch alkanes C– stretch alkanes |
Medium |
| 1436.22 | C–H bend alkanes | Medium |
| 1409.89 | C–C stretch aromatics | Medium |
| 1315.64 | C–O stretch alcohols, carboxylic acids, esters, ethers | Sharp |
| 1021.42 | C–N stretch aliphatic amines | Medium |
| 953.59 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bending alkenes C–H bending alkenes |
Sharp |
| 901.85 | N–H wagging primary amines | Strong, broad |
| 706.10 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketone O ketone |
Sharp |
The GC-MS results obtained from the ethyl acetate extract of B. bassiana indicated the presence of six major compounds viz. 9,12-octadecadienoic acid (ZZ)– (63.16%), n-hexadecanoic acid (21.28%), octadecanoic acid, phenyl methyl ester (10.45%), dehydroegosterol 3,5-dinitrobenzoate (1.86%), squalene (1.66%), and bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate (1.56%) (Fig. 3 and Table 6). Hence, the isolated bioactive compounds obtained from the B. bassiana derived products, with proven potential as an insecticide, can play an important role in the interruption of the transmission of mosquito-borne diseases. The larvicidal and pupicidal activity of the ethyl acetate extract may be due to the presence of major bioinsecticide constituents such as 9,12-octadecadienoic acid (ZZ)– and n-hexadecanoic acid.
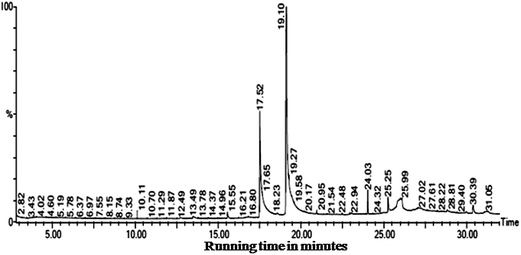 | ||
| Fig. 3 The insecticidal compounds identified in the ethyl acetate mycelium extracts obtained from B. bassiana. | ||
| Rt | Area | Area% | Molecular weight/formula | Compound name | Biological activity | References |
|---|---|---|---|---|---|---|
| a Components identified based on computer matching of the mass peaks with the NIST-2008 Library. | ||||||
| 17.519 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 887 887![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 080.0 080.0 |
21.286 | 256, C16H32O2 | n-Hexadecanoic acid | Nematicide, pesticide | Ragavendran and Natarajan 2015,60 Rajeswari et al. 2012,73 Zahir Hussain et al. 2010 (ref. 74) |
| 19.120 | 240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 006 006![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224.0 224.0 |
63.160 | 280, C18H32O2 | 9,12-Octadecadienoic acid (ZZ)– | Larvicide | Velu et al. 2014 (ref. 75) |
| 24.032 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307.5 307.5 |
1.663 | 410, C3CH50 | Squalene | Pesticide, antioxidant and antitumor | Rajeswari et al. 2012,73 WHO 1997 ref. 76 |
| 25.253 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480.0 480.0 |
1.865 | 588, C35H44O6N2 | Dehydroegosterol 3,5-dinitrobenzoate | Not known | Nil |
| 26.098 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176.0 176.0 |
10.458 | 374, C25H42O2 | Octadecanoic acid, phenyl methyl ester | Hypocholesterolemic and nematicide | Dr Duke's Phytochemical and Ethnobotanical Database77 |
| 30.390 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 952![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307.5 307.5 |
1.566 | 608, C38H56O6 | Bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate | Not known | Nil |
HPLC analysis of the ethyl acetate mycelium extract of B. bassiana and the n-hexadecanoic acid standard showed a similar chromatographic peak (at the retention time 3.383 and 3.378 min) (Fig. 4a and b).
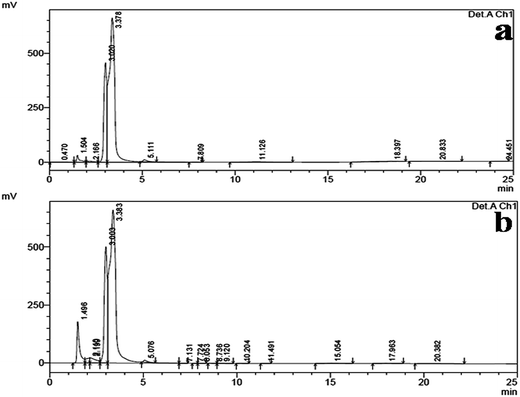 | ||
| Fig. 4 (a) The HPLC chromatogram of the n-hexadecanoic acid standard and (b) the HPLC chromatogram of the ethyl acetate mycelium extract obtained from B. bassiana. | ||
Discussion
Microbial sources serve as a guide for the isolation of several bioactive compounds particularly mosquito control agents. The entomopathogenic fungi have the ability to directly infect the host insect by penetrating into the cuticle and do not require ingesting by the insect to cause diseases. The fungi have a very narrow range and significant progress has been made in recent years towards the improvement of environmentally benign spores and mycelium-based biocontrol agents for mosquito populations. Fungal biocontrol agents have cheap inputs of unsafe synthetic chemical pesticides in agriculture, horticultural and forest systems.14 The results of fungal identification showed conidiogenous cells of B. bassiana densely clustered in whorls, globose or flask-like base, hyaline, smooth and short. The new conidium, giving a distinct zig-zag appearance in its colonies on PDA were round and flat, like a hyaline film from the radial growing mycelium. Similar results from B. bassiana were reported by Draganova et al.38 B. bassiana (Balsamo) is considered a very important and promising fungal agent for use in the control of insects.39 The fungus causes high mortalities in mosquito populations, as tested in numerous laboratories; Neetu Vyas et al.40 reported that Lagenidium giganteum fungus metabolites showed 100% mortality in first instar larvae against An. stephensi, Ae. aegypti, and Cx. quinquefasciatus. Mohanty and Prakash41 have described that the filtrate metabolites of Trichophyton ajelloi are effective on the larvae of two mosquito species, Cx. quinquefasciatus and An. stephensi. The culture filtrate metabolites of Chrysosporium tropicum were also found to be toxic and showed an LC50 and LC90 toxicity for all larval instars of An. stephensi tested at different concentrations.The present study exhibited that the bioactive metabolites of B. bassiana have larvicidal and pupicidal activity against Anopheles, Culex and Aedes mosquitoes. These metabolites may destroy the cuticle layer of the larvae and pupae, which leads to the death of the larvae and pupa. A similar study has been reported by Ababutain et al.,42 which identified Streptomyces sp. having better mosquitocidal properties. The use of fungus and their products are virulent and are a promising alternative insecticidal control agent.43 The efficacy of the insecticidal activity of B. bassiana products against the larvae of An. stephensi, Cx. quinquefasciatus and Ae. aegypti larvae showed that the LC50 and LC90 values for Cx. quinquefasciatus and Ae. aegypti were higher than An. stephensi. The LC50 values for the 1st to 4th instar larvae values were observed to be as follows: 65.22, 68.96, 67.64 and 52.95; LC90 = 317.77, 431.59, 345.35 and 687.70 μg mL−1, respectively. In the present study, after the treatment of the various larval stages of An. stephensi, Cx. quinquefasciatus and Ae. aegypti with the B. bassiana mycelia extracts at different concentrations, 100% mortality was observed based on the dose-dependent manner. Recently, Kovendan et al.,44 studied B. thuringiensis var. israelensis against the larvae of Cx. quinquefasciatus at different concentrations. The LC50 and LC90 values were reported as follows: the LC50 value of I instar was 9.332%, II instar was 9.832%, III instar was 10.212%, and IV instar was 10.622%, whereas the LC90 value of I instar was 15.225%, II instar was 15.508%, III instar was 15.887% and IV instar was 15.986%. Similar studies have been carried out by several researchers using bacteria Bacillus thuringiensis,45,46 Bacillus sphaericus47 and fungus Trichoderma viride48 and Actinobacteria,49 entomopathogenic fungi Metarhizium,50 Trichophyton,41 Tolypocladium,51 Chrysosporium52 and Lagenidium53 were reported as potential insecticidal agents.
The outcome of present study proved that mycelium extract of B. bassiana had a broad spectrum larval mortality against An. stephensi, Cx. quinquefasciatus and Ae. aegypti and the values were found to be as follows: for An. stephensi, LC50 = 65.22, 68.96, 67.64, and 52.95; LC90 = 317.77, 431.59, 345.35 and 687.70 μg mL−1; for Cx. quinquefasciatus, LC50 = 98.56, 80.85, 61.72, and 41.16; LC90 = 678.66, 399.97, 336.85 and 470.47 μg mL−1 and for Ae. aegypti, LC50 = 64.94, 72.61, 61.90 and 57.65; LC90 = 961.97, 901.21, 439.32 and 916.04 μg mL−1. Similarly, Vijayan and Balaraman54 isolated 94 actinomycetes from marine soil samples collected at different locations, out of which 35 samples exhibited improved larvicidal activity against Cx. quinquefasciatus, An. stephensi and Ae. aegypti with LC50 values in the range of 1–3 μL mL−1.
The larval and pupal mortality of Cx. quinquefasciatus after 24 h of treatment with the n-hexadecanoic acid standard showed the highest larvicidal (LC50 = 2.27 and LC90 = 15.91 μg mL−1) and pupal toxicity (LC50 = 0.69 and LC90 = 4.38 μg mL−1) than An. stephensi and Ae. aegypti. Similarly, Rahuman et al.55 reported a bioassay-guided fractionation of the acetone extract of Feronia limonia, which was shown as a potent mosquito larvicide, identified as n-hexadecanoic acid and found to be effective against fourth instar larvae of Ae. aegypti, Cx. quinquefasciatus and An. stephensi. Similarly, Sivakumar et al.31 found the larvicidal and repellent activity of pure tetradecanoic acid against Ae. aegypti and Cx. quinquefasciatus. The LC50 values were 14.08 and 25.10 μg mL−1. More recently, Srinivasan et al.56 reported the larvicidal potential of isolated thujone against the 4th instar larvae of Ae. aegypti (LC50 = 4.23 mg L−1) and An. stephensi (LC50 = 3.30 mg L−1). Fungal secondary metabolites have play an important roles in pathogenesis and the larvicidal activity, which can help in controlling mosquito populations and reduce the spread of vector borne diseases. Acremonium ethyl acetate metabolites were found to be more effective against Ae. aegypti and Cx. quinquefasciatus, followed by An. stephensi larvae. Furthermore, the pathogenicity of Acremonium sp. was also reported to possess good parasitic properties.57 Similarly, Stanly Pradeep et al.58 proved that F. oxysporum metabolites are more effective against An. stephensi than Cx. quinquefasciatus larvae.
The FTIR results indicated that the ethyl acetate mycelium extract showed the presence of chemical bands due to O–H group hydrogen-bonded alcohols or phenols (3420.94), ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H aromatics (3002.58), C–H alkanes (2916.88), –C
C–H aromatics (3002.58), C–H alkanes (2916.88), –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– nitriles (2122.99), –C
C– nitriles (2122.99), –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– alkanes (1654.84), C–C aromatics (1409.89), C–O carboxylic acids or alcohols (1315.64), C–N aliphatic amines (1021.42), N–H primary amines (901.85) and C
C– alkanes (1654.84), C–C aromatics (1409.89), C–O carboxylic acids or alcohols (1315.64), C–N aliphatic amines (1021.42), N–H primary amines (901.85) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ketones (706.10) cm−1. Similar functional groups were obtained by Nagajyothi et al.59 The GC-MS analysis results revealed that the larvicidal and pupicidal activity of mycelium ethyl acetate extracts from B. bassiana were exhibited due to six major compounds, namely 9,12-octadecadienoic acid (ZZ)– (63.16%), n-hexadecanoic acid (21.28%), octadecanoic acid, phenyl methyl ester (10.45%), dehydroegosterol 3,5-dinitrobenzoate (1.86%), squalene (1.66%), bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate (1.56%). Earlier, Ragavendran and Natarajan60 reported that the Aspergillus terreus ethyl acetate extract contains six bioactive compounds and its constituents showed better larvicidal and the pupicidal effects on selected mosquito vectors, namely An. stephensi (LC50 = 97.410, 102.551, 29.802 and 8.907; LC90 = 767.957, 552.546, 535.474 and 195.677 μg mL−1), Cx. quinquefasciatus (LC50 = 89.584, 74.689, 68.265 and 67.40; LC90 = 449.091, 337.355, 518.793 and 237.347 μg mL−1) and Ae. aegypti (LC50 = 83.541, 84.418, 80.407 and 95.926; LC90 = 515.464, 443.167, 387.910 and 473.998 μg mL−1). Pupicidal activity was also reported against An. stephensi (LC50 = 25.228; LC90 = 140.487 μg mL−1), Cx. quinquefasciatus (LC50 = 54.525; LC90 = 145.366 μg mL−1) and Ae. aegypti (LC50 = 10.536; LC90 = 63.762 μg mL−1). Squalene is considered as an important substance for practical and clinical use with huge potential in the nutraceutical and pharmaceutical industries.61 Similarly, Thimiri et al.62 reported that the Streptomyces sp. produced the isolated compound (2S,5R,6R)-2-hydroxy-3,5,6-trimethyloctan-4-one observed against the larvae of R. microplus (LC50 = 88.74 ppm; r2 = 0.865), An. subpictus (LC50 = 162.59 ppm; r2 = 0.817) and Cx. quinquefasciatus (LC50 = 120.15 ppm; r2 = 0.782). Kumar Saurav et al.63 reported that Streptomyces VITSVK5 sp. yielded the bioactive/isolated compound 5-(2,4-dimethylbenzyl) pyrrolidin-2-one, which had larvicidal activity against the larvae of R. microplus (LC50 = 210.39 ppm, r2 = 0.873), An. stephensi (LC50 = 169.38 ppm, r2 = 0.840) and Cx. tritaeniorhynchus (LC50 = 198.75 ppm, r2 = 0.887). Previously, some researchers have reported the insecticidal activity of isolated compounds obtained from the species of Streptomyces, namely tetranectin,64 avermectins,65 faeriefungin66 and macrotetrolides.67
O ketones (706.10) cm−1. Similar functional groups were obtained by Nagajyothi et al.59 The GC-MS analysis results revealed that the larvicidal and pupicidal activity of mycelium ethyl acetate extracts from B. bassiana were exhibited due to six major compounds, namely 9,12-octadecadienoic acid (ZZ)– (63.16%), n-hexadecanoic acid (21.28%), octadecanoic acid, phenyl methyl ester (10.45%), dehydroegosterol 3,5-dinitrobenzoate (1.86%), squalene (1.66%), bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate (1.56%). Earlier, Ragavendran and Natarajan60 reported that the Aspergillus terreus ethyl acetate extract contains six bioactive compounds and its constituents showed better larvicidal and the pupicidal effects on selected mosquito vectors, namely An. stephensi (LC50 = 97.410, 102.551, 29.802 and 8.907; LC90 = 767.957, 552.546, 535.474 and 195.677 μg mL−1), Cx. quinquefasciatus (LC50 = 89.584, 74.689, 68.265 and 67.40; LC90 = 449.091, 337.355, 518.793 and 237.347 μg mL−1) and Ae. aegypti (LC50 = 83.541, 84.418, 80.407 and 95.926; LC90 = 515.464, 443.167, 387.910 and 473.998 μg mL−1). Pupicidal activity was also reported against An. stephensi (LC50 = 25.228; LC90 = 140.487 μg mL−1), Cx. quinquefasciatus (LC50 = 54.525; LC90 = 145.366 μg mL−1) and Ae. aegypti (LC50 = 10.536; LC90 = 63.762 μg mL−1). Squalene is considered as an important substance for practical and clinical use with huge potential in the nutraceutical and pharmaceutical industries.61 Similarly, Thimiri et al.62 reported that the Streptomyces sp. produced the isolated compound (2S,5R,6R)-2-hydroxy-3,5,6-trimethyloctan-4-one observed against the larvae of R. microplus (LC50 = 88.74 ppm; r2 = 0.865), An. subpictus (LC50 = 162.59 ppm; r2 = 0.817) and Cx. quinquefasciatus (LC50 = 120.15 ppm; r2 = 0.782). Kumar Saurav et al.63 reported that Streptomyces VITSVK5 sp. yielded the bioactive/isolated compound 5-(2,4-dimethylbenzyl) pyrrolidin-2-one, which had larvicidal activity against the larvae of R. microplus (LC50 = 210.39 ppm, r2 = 0.873), An. stephensi (LC50 = 169.38 ppm, r2 = 0.840) and Cx. tritaeniorhynchus (LC50 = 198.75 ppm, r2 = 0.887). Previously, some researchers have reported the insecticidal activity of isolated compounds obtained from the species of Streptomyces, namely tetranectin,64 avermectins,65 faeriefungin66 and macrotetrolides.67
The HPLC analysis of the ethyl acetate mycelium extract was compared with the n-hexadecanoic acid standard and they showed a similar chromatographic peak (at a retention time of 3.383 and 3.378 min). The HPLC results were in agreement with the earlier reports of Ragavendran and Natarajan,60 and Manilal et al.68 who obtained (15.31 and 42%) n-hexadeconoic acid using different extracts. Previously, several researchers have isolated n-hexadecanoic acid from different plants and microbes i.e. Vitex altissima, V. negundo and V. trifolia,69 Aspergillus fumigatus,70 A. versicolor71 and Pestalotiopsis sp.72 The use of fungus based products would be cheaper, target-specific, self-sustained and highly toxic to mosquitoes, even at low doses.
Conclusion
Our findings confirm a promising as well as a novel biological based strategy to be integrated with additional control measures to reduce the global rate of vector-borne disease transmission. At a concentration of 300 μg mL−1 of B. bassiana ethyl acetate extract, 90% mortality was observed within 18 h against An. stephensi and Cx. quinquefasciatus, followed by Ae. aegypti and 100% pupal mortality was observed at higher concentrations. The pupal toxicity of the mosquitoes was mainly based on the dose-dependent effect against An. stephensi, Cx. quinquefasciatus and Ae. aegypti. GC-MS analysis of the ethyl acetate extract of B. bassiana identified six major components, i.e. 9,12-octadecadienoic acid (ZZ)– (63.16%), n-hexadecanoic acid (21.28%), octadecanoic acid, phenyl methyl ester (10.45%), dehydroegosterol 3,5-dinitrobenzoate (1.86%), squalene (1.66%) and bis[3-(3,5-di-tert-butyl-4-hydroxyphenyl)prophyl]maleate (1.56%). The bioactive compounds may be responsible for the larvicidal and pupicidal activity against An. stephensi, Cx. quinquefasciatus and Ae. aegypti mosquitoes. In addition, HPLC analysis of the ethyl acetate mycelium extract of B. bassiana and the n-hexadecanoic acid standard show a similar chromatographic peak (at a retention time of 3.383 and 3.378 min). Moreover, these metabolites can be used for the development of new insecticidal formulations to control vector borne diseases because they constitute a rich source of bioactive compounds that are more effective, eco-friendly, non-toxic, and potentially suitable for use in the management of target insects/pests. Further studies are ongoing for the isolation of pure active compounds and determination of the mode of action so as to recommend an eco-friendly measure for the control of mosquitoes.Authors' contributions
Conceived and designed the experiments: CR and DN Performed the experiments and analyzed the data: CR. CR and DN analyzed and interpreted the data and wrote the manuscript. Revision of the manuscript: DN and NKD Finally, all authors have read and approved the final manuscript.Compliance with ethical standards
All applicable international and national guidelines for the care and use of animals were followed. All procedures performed in the studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.Conflict of interest
The authors declare that they have no conflict of interest in this research article.Acknowledgements
The first author acknowledged to the Periyar University for providing financial support under University Fellowship Scheme (Ref No. PU/A&A-3/URF/2014). We would like to thank the Department of Biotechnology, School of Biosciences, Periyar University for providing necessary infrastructural facility for carrying out this study successfully. We also thank the Institute of Vector Control and Zoonoses (IVCZ) Hosur for supplying mosquitoes and we thank the Vellore Institute of Technology (VIT) for GC-MS analysis of our bio-samples. The authors would like to express their sincere thanks to Prof. P. Perumal, Marine Biotechnology Laboratory, Department of Biotechnology, Periyar University, Salem, Tamilnadu, India for providing the HPLC instrumental facility for analysis of the samples.References
- V. Corby-Harris, A. Drexler, L. Watkins de Jong, Y. N. Antonova and N. Pakpour, PLoS Pathog., 2010, 6, 1–10 CrossRef PubMed , e1001003.
- WHO, Larval source management: a supplementary measure for malaria vector controls an operational manual, 2013, pp. 1–116 Search PubMed.
- M. Govindarajan, T. Mathivanan, K. Elumalai, K. Krishnappa and A. Anandan, Asian Pac. J. Trop. Biomed., 2011, 1, 43–48 CrossRef CAS PubMed.
- WHO, http://www.who.int/media%20centre/factsheets/fs102/en/, 2012.
- Z. Peng, J. Yang, H. Wang and F. E. R. Simons, Insect Biochem. Mol. Biol., 1999, 29, 909–914 CrossRef CAS PubMed.
- WHO, http://www.who.int/features/factfiles/malaria/en/index.html, 2013.
- M. Z. Hossam El-Din and S. A. M. Abdelgaleil, J. Agric Res., 2010, 36, 385–401 Search PubMed.
- T. Su and M. S. Mulla, J. Am. Mosq. Control Assoc., 1998, 14, 204–209 CAS.
- S. Poopathi, Journal of Biofertilizers & Biopesticides, 2012, 3, 1–14 Search PubMed.
- M. Maheu-Giroux and M. C. Castro, PLoS One, 2013, 8, 1–11 Search PubMed.
- EPA, Biopesticide Fact Sheet: Beauveria bassiana strain ATCC. 74040, 2000 128818 Search PubMed.
- F. V. A. Howard, R. Guessan, C. J. M. Koenraadt, A. Asidi, F. Marit, M. Akoqbeto, M. B. Thomas, B. G. H. Knols and W. Takken, Malar J., 2010, 3, 1–11 Search PubMed.
- EPA, Biopesticide Registration Action Document: Beauveria bassiana HF23, 2006 Search PubMed.
- H. Strasser, A. Vey and T. Butt, Biocontrol Science and Technology, 2000, 10, 717–735 CrossRef.
- A. Vey, R. E. Hoagland and T. M. Butt, Toxic metabolites of fungal biocontrol agents. Fungi as biocontrol agents. progress, problems and potential, ed. T. M. Butt, C. Jackson and N. Magan, CABI Publishing, Oxford, UK, 2001, pp. 311–346 Search PubMed.
- E. Quesada-Moraga and A. Vey, Mycol. Res., 2004, 108, 441–452 CrossRef CAS PubMed.
- A. K. Charnley and R. J. S. Leger, The role of cuticle-degrading enzymes in fungal pathogenesis in insects, p. 267–287, in, Fungal Spore Disease Initiation in Plants and Animals, ed. E. T. Cole and H. C. Hoch, Plenum Press, New York, 1991, p. 555 Search PubMed.
- M. Govindarajan, A. Jebanesan and D. Reetha, Trop. Biomed., 2005, 22, 1–3 CAS.
- N. Haraprasad, S. R. Niranjans, H. S. Prakash, H. S. Shetty and W. Seema, Ind. Biocontrol Sci. Tech., 2001, 11, 251–260 CrossRef.
- R. A. Samson, Identification: Entomopathogenic Deuteromycetes, in, Microbial Control of Pests and Plant Diseases, ed. H. D. Burges, Academic press, London, 1981, pp. 93–106 Search PubMed.
- R. A. Samson, H. C. Evans and J. P. Latgé, Atlas of Entomopathogenic Fungi, Springer-Verlag, Berlin, 1988 Search PubMed.
- G. S. Hoog de, Stud. Mycol., 1972, 1, 1–41 Search PubMed.
- J. M. Gardner and J. S. Pillai, Mycopathologia, 1987, 97, 77–82 CrossRef CAS PubMed.
- G. N. Belofsky, M. Anguera, P. R. Jensen, W. Fenical and M. Kock, Chem.–Eur. J., 2000, 6, 1355–1360 CrossRef CAS.
- S. V. Patil, C. D. Patil, B. K. Salunke and R. B. Salunkhe, Trop. Biomed., 2010, 27, 360–365 CAS.
- WHO, 2005, WHO/CDS/WHOPES/GCDPP/2005:13.
- M. Govindarajan and G. Benelli, J. Parasitol. Res., 2016, 115, 925–935 CrossRef PubMed.
- W. S. Abbott, J. Econ. Entomol., 1925, 18, 265–266 CrossRef CAS.
- D. J. Finney, Probit analysis, Cambridge University Press, Cambridge, 1971, pp. 76–80 Search PubMed.
- R. R. Rahuman and P. Venketesan, J. Parasitol. Res., 2008, 103, 133–139 CrossRef PubMed.
- R. Sivakumar, A. Jebanesan, M. Govindarajan and P. Rajasekar, Asian Pac. J. Trop. Med., 2011, 4, 706–710 CrossRef CAS PubMed.
- K. V. Bhaskara Rao, K. S. Hemath Naveen, G. Kumar and L. Karthik, Arch. Appl. Sci. Res., 2010, 2, 161–167 Search PubMed.
- M. A. Hossain and M. D. M. Shah Sakari, Asian Pac. J. Trop. Med., 2011, 4, 637–641 CrossRef CAS PubMed.
- M. Junaid Khan, S. Swarnlata and S. Shailendra, J. Ethnopharmacol., 2016 DOI:10.1016/j.jep.2016.08.021.
- P. J. Reddy, D. Krishna, U. S. Murthy and K. Jamil, CABIOS, Comput. Appl. Biosci., 1992, 8, 209–213 CAS.
- E. J. Hermanides-Nijihof, (Stud. Mycol.15) Centraalbureau voor Schimecultures, Baarn, 1977, vol. 1, pp. 141–177 Search PubMed.
- S. Seyed Ali, J. Plant Prot. Res., 2010, 50, 159–163 Search PubMed.
- S. A. Draganova, I. Danail, D. Takov and D. Doychev, Pestic. Phytomed., 2010, 25, 59–63 CrossRef.
- W. B. Shi and M. G. Feng, Biol. Control, 2004, 30, 165–173 CrossRef.
- N. Vyas, K. K. Dua and S. Prakash, J. Parasitol. Res., 2007, 101, 385–390 CrossRef PubMed.
- S. S. Mohanty and S. Prakash, Curr. Sci., 2004, 86, 323–325 CAS.
- M. Ababutain, K. Zeinab, A. Abdul Azizand Nijla and A. L. Meshhen, Can. J. Pure Appl. Sci., 2012, 6, 1739–1748 Search PubMed.
- E. J. Scholte, K. Ng'habi, J. Kihonda, W. Takken, K. Paaijmans, S. Abdulla and G. F. Killeen, B. G. J. Knols Science, 2005, 10, 1641–1642 Search PubMed.
- K. Kovendan, K. Murugan, S. Vincent and S. Kamalakannan, J. Parasitol. Res., 2011, 109, 1251–1257 CrossRef PubMed.
- K. Balaraman, ICMR Bulletin, 1995, 25, 45–51 Search PubMed.
- G. Prabhakaran, V. Padmanaban and K. Balaraman, J. Biol. Contr., 2000, 14, 63–66 Search PubMed.
- G. Rajendran, S. Sabesan, K. Kuppusamy and K. Balaraman, Entomon, 1991, 16, 213 Search PubMed.
- I. Geetha and K. Balaraman, J Biol Contr, 2001, 15, 93 Search PubMed.
- D. Dhanasekaran, V. Sakthi, N. Thajuddin and A. Panneerselvam, Int. J. Appl. Biol. Pharm. Technol., 2010, 1, 374–381 Search PubMed.
- D. W. Roberts, Some effects of Metarhizium anisopliae and its toxins on mosquito larvae, in Insect pathology and microbial control, ed. Laan PA van der, North-Holland Publishing Company, 1967, pp. 243–246 Search PubMed.
- V. Matha, J. Weiser and J. Olejnicek, Parasitology, 1988, 35, 379–381 CAS.
- A. Priyanka and S. Prakash, J. Am. Mosq. Control Assoc., 2003, 19, 404–407 Search PubMed.
- N. Vyas, K. K. Dua and S. Prakash, Bull. Bio. Sci., 2006, 4, 65–69 Search PubMed.
- V. Vijayan and K. Balaraman, Indian J. Med. Res., 1991, 93, 115–117 CAS.
- A. A. Rahuman, G. Gopalakrishnan, B. S. Ghouse, S. Arumugam and B. Himalayan, Fitoterapia, 2000, 71, 553–555 CrossRef CAS PubMed.
- R. Srinivasan, D. Natarajan, M. S. Shivakumar, T. Vinuchakkaravarthy and D. Velmurugan, Ind. Crops Prod., 2015, 76, 394–401 CrossRef CAS.
- G. F. Atkinson, Some diseases of cotton, Albama Polytechnical Inst. Expt. Sta., 1892, 41, 61–65 Search PubMed.
- F. Stanly Pradeep, M. Palaniswamya, S. Ravi, A. Thangamani and B. V. Pradeep, Process Biochem., 2015, 50, 1479–1486 CrossRef.
- P. C. Nagajyothi, T. V. M. Sreekanth, J. L. Lee and K. D. Lee, J. Photochem. Photobiol., B, 2014, 130, 299–304 CrossRef CAS PubMed.
- C. Ragavendran and D. Natarajan, Environ. Sci. Pollut. Res., 2015, 22, 17224–17237 CrossRef PubMed.
- S. K. Kim and F. Karadeniz, Adv. Food Nutr. Res., 2012, 65, 223–233 Search PubMed.
- L. D. Thimiri, K. Kannabiran, V. Gopiesh Khanna, G. Rajakumar, C. Jayaseelan, T. Santhoshkumar and A. A. Rahuman, J. Parasitol. Res., 2012, 111, 1151–1163 CrossRef PubMed.
- S. Kumar, G. Rajakumar, K. Kannabiran, A. A. Rahuman, K. Velayutham, G. Elango, C. Kamaraj and A. A. Zahir, J. Parasitol. Res., 2013, 112, 215–226 CrossRef PubMed.
- K. Ando, How to discover new antibiotics for insecticidal use, in Pesticide chemistry: human welfare and the environment. Natural products, ed. T. Takahashi, H. Yoshioka, T. Misato and S. Matusunaka, Pergamon, New York, 1983, pp. 253–259 Search PubMed.
- S. Pampiglione, G. Majori, G. Petrangeli and R. Romi, Trans. R. Soc. Trop. Med. Hyg., 1985, 79, 797–799 CrossRef CAS PubMed.
- Anonymous, Biotechnol Abstr., 1990, 9, 58 Search PubMed.
- Z. Zizka, J. Weiser, M. Blumauerova and J. Jizba, Cytobios, 1989, 58, 85–91 CAS.
- A. Manilal, S. Sujitha, J. Selvin, C. Shakir and G. Seghal Kiran, Indian J. Exp. Biol., 2009, 78, 161–166 Search PubMed.
- K. Kannathasan, A. Senthilkumar, V. Venkatesalu and M. Chandrasekaran, J. Parasitol. Res., 2008, 103, 999–1001 CrossRef PubMed.
- J. Xu, L. Xianqun, Z. J. Z. Wenhua and T. Renxiang, J. Chem. Pharm. Res., 2014, 6, 893–897 Search PubMed.
- G. Senthilkumar, P. Madhanraj and S. Panneerselvam, Asian J. Pharm. Sci., 2011, 1, 19–21 Search PubMed.
- D. Li, Y. Shihong, P. Proksch, Z. Liang, Q. Li and J. Xu, Afr. J. Biotechnol., 2013, 12, 3802–3806 Search PubMed.
- G. Rajeswari, M. Murugan and V. R. Mohan, Res. J. Pharm., Biol. Chem. Sci., 2012, 3, 301–307 CAS.
- A. Zahir Hussain, I. Aruna and J. Asian, J. Chem., 2010, 22, 3591–3595 Search PubMed.
- K. Velu, D. Elumalai, P. Hemalatha, M. Babu, A. Janaki and P. K. Kaleena, Int. J. Mosq. Res., 2015, 2, 1–08 Search PubMed.
- WHO, Squalene-based adjuvants in vaccines, Global Advisory Committee on Vaccine Safety, 1997 Search PubMed.
- Dr. Duke's Phytochemical and Ethnobotanical Databases compiled by Dr. Jim Duke of the Agricultural Research Service/USDA.
| This journal is © The Royal Society of Chemistry 2017 |