Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molybdenum trioxide nanopaper as a dual gas sensor for detecting trimethylamine and hydrogen sulfide†
Hua-Yao Li a,
Liang Huangb,
Xiao-Xue Wangc,
Chul-Soon Leea,
Ji-Wook Yoona,
Jun Zhou
a,
Liang Huangb,
Xiao-Xue Wangc,
Chul-Soon Leea,
Ji-Wook Yoona,
Jun Zhou b,
Xin Guoc and
Jong-Heun Lee
b,
Xin Guoc and
Jong-Heun Lee *a
*a
aDepartment of Materials Science and Engineering, Korea University, Seoul 02841, Republic of Korea. E-mail: jongheun@korea.ac.kr
bWuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan 430074, P. R. China
cLaboratory of Solid State Ionics, School of Materials Science and Engineering, Huazhong University of Science and Technology, Wuhan 430074, P. R. China
First published on 17th January 2017
Abstract
A free-standing, flexible, and semi-transparent MoO3 nanopaper was fabricated using ultralong MoO3 nanobelts (length ∼ 200 μm; width 200–400 nm), and its gas-sensing characteristics were investigated. The sensor exhibited high responses (resistance ratio) of 49 to 5 parts per million (ppm) hydrogen sulfide (H2S) at 250 °C and 121 to 5 ppm trimethylamine (TMA) at 325 °C with excellent gas selectivity, demonstrating its dual function for gas detection. Moreover, the sensor showed promising potential for the all-in-one detection of three representative offensive odors (TMA, H2S, and NH3) simply by tuning of the sensing temperature. This particular performance is attributed to the high chemical affinity of MoO3 to H2S and the acid–base interaction between basic TMA/NH3 and acidic MoO3. The mechanism underlying the control of gas selectivity by modulating the sensor temperature was investigated by Diffuse Reflectance Infrared Fourier Transform (DRIFT) measurements.
Introduction
Trimethylamine (TMA) and hydrogen sulfide (H2S), which are generated by the decomposition of plants and animals, are colorless, flammable, and poisonous gases with unpleasant odors that are described as “fishy” and “rotten-eggs”, respectively.1–4 Although the smell is initially pungent, it rapidly paralyzes the olfactory system, resulting in unawareness of its presence. Exposure to a certain concentration (∼10 parts per million (ppm)) of either TMA or H2S can cause not only discomfort but also headaches, nausea, and irritation to the eyes and respiratory system.5,6 TMA and H2S are trace biomarkers (sub-ppm level)—detected in human exhaled breath—of renal-system imbalance and periodontal disease, respectively.7,8 Accordingly, the detection of sub-ppm to ppm levels of TMA and H2S is of vital importance in food quality control, environmental monitoring, and medical diagnosis.Metal-oxide semiconductors are widely used in gas sensors to detect trace concentrations of reducing gases because of their high response, simple structure, facile integration into small devices, and cost effectiveness.9–11 However, oxide-semiconductor gas sensors often exhibit similar gas responses to a range of different gases, which hampers the selective detection of a specific gas. Many efforts have been made to enhance the selectivity, including the modulation of the sensor temperature,12 the loading of noble-metal catalysts,13,14 the doping of catalytic oxides,15,16 and control of acid–base interactions.17,18 Although pattern recognition using an array of sensors is one solution for gas discrimination, the number of sensors should be minimized to reduce the size, cost, power consumption, and sensing circuit of devices.19,20 From this viewpoint, the detection of multiple gases using a single sensor is the ideal approach for realizing simple and reliable gas sensors.
To date, various metal-oxide semiconductors have been investigated for the detection of TMA and H2S. TiO2, WO3, MoO3, and LaFeO3 exhibit good sensing performance toward TMA,21–25 and ZnO, SnO2, WO3, CuO, MoO3, and BaCoO3 have been used to detect H2S.26–33 Note that, molybdenum trioxide (MoO3), which has abundant Lewis-acid sites, exhibits a high reactivity to both TMA and H2S, probably because of its high chemical affinity to H2S and strong interaction with basic TMA.15,24 This suggests the possibility of the simultaneous detection of ubiquitous offensive odors (TMA and H2S) using a single gas sensor.
Yang et al.34 fabricated a gas sensor by drop coating a slurry containing MoO3 nanobelts onto a substrate and demonstrated its potential for detecting TMA. However, neither the control of the selectivity to different offensive odors such as TMA, H2S, and NH3 nor the detailed gas-sensing mechanism was investigated. In the present study, MoO3 nanobelts with an average length of 200 μm were synthesized by a modified hydrothermal method and fabricated into a free-standing, flexible, semi-transparent ultrathin paper to increase the gas accessibility and the gas-sensing reliability. The sensor using MoO3 nanopapers showed the dual functionality to detect H2S at 250 °C and TMA 325 °C both with excellent selectivity. The control of the gas selectivity, the gas response, and dual gas sensing by the modulation of the sensor temperature was investigated in relation to the gas-sensing mechanism and surface-reaction analysis using Diffuse Reflectance Infrared Fourier Transform (DRIFT) spectra.
Experimental
Ultralong MoO3 nanobelts were synthesized via a modified hydrothermal method.35 2 g of molybdenum powder (Aladdin, 99.5%) to 10 mL of deionized (DI) water and 20 mL of 30 wt% H2O2 until the solution became light yellow. The solution was stirred for 30 min to react thoroughly and then transferred to a Teflon-lined stainless-steel autoclave and heated to 220 °C for 100 h. The precipitate was filtered and rinsed by DI water and ethanol several times.Vacuum filtration was employed to fabricate MoO3 nanopapers. First, 0.82 mg of MoO3 nanobelts was dispersed into 50 mL of DI water to form a homogeneous suspension. The as-prepared suspension was filtered through a membrane (pore size of 220 nm). After filtration, the nanopaper formed on the membrane was dried in air for 5 min and then peeled from the filter membrane to obtain a free-standing and semi-transparent MoO3 nanopaper.
The MoO3 nanopaper was cut into ∼1.5 mm × 1.5 mm pieces using scissors. A SiO2/Si substrate (area = 10 × 10 mm2, thickness = 0.5 mm) with two platinum electrodes on its top surface (electrode widths of 50 μm and separation of 100 μm) was used as the sensor substrate. To enhance the adhesion between the MoO3 nanopaper and the silicon-wafer substrate, a droplet of DI water (20 μL) was dropped on the substrate using a micro-pipette, and then the MoO3 nanopaper was placed on the substrate. After drying at 60 °C for 20 min, the sensor was annealed at 450 °C for 2 h to remove any residual organic content and to stabilize the sensor at the operation temperature. A schematic image of the MoO3-nanopaper sensor is shown in Fig. 1a.
 | ||
| Fig. 1 Schematic figure (a), X-ray diffraction (XRD) pattern (b), and SEM images (c)–(f) of the MoO3-nanopaper gas sensor. | ||
The crystal structure of the MoO3 nanopaper was investigated using an X-ray diffractometer (Philips X'Pert Pro) with Cu Kα radiation (λ = 1.54184 Å). The morphologies of the sensor were characterized using scanning electron microscopy (SEM, JEOL JSM-6060) and transmission electron microscopy (TEM, Tecnai G20). The reaction between the gas molecules and the sensing materials was analyzed using DRIFT spectra, which were recorded with a VERTEX 70-FTIR spectrometer equipped with a SMART collector and an MCT detector.
The sensors were placed in a quartz tube, and their gas-sensing characteristics were measured at 100–350 °C. A flow-through technique with a constant flow rate of 200 cm3 min−1 was used. The gas concentrations were controlled by changing the mixing ratio of the parent gases (TMA, H2S, NH3, NO2, acetone, benzene, ethanol, formaldehyde, toluene, xylene; all 5 ppm in dry synthetic air balance) and dry synthetic air. The interference gases are chosen for the practical applications of sensors in food quality control, environment monitoring and medical diagnosis. A thermocouple was placed near the sample to accurately monitor the sensor temperature. The direct-current two-point probe resistance of the sensor was measured using an electrometer with a computer interface.
Results and discussion
According to the XRD pattern shown in Fig. 1b, the nanopaper was identified as orthorhombic MoO3 (JCPDS no. 05-0508) with a highly ordered orientation. SEM images of the MoO3 nanobelt sensor (Fig. 1c–f) confirm that uniform MoO3 nanopapers consisting of highly interconnected MoO3 nanobelts were coated on the electrodes. The MoO3 nanobelts were 200–400 nm wide and 100–200 μm long, indicating that almost every MoO3 nanobelt could bridge two electrodes over the gap (100 μm). The highly interconnected and gas-accessible structures surely promote the gas-sensing reaction and carrier transportation. TEM and SAED images of the MoO3 nanobelts (Fig. 2) reveal that the nanobelts were single-crystalline with interplanar spacings of 0.37 and 0.40 nm along the perpendicular directions, which are consistent with the (002) and (200) d-spaces of MoO3.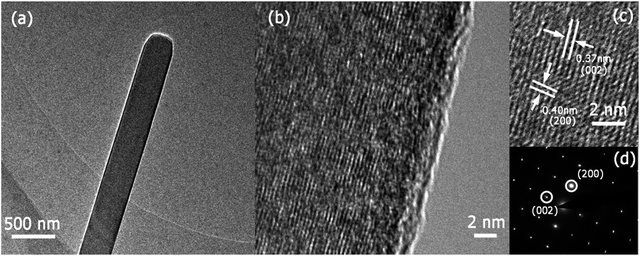 | ||
| Fig. 2 TEM image (a), high-resolution TEM images (b) and (c), and selected-area electron diffraction (SAED) pattern (d) of MoO3 nanobelts. | ||
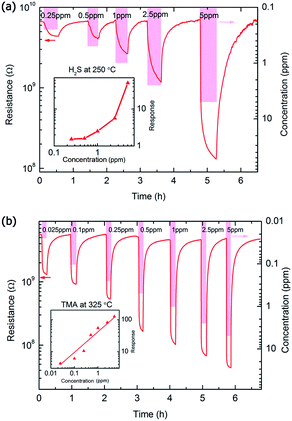
The responses of the MoO3 nanopaper to 5 ppm H2S and TMA at 250 and 325 °C are shown in Fig. S1.† The sensor resistance decreased when reducing analyte gas was introduced, which indicates typical n-type sensing. The gas response (S) is defined as Ra/Rg for reducing gas; and −Rg/Ra for oxidizing gas, where Ra and Rg are the sensor resistances in air and gas, respectively. The response and recovery time is defined as the time required to reach 90% variation of the sensor resistance upon exposure to the analyte gas or air. To better illustrate the sensor performance, the gas-sensing characteristics, including the gas response and response and recovery time, are listed in Table S1.†15,23–25,31,32,34 The MoO3-nanopaper sensor shows relatively rapid gas responses and complete recovery upon exposure to air.
The gas responses of the MoO3-nanopaper sensor to 5 ppm TMA and H2S were measured at 100–350 °C (Fig. 3). The maximum responses to TMA and H2S occurred at 325 and 250 °C, respectively. The selectivity of the sensors at different operating temperatures is presented in Fig. 4. At 250 and 325 °C, the MoO3-nanopaper sensor showed good selectivity toward H2S and TMA, respectively (Fig. 4b and d). The sensor showed a relatively high response to NH3 in the entire range of the sensor temperature (225–325 °C). This demonstrates the potential of the sensor to detect offensive odors. In particular, the comparable and high responses to 5 ppm TMA, H2S, and NH3 at 225 and 275 °C (Fig. 4a and c) indicate that the sensor can be used to detect total irritant gases with unpleasant smells in a selective manner. The all-in-one detection of TMA, H2S, and NH3 can be used in environment monitoring and food quality control.
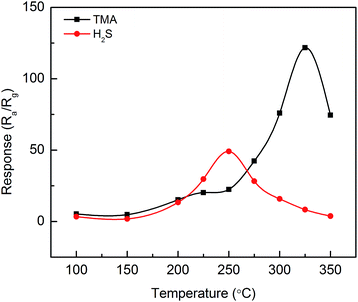 | ||
| Fig. 3 Responses of the MoO3-nanopaper sensor to 5 ppm TMA and H2S with respect to the sensing temperature. | ||
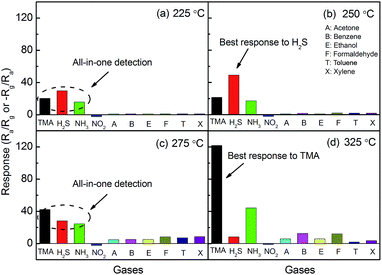 | ||
| Fig. 4 Selectivity of the MoO3-nanopaper sensor at different operation temperatures: (a) 225 °C, (b) 250 °C, (c) 275 °C, and (d) 325 °C. | ||
The response–recovery curves of the MoO3-nanopaper sensor for different gas concentrations are shown in Fig. 5. At 250 °C, the sensor detected 250 ppb H2S gas with a response of 1.53. At 325 °C, the response to 25 ppb TMA was 4.26, demonstrating that the detection limit was lower. According to the U.S. National Institute for Occupational Safety and Health, the permissible exposure limits of TMA and H2S are both 10 ppm.36,37 Although humans can recognize the presence of H2S at concentrations as low as 10–50 ppb, the human nose cannot quantify the concentration of H2S and is susceptible to olfactory fatigue.20 The presence of both gases at a concentration higher than 10 ppm in the ambient atmosphere is known to cause severe irritation to human beings. The presence of TMA and H2S in human exhaled breath (0.1–0.2 ppm for TMA, 0.1–0.5 ppm for H2S) indicates renal-system imbalance and periodontal disease, respectively.7,8 Thus, a MoO3-nanopaper sensor with an ultralow gas-detection limit, such as the proposed senor, can be used in various applications, including food quality control, environmental monitoring, and medical diagnosis.
The MoO3 sensor showed stable and reversible sensing characteristics upon repetitive exposure to 5 ppm H2S and TMA, as shown in Fig. S2,† confirming its good stability and reversibility. In general, CuO-loaded n-type semiconductors (SnO2, ZnO, and WO3) show high response and selectivity to H2S because of the strong chemical interaction between CuO and H2S and the conversion of semiconducting CuO into metallic CuS.26,29,38 However, the recovery is sluggish and often incomplete, which is mainly ascribed to the sluggish oxidation of CuS into CuO and/or the deterioration of the sensing surface by sulfur compounds.39,40 In contrast, MoO3 is known to form non-reactive adsorption compounds such as MoO3·SH2 and MoO2·S at 100–300 °C, and the material surface can be regenerated by the desorption of sulfur-containing surface species.41 This shows that MoO3 is a promising material for detecting H2S in a highly reversible manner. Moreover, acidic MoO3 can enable the detection of other offensive odors with alkaline nature, such as those of TMA and NH3.
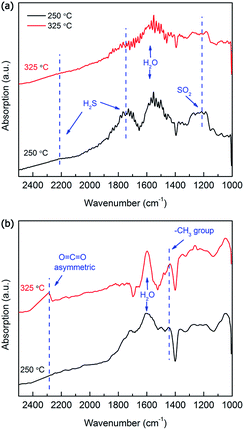
The surface reaction of the MoO3 nanopaper was investigated using DRIFT to investigate the gas-sensing mechanism (Fig. 6). Note that gas concentrations 10 times higher (50 ppm H2S and 50 ppm TMA) were used during the DRIFT measurements in order to observe the interaction between the analyte gas and the sensor surface more clearly. The absorption bands around 2200 and 1750 cm−1 are related to the H–S bond; the bands around 1250 cm−1 and 1600 cm−1 are related to the SO2 and H2O, respectively (Fig. 6a).42,43 The absorption bands at 1750, 1600, and 1250 cm−1 at 250 °C are substantially larger than those at 325 °C. The absorption at 1750 cm−1 suggests the active adsorption of H2S, and the absorption at 1600 and 1250 cm−1 indicates the promotion of the following gas-sensing reaction to form H2O and SO2:
| H2S + 3O− → H2O + SO2 + 3e− | (1) |
In Fig. 6b, the absorption band around 1450 cm−1 is related to the methyl groups,45 and that around 2280 cm−1 shows the presence of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, which were due to the asymmetric stretching vibration of CO2 molecules.46 As explained in Fig. 6a, the absorption band around 1600 cm−1 is related to H2O molecules. The intensities of the three bands are substantially higher at 325 °C than at 250 °C, indicating that an active surface reaction involving the methyl group occurred at 325 °C. The oxidation of TMA into CO2 and H2O by the reaction with negatively charged surface oxygen can be expressed as a probable sensing reaction:
O bonds, which were due to the asymmetric stretching vibration of CO2 molecules.46 As explained in Fig. 6a, the absorption band around 1600 cm−1 is related to H2O molecules. The intensities of the three bands are substantially higher at 325 °C than at 250 °C, indicating that an active surface reaction involving the methyl group occurred at 325 °C. The oxidation of TMA into CO2 and H2O by the reaction with negatively charged surface oxygen can be expressed as a probable sensing reaction:
| 2(CH3)3N + 21O− → N2 + 6CO2 + 9H2O + 21e− | (2) |
This accords with gas-chromatography results from the literature.34
The basic mechanism of chemiresistive metal-oxide gas sensing includes the adsorption and desorption of gas molecules, surface reaction, and charge transportation. The MoO3 nanopaper comprised a network of nanobelts that facilitated the adsorption, surface reaction, and desorption of the testing/product gases because of the high gas accessibility and large surface-to-volume ratio. When the MoO3 was exposed to air, oxygen molecules absorbed on the surface and formed chemisorbed oxygen species. Thus, a depletion layer was formed, which led to a high sensor resistance. When the MoO3 was exposed to H2S gas, its high chemical affinity to H2S promoted the adsorption of H2S and the gas-sensing reaction (1). DRIFT results clearly show that the H2S-sensing reaction involving the adsorption and oxidation of H2S was more active at 250 °C (Fig. 6a). When the MoO3 was exposed to TMA, the nitrogen atoms in TMA had a lone pair of electrons that could be donated to form bonds with the Lewis-acid (Mo ion) sites of MoO3. In addition, the methyl group in TMA easily reacted with the negatively charged oxygen on the surface. According to the DRIFT results (Fig. 6b), the gas-sensing reaction (2) was active at 325 °C, confirming that the MoO3 nanopaper had the best response to TMA at 325 °C.
Conclusions
Free-standing, semi-transparent, flexible MoO3 nanopapers were synthesized by a modified hydrothermal method and filtration. The nanopapers exhibited excellent dual-sensing properties toward H2S and TMA at different sensing temperatures. The sensor also demonstrated the potential for the all-in-one detection of three representative offensive odors (TMA, H2S, and NH3). The high chemical affinity of MoO3 to H2S and the regenerative refreshing of the sensor surface are suggested as reasons for the selective, sensitive, and reversible detection of H2S at 250 °C, while the acid–base interaction between acidic MoO3 and basic TMA was responsible for the selective and sensitive detection of TMA. The mechanism underlying the dual sensing was elucidated using DRIFT analysis. The proposed sensor provides a simple solution to detect various irritant gases in a highly selective manner.Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea, which is funded by the Korean government (Ministry of Education, Science, and Technology (MEST): Grant No. 2016R1A2A1A05005331).Notes and references
- W. Zhang and W. Zhang, Sens. Actuators, B, 2008, 134, 403–408 CrossRef CAS.
- G. Moula, M. Bose and S. Sarkar, Inorg. Chem., 2013, 52, 5316–5327 CrossRef CAS PubMed.
- R. J. Reiffenstein, W. C. Hulbert and S. H. Roth, Annu. Rev. Pharmacol., 1992, 32, 109–134 CrossRef CAS PubMed.
- L. Zhang, P. De Schryver, B. De Gusseme, W. De Muynck, N. Boon and W. Verstraete, Water Res., 2008, 42, 1–12 CrossRef CAS PubMed.
- H. U. Rehman, Postgrad. Med. J., 1999, 75, 451–452 CrossRef CAS PubMed.
- Y. Sun, X. M. Wang, Y. H. Chen, R. X. Zhu and C. C. Liao, Chin. Med. J., 2013, 126, 3240–3244 CAS.
- M. A. Bain, R. Faull, G. Fornasini, R. W. Milne and A. M. Evans, Nephrol., Dial., Transplant., 2006, 21, 1300–1304 CrossRef CAS PubMed.
- A. M. W. T. van den Broek, L. Feenstra and C. de Baat, J. Dent., 2007, 35, 627–635 CrossRef PubMed.
- I. D. Kim, A. Rothschild and H. L. Tuller, Acta Mater., 2013, 61, 974–1000 CrossRef CAS.
- A. Rothschild and H. L. Tuller, J. Electroceram., 2006, 17, 1005–1012 CrossRef CAS.
- H. J. Kim and J. H. Lee, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS.
- C. W. Na, H. Woo, I. Kim and J. Lee, Chem. Commun., 2011, 47, 5148–5150 RSC.
- S. J. Kim, I. S. Hwang, C. W. Na, I. D. Kim, Y. C. Kang and J. H. Lee, J. Mater. Chem., 2011, 21, 18560–18567 RSC.
- Y. J. Hong, J. W. Yoon, J. H. Lee and Y. C. Kang, Chem.–Eur. J., 2014, 20, 2737–2741 CrossRef CAS PubMed.
- H. S. Woo, C. H. Kwak, I. D. Kim and J. H. Lee, J. Mater. Chem. A, 2014, 2, 6412–6418 CAS.
- H. M. Jeong, H. J. Kim, P. Rai, J. W. Yoon and J. H. Lee, Sens. Actuators, B, 2014, 201, 482–489 CrossRef CAS.
- H. Li, Z. Cai, J. Ding and X. Guo, Sens. Actuators, B, 2015, 220, 398–405 CrossRef CAS.
- K. W. Kim, P. S. Cho, S. J. Kim, J. H. Lee, C. Y. Kang, J. S. Kim and S. J. Yoon, Sens. Actuators, B, 2007, 123, 318–324 CrossRef CAS.
- D. Li, T. Lei, S. Zhang, X. Shao and C. Xie, Sens. Actuators, B, 2015, 221, 556–563 CrossRef CAS.
- S. P. Zhang, C. S. Xie, D. W. Zeng, H. Y. Li, Y. Liu and S. Z. Cai, Sens. Actuators, B, 2009, 142, 243–252 CrossRef CAS.
- P. M. Perillo and D. F. Rodríguez, J. Alloys Compd., 2016, 657, 765–769 CrossRef CAS.
- S. Zhu, X. Liu, Z. Chen, C. Liu, C. Feng, J. Gu, Q. Liu and D. Zhang, J. Mater. Chem., 2010, 20, 9126–9132 RSC.
- Y. H. Cho, Y. C. Kang and J. H. Lee, Sens. Actuators, B, 2013, 176, 971–977 CrossRef CAS.
- Y. H. Cho, Y. N. Ko, Y. C. Kang, I. Kim and J. Lee, Sens. Actuators, B, 2014, 195, 189–196 CrossRef CAS.
- X. Chu, S. Zhou, W. Zhang and H. Shui, Mater. Sci. Eng., B, 2009, 164, 65–69 CrossRef CAS.
- S. J. Kim, C. W. Na, I. S. Hwang and J. H. Lee, Sens. Actuators, B, 2012, 168, 83–89 CrossRef CAS.
- J. W. Yoon, Y. J. Hong, Y. C. Kang and J. H. Lee, RSC Adv., 2014, 4, 16067–16074 RSC.
- A. Chowdhuri, V. Gupta, K. Sreenivas, R. Kumar, S. Mozumdar and P. K. Patanjali, Appl. Phys. Lett., 2004, 84, 1180–1182 CrossRef CAS.
- N. S. Ramgir, C. P. Goyal, P. K. Sharma, U. K. Goutam, S. Bhattacharya, N. Datta, M. Kaur, A. K. Debnath, D. K. Aswal and S. K. Gupta, Sens. Actuators, B, 2013, 188, 525–532 CrossRef CAS.
- J. Chen, K. Wang, L. Hartman and W. Zhou, J. Mater. Chem. C, 2008, 112, 16017–16021 CAS.
- Q. Ouyang, L. Li, Q. Wang, Y. Zhang, T. Wang, F. Meng, Y. Chen and P. Gao, Sens. Actuators, B, 2012, 169, 17–25 CrossRef CAS.
- W. Kim, H. Kim and S. Hong, J. Nanopart. Res., 2010, 12, 1889–1896 CrossRef CAS.
- H. Huang, H. Li, X. Wang and X. Guo, Sens. Actuators, B, 2017, 238, 16–23 CrossRef CAS.
- S. Yang, Y. Liu, W. Chen, W. Jin, J. Zhou, H. Zhang and G. S. Zakharova, Sens. Actuators, B, 2016, 226, 478–485 CrossRef CAS.
- B. Yao, L. Huang, J. Zhang, X. Gao, J. Wu, Y. Cheng, X. Xiao, B. Wang, Y. Li and J. Zhou, Adv. Mater., 2016, 28, 6353–6358 CrossRef CAS PubMed.
- The National Institute for Occupational Safety and Health (NIOSH), Trimethylamine, Centers for Disease Control and Prevention, Atlanta, GA, USA, 2006, http://www.cdc.gov/niosh/npg/npgd0636.html, accessed on October 2016.
- The National Institute for Occupational Safety and Health (NIOSH), Hydrogen Sulfide, Centers for Disease Control and Prevention, Atlanta, GA, USA, 2006, http://www.cdc.gov/niosh/idlh/7783064.html, accessed on October 2016.
- X. Xue, L. Xing, Y. Chen, S. Shi, Y. Wang and T. Wang, J. Mater. Chem. C, 2008, 112, 12157–12160 CAS.
- F. Zhang, A. Zhu, Y. Luo, Y. Tian, J. Yang and Y. Qin, J. Mater. Chem. C, 2010, 114, 19214–19219 CAS.
- C. S. Lee, Y. S. Kim, W. B. Hwang, Y. S. Jung, D. Ragupathy, S. I. Son, D. D. Lee and C. J. Kim, Sensors, 2013, 13, 3889–3901 CrossRef PubMed.
- S. Matsuda, T. Kamo, J. Imahashi and F. Nakajima, Ind. Eng. Chem. Res., 1982, 21, 18–22 CrossRef CAS.
- T. J. Toops and M. Crocker, Appl. Catal., B, 2008, 82, 199–207 CrossRef CAS.
- R. V. Siriwardane and S. Woodruff, Ind. Eng. Chem. Res., 1997, 36, 5277–5281 CrossRef CAS.
- S. Bai, C. Chen, R. Luo, A. Chen and D. Li, Sens. Actuators, B, 2015, 216, 113–120 CrossRef CAS.
- N. Nishino, K. D. Arquero, M. L. Dawson and B. J. Finlayson-Pitts, Environ. Sci. Technol., 2014, 48, 323–330 CrossRef CAS PubMed.
- J. Ding, H. Li and X. Guo, Solid State Ionics, 2015, 272, 155–159 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Response and recovery curves of MoO3-nanopaper gas sensor, stability of the MoO3-nanopaper sensor, and Gas-sensing performance of various sensors. See DOI: 10.1039/c6ra26280e |
| This journal is © The Royal Society of Chemistry 2017 |