Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced acaricidal activity of ricinine achieved by the construction of nano-formulation using amphiphilic block copolymer
Yingqiang Zhang†
a,
Jun Cheng†*b,
Saina Yangc,
Fuxin Liang*c and
Xiaozhong Qu *a
*a
aCollege of Materials Science and Opto-Electronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China. E-mail: quxz@iccas.ac.cn
bCollege of Biological Science and Engineering, Beijing University of Agriculture, Beijing 102206, China. E-mail: chengjun@bua.edu.cn
cState Key Laboratory of Polymer Physics and Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: liangfuxin@iccas.ac.cn
First published on 17th January 2017
Abstract
Efficient control of Tetranychus cinnabarinus (B.) is in challenge worldwide. Herein we report the use of an amphiphilic block copolymer, poly(ethylene oxide)-b-poly(caprolactone) (PEO–PCL), as a micellar carrier to make formulations of ricinine, a water insoluble botanical pesticide, and the tests of their physical properties and moreover the acaricidal activity on Vigna unguiculata (L). Compared to the formulations made from small molecular surfactant, e.g. Tween-80, the polymer formulations showed differentiated spreading property on T. cinnabarinus (B.) and V. unguiculata (L.) leaf surfaces, i.e. having slightly lower contact angle on the mite's integument. This contributes a relatively easy wash-off performance of the polymer formulations from the V. unguiculata (L.) leaf and meanwhile an enhanced protection to the plant in the simulated field trial. Our work thus suggests favorable characteristic of amphiphilic polymer in the future development of insecticides.
Introduction
The carmine spider mite, Tetranychus cinnabarinus (B.), is an important phytophagous pest which attacks more than one hundred agriculture crops or plants like fruits, cotton, beans, peppers, tomatoes, cucurbits and so on.1–3 However, due to its short life cycle, high reproductive capacity and strong adaptability, T. cinnabarinus (B.) had rapidly developed high resistance to chemical acaricides.4 To date, T. cinnabarinus (B.) has proven highly resistant to at least 25 chemical pesticides, such as bifenazate and hexythiazox, in China and other countries.5 Thus the mite population control encounters a great challenge.4,6,7Ricinine (Nr-methyl-3-cyano-4-methoxy-2-pyridone) is an alkaloid found in the leaves and seeds of Ricinus communis (L.), which showed excellent insecticidal activity against Callosobruchus chinensis (L.), Atta sexdens rubropilosa (F.), Anopheles arabiensis (P.), Culex quinquefasciatus (S.), Meloidogyne incognita (K. & W.) and Rhipicephalus sanguineus (L.).8–12 And more important, as a botanical pesticide, ricinine enables to maintain bioactivity to pests for much longer time than chemical insecticides,11 and was found to have strong contact toxicity on T. cinnabarinus (B.) in our laboratory recently. However, because of its poor water solubility, delivery system is necessary to allow the pesticide to penetrate through the mite skin and thus influence its nervous system.13 Micelle and emulsion formulations are now popular in the application of water insoluble pesticides, with the addition of surfactants, to solubilize lipophilic compounds and stabilize the liquid–solid or liquid–liquid interface.14 Besides, surfactants also play as a wetting agent that helps the spreading of the aqueous dispersion onto the surface of plants, in case the surface is hydrophobic due to the secretion of waxy and rough substance such as on certain amount of crops, and hence to transport to the pests.15 Nevertheless, so far drawbacks are still revealed from the traditional pesticide dispersions.16–18 (1) Low bioavailability, related to the poor control of spreadability, leads to ca. 90% of the pesticides missing the objects due to the surface runoff, evaporation, degradation and photolysis. (2) Contamination, with high affinity of the pesticides on the plants, results in the loss of edibility especially for vegetables. (3) Environmental pollution, will still happen caused by the additives, e.g. the surfactants, due to the uncontrollable deposition and degradation (into harmful produces). Therefore, in the past two decades, great efforts have been made on the development of efficient, safe and “green” pesticide formulations.
Amphiphilic copolymers have been widely applied in drug delivery systems,19 and recently are also focused as a carrier for the delivery of pesticides.20,21 Such polymer can form aggregates with various microstructures, e.g. micelle, vesicle and nanoparticle, which involve hydrophobic microdomains that are capable to entrap water insoluble stuffs including the hydrophobic pesticides. With higher molecular weight and preferable thermodynamic stability, the assemblies formed by amphiphilic copolymers are recognized to have lower critical aggregation concentration (CAC) and higher resistance against dilution compared to the surfactant-based micelles.22,23 In addition, the solubilization capacity of amphiphilic copolymers could be more sufficient if reactive and functional groups are introduced into the polymer chain.24 Besides, it is also facile to adjust the amphiphilicity of a copolymer, e.g. the HLB value, for example by changing the chemical structure as well as the molecular weight of the blocks, and thus to tune the spreading property/surface tension of its dispersion.25,26 Nevertheless, up to now only a few works have been reported on the use of amphiphilic copolymers, e.g. Pluronic F127 and poly(ethylene oxide)-b-poly(caprolactone), in the formulation of fungicide agents.21 Deep understanding on the characteristics of polymer formulations for pesticide delivery is still necessary.
The main aim of this work was to develop ricinine formulations using an amphiphilic copolymer, i.e. amine-functionalized poly(ethylene oxide)-b-poly(caprolactone) (PEO–PCL), as the solubilizer. While PEO–PCL is well-known on its biodegradation and carrier properties in pharmaceutic field,27 the objective of the current study was not limited to the increase of the ricinine solubilization by the copolymer, but also to gain a selectively enhanced spreading of the formulation on the T. cinnabarinus (B.), by regulating stronger adhesion of the copolymer with the mite surface than that with the surface of a plant, i.e. Vigna unguiculata (L). For the investigation, Tween-80, a typical surfactant that is commonly used in pesticides, was selected and examined as a control sample. It was known that small molecular surfactants normally have hydrophobic tails composed of unsaturated fatty acid ester, which shows structural similarity to the compounds found on the surface of crops like V. unguiculata (L).28,29 In contrast, despite also having a hydrophobic surface, the integument of the mites, e.g. the T. cinnabarinus (B.), is constructed by various proteins such as chloride channel protein, cytochrome b, carboxypeptidase and chitin binding protein in the peritrophic membrane.30,31 Therefore, we could hypothesize that the multiple ester bonds in the PEG–PCL, i.e. the PCL block, would favor the generation of non-covalent interactions of the copolymer with the mites. The enhanced interactions would further decrease the surface energy of the polymer formulation on the mite surface and thus lead to a passive acceleration of the pesticide to the mites.
Experimental
Materials
Ethylene oxide (EO, BDH Limited Poole, England) and ε-caprolactone (ε-CL, Acros Organics) were dried with calcium hydride (CaH2) and distilled before use. Potassium bis(trimethylsilyl)amide ((CH3)3Si2NK) was purchased from Alfa Aesar. Other compounds and solvents were obtained from J&K Chemical and used without further purification.Preparation of ricinine
The dry castor cake (1 kg) was extracted with 2 liters boiling water for 3 times, and the crude extract (68 g) was obtained after concentration under vacuum. The crude extract (65 g) was extracted with hot chloroform (CHCl3) in Soxhlet extraction tube for 4 h, 3 times. The CHCl3 extract was combined and concentrated under vacuum, then the yellow solid (1.8 g) were obtained. The yellow solid (1.5 g) was dissolved in 20% hot ethanol for an immediate filtration. The filtrate was put in refrigerator overnight, then the ricinine crystals (580 mg) was obtained in the filtrate.Synthesis of amine-functionalized poly(ethylene oxide)-b-poly(caprolactone) (PEO–PCL)
Amine functionalized PEO–PCL was synthesized by a sequential ring-opening polymerization procedure.32 Briefly, silicane protected amine-terminated poly(ethylene oxide) ((CH3)3Si2N-PEO) was first prepared by initiating EO with (CH3)3Si2NK in anhydrous THF. Then, ε-CL was added and polymerized as the second segment to get (CH3)3Si2N-PEO-b-PCL. The copolymer was isolated by precipitation in cold ethyl acetate for three times and drying in vacuum. The protection group of (CH3)3Si2N-PEO-b-PCL was removed by adding moderate acetic acid, achieving H2N-PEO-b-PCL (PEO–PCL) in a yield of 85% to the total mass of monomers.Mite preparation
A rearing stock of Tetranychus cinnabarinus (B.) was established at the Beijing University of Agriculture by collecting naturally occurring mites from flowering peach and transferring them to cowpeas, i.e. V. unguiculata (L.), that were sown in plastic pots filled with pot soil (10 by 10 cm) and grown in a greenhouse. The plants used for rearing were 15–20 days old and had six true leaves. The infested cowpeas were held at 27 ± 2 °C, 60% RH, and a photoperiod of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h (L/D) and 50
8 h (L/D) and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Im m−2. The female adults T. cinnabarinus (B.) as experimental mites are collected from infested V. unguiculata (L.) leaves in accordance with similar age (around 10 days) and size.
000 Im m−2. The female adults T. cinnabarinus (B.) as experimental mites are collected from infested V. unguiculata (L.) leaves in accordance with similar age (around 10 days) and size.
Preparation of PEO–PCL micelle and ricinine loading
Desired amount of PEO–PCL was dispersed in double distilled water (DDI water) and probe-sonicated for 1 min using a JY96-II probe sonicator (Zhejiang Xin-Zhi, China) with the output set at 150 W. Then the dispersion was diluted to predicted concentrations by adding calculated volume of DDI water. The samples were stood at room temperature for 4 h before the loading of the ricinine.Ricinine powder was weighed in glass vials, to which designed volume of polymer dispersion was pipetted to reach the calculated concentration of the pesticide. The mixtures were further dispersed by probe-sonication for 1 min at 150 W and cooled down to ambient temperature to get ricinine solubilized polymeric micelle dispersions. In separate experiment, 60 mg of ricinine was dissolved in 10 mL of ethyl acetate to make a stock solution of the pesticide. Subsequently calculated volume of the stock solution was pipette into glass vials followed by the addition of desired amount of polymer dispersion. The mixture was probe-sonicated for 1 min to gain ricinine emulsion.
For the preparation of control formulations, Tween-80 was used to replace the polymer to prepare ricinine/Tween-80 micelle dispersions and ricinine/Tween-80 emulsions under the same procedures as described above. The content of the as-prepared ricinine formulations is listed in Table 1.
| Sample | Ricinine (mg mL−1) | PEO–PCL (mg mL−1) | Tween-80 (mg mL−1) | Ethyl acetate (v/v) | LEa (%) | LCa (%) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Measured after 24 h equilibrium at RT. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| C1 | 1.5 | 0 | 0 | 0 | — | — | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P2 | 1.5 | 5 | 0 | 25% | 94.6 | 20.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P3 | 1.5 | 5 | 0 | 0 | 91.3 | 21.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| P6 | 10 | 5 | 0 | 25% | 96.6 | 63.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T4 | 1.5 | 0 | 5 | 25% | 95.0 | 20.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T5 | 1.5 | 0 | 5 | 0 | 89.2 | 21.1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| T7 | 10 | 0 | 5 | 25% | 96.7 | 63.7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The ricinine loading efficiency (LE) and loading capacity (LC) was determined using HPLC by filtration method.23 After the pesticide dispersions were held at room temperature for 24 h, the formulations were filtered by 200 nm syringe filters. The filtrate was diluted by 100 times and 20 μL of the samples were injected to a HPLC (Agilent Technologies 1200 Series) equipped with a ZORBAX Eclipse Plus C18 column (150 mm × 4.6 mm, 5.0 μm, Agilent Corp., USA) at 37 °C and a UV detector set at 321 nm. A mobile phase of acetonitrile and water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v) was used and elution rate was at 1 mL min−1. The LC and LE are defined as the percentage of ricinine in the filtrate to total amount of pesticide loaded and the percentage of the ricinine to the mass of copolymer or Tween-80 used in the formulation, respectively.
7 v/v) was used and elution rate was at 1 mL min−1. The LC and LE are defined as the percentage of ricinine in the filtrate to total amount of pesticide loaded and the percentage of the ricinine to the mass of copolymer or Tween-80 used in the formulation, respectively.
Characterizations
Molecular weight (MW) and polydispersity index (PDI) of PEO–PCL were determined using a Waters 515 gel permeation chromatography (GPC) instrument with three linear Styragel columns and a 2411 refractive index detector with THF at a flow rate of 1.0 mL min−1 at 40 °C. Critical micellization concentration (CMC) of the PEO–PCL was determined by fluorescence method using pyrene as a probe. Size distribution of the formulations was measured using a Zetasizer (Nano Series, Malvern Instruments, UK) at 25 °C with a scattering angle of 176.1°. Scanning electron microscopy (SEM) was taken on Hitachi S-4800 at 15 kV. V. unguiculata (L.) leaves were collected, immediately washed with water, wiped and dropped by ricinine formulations using a sprayer. The formulation wetted leaves were held in desiccators to allow the evaporation of water. And the dried leaves were cut into pieces and mounted on SEM stubs using conductive adhesive tape and sputter coated with a 10 nm layer of gold for the SEM observation.Contact angle measurement was conducted using a Krüss DSA 100 analyzer by dropping 2 μL of specimen onto the substrate, i.e. a piece V. unguiculata (L.) leaf pasted on a glass slide. The contact angle was then determined with the Drop Shape Analysis software. Contact angle hysteresis was measured by carefully tilting the substrate to 60° to the horizontal plane at which the difference between the advancing and the receding angle, i.e. the hysteresis value, was recorded. No roll-off of the drops was observed from samples tested in this work up to a maximum tilting angle of 80°. The measurement was done in triplicates.
Dynamic retention of ricinine on V. unguiculata (L.) leaf
Fresh V. unguiculata (L.) leaves were cut into squares at 3 × 3 cm. A piece of the cut leaf was put into the ricinine formulation perpendicularly to immerse for 5 s and lifted out carefully. The wet leaf was hanged for 15 min to dry the surface water, and then covered by filter paper to extract in methanol using a Soxhlet extractor for 48 h. The amount of ricinine was quantitatively determined by HPLC. The dynamic retention level was then calculated based on the average of three repeated measurements and described as the mass of extracted ricinine to the mass of the V. unguiculata (L.) leaf.Ricinine retention against washing
V. unguiculata (L.) were planted in greenhouse and used for rearing 15–20 days after seeding, then have six true leaves. Ricinine formulation was sprayed on the leaves at the level of 1 mL per leaf in average. The plants allowed to grow for another 12 days, and were watered every day, before all six leaves, with an average weight of 6.0 ± 0.2 g, were collected per plant and were immersed in 1 L of DDI water with four changes in every 20 min. The cleaned leaves were then wiped to remove surface water, weighted and extracted using the Soxhlet extractor to determine the residue amount of ricinine. The experiment was done with three repeats on each formulation and the residue retention was calculated as the average mass of ricinine on one gram of the V. unguiculata (L.) leaf.Acaricidal activity assay
Acaricidal activities of the ricinine formulations were determined by glass slide-dipping method. Female adult T. cinnabarinus (B.) were affixed on double-sided adhesive tape, which was pre-attached to one end of a 10 × 2 cm glass slide. Afterwards, the mites were individually dipped into various formulations and different diluting times for 5 s. Once the slide was removed, any extra solution was absorbed with filter papers. Total 30 females were used in each concentration assay. All treated mites were maintained at a temperature of (27 ± 2) °C, 60% RH and a photoperiod of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h (L/D). The mite mortality rates were monitored 24 h after each treatment. The tested mite was considered dead if mite did not move when touched gently with a camel hair brush. All treatments were replicated for three times.
8 h (L/D). The mite mortality rates were monitored 24 h after each treatment. The tested mite was considered dead if mite did not move when touched gently with a camel hair brush. All treatments were replicated for three times.
T. cinnabarinus (B.) inhibition of ricinine formulation in simulated field trial
After 15 V. unguiculata (L.) plants were sown and grown in greenhouse for 15–20 days, adult T. cinnabarinus (B.) were transferred onto the leaves of the plants at 60 mites per leaf on both top and bottom sides. Then the plants were randomly divided into 5 groups (three plants in each group). Selected ricinine formulations, i.e. C1, P2, P3, T4 and T5 (Table 1), were diluted using DDI water for 15 times, i.e. to get a ricinine concentration of 0.1 mg mL−1, and loaded at 10 mL to each plant which were homogeneously sprayed to both sides of the leaves. The plants further grew for over 15 days, without additional treatment. At predetermined time points, the disease index (DI) of each plant was evaluated according to the following formula:33 DI = [∑(Ni × i)/(N × 5)] × 100, where i means a 0–5 disease level of the leaf, Ni means number of the leaves on i and N means the number of total leaves measured. The disease level is defined as: 0 grade, leaf without scab; 1 grade, leaf with 1–5% scab area; 2 grade, leaf with 6–10% scab area; 3 grade, leaf with 11–25% scab area; 4 grade, leaf with 26–50% scab area; 5 grade, leaf with >50% scab area to the total area of the piece of leaf. The experiment was repeated for four times separately (with total 12 plants for each group).Statistical analysis
The mortality data were corrected for control mortality using Abbott's formula.34 Concentration–mortality data were subjected to probit analysis for medium lethal concentration (LC50) and toxicity regression equation (SPSS Institute, 2004). The LC50 values for each acaricidal preparation and their treatments were considered to be significantly different from one another when their 95% confidence limits (CLs) did not overlap.Results and discussion
Preparation of PEO–PCL micelle
The synthesis procedure of PEO–PCL was according to literature, which followed ring-opening polymerization mechanism of ethyl oxide (EO) and ε-caprolactone (CL) using potassium bis(trimethylsilyl)amide ([(CH3)3Si]2NK) as the initiator.32 The advantage of the synthesis route is the introduction of amine functional group to the terminal end of the polymer chain, which includes positive charge to the copolymer in aqueous solution and furthermore would favor the future functionalization of the copolymer for active targeting utilities. The chemical structure and molecular weight of the block copolymer were investigated by 1H NMR and GPC.27 The copolymer synthesized in this work has a PEO and a PCL MW of 4.2 kDa and 5.3 kDa respectively with polydispersity index of 1.2 for the whole copolymer. The copolymer self-assembles into micellar structured aggregates in water with a critical micellization concentration of 2.2 × 10−3 mg mL−1, measured by fluorescence method. The particle size of the PEO–PCL micelle is 97.2 ± 0.7 nm as determined using DLS at 1 mg mL−1 in water. Comparably, Tween-80 shows a CMC of 2.0 × 10−2 mg mL−1 in water which is ca. 10 times higher than that of the copolymer, indicating better thermodynamic stability of the later. The critical micellization concentration of PEO–PCL could be tuned by changing the molecular weight of either the blocks or the entire polymer chain.27Ricinine solubilisation
As listed in Table 1, two types of ricinine formulations were prepared in forms of ricinine solubilized micelle dispersion and emulsion. The concentration of PEO–PCL and Tween-80 was fixed at 5 mg mL−1. And the ricinine feeding level was 1.5 mg mL−1 and 10 mg mL−1. For the preparation of emulsion, ethyl acetate (EtAc) was used as the oil phase at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to water.
3 to water.
The size of the dispersed systems was first monitored using DLS and the results are listed in Table 2. It is seen that the ricinine solubilized micelle dispersions, i.e. P3 and T5, has hydrodynamic diameters (Dh) ranging from 150 to 230 nm shortly after the preparation procedure. Upon a feeding content of 1.5 mg mL−1, the loading efficiency of ricinine in both the copolymer and the surfactant is higher than 90%, measured through filtration method, resulting in a loading capacity of >20% (Table 1). Meanwhile, although the DLS displays an apparent particle size of ca. 100 nm to the neat ricinine dispersion (Table 2), precipitate was obviously seen within 1 h after the probe-sonication for the dispersion. The solubility of ricinine in water was tested to be 8.6 μg mL−1 by HPLC after filtration. Therefore the solubilization data clearly prove the efficiency of the amphiphiles on the solubilization of the pesticide. Comparing the two solubilizers, the micelle formulation from the amphiphilic copolymer shows larger particle size than Tween 80 (Table 2), which is attributed to the tendency of entanglement of polymer chains that leads to the formation of multicore structure in the micellar aggregates.23 With both amphiphiles, the stability of the micelle formulations is desirable since only limit increase of particles size was observed within 12 h, i.e. from 224 to 252 nm for PEO–PCL and from 157 to 181 nm for Tween-80 (Table 2). However, the stability against dilution was very different to the two micelle formulations. After diluted for 15 times, very limited change of particle size was observed from the polymer formulation (P3), whereas a significant increase was recorded from the Tween-80 formulation (T5) (Table 2). Besides, after dilution, the P3 formulation showed better stability than the diluted T5, evidenced by the size change after a standing for 24 h (Table 2). It should be noted that upon a 15 time-dilution, the concentration of both amphiphiles, i.e. 0.33 mg mL−1, is still above the CMC values. Since the increase of size normally indicates the fusion and/or the hydration of the micelles,35,36 the results imply that the interaction of the solubilizate with the core forming material relatively stronger to the PEO–PCL micelles, which is reasonable because of the inclusion of polar groups, in the ester bonds, in the polymer chain so that enhances physical interactions rather than the van der Waals force generated among the hydrophobic groups.
| Sample | 0.5 h | 1 h | 12 h | After 15× dilution | 24 h later |
|---|---|---|---|---|---|
| C1 | 107.7 | 106.1 | 102.3 | — | — |
| P2 | 291.3 | 300.7 | 295.5 | 281.0 | 291.5 |
| P3 | 224.0 | 288.2 | 251.6 | 234.0 | 333.9 |
| T4 | 576.5 | 831.0 | 820.0 | 531.9 | 801.4 |
| T5 | 157.0 | 157.4 | 180.6 | 408.6 | 555.0 |
Ricinine emulsions were gained by the addition of ethyl acetate. The organic solvent serves as the oil phase to solute the pesticide in molecular level. Using PEO–PCL, the copolymer favorably stabilized the liquid–liquid interface in the emulsified system, giving an average size of 300 nm for the droplets (P2 in Table 2). It is notable that the polymer emulsion shows ideal stability and dilution resistance as proven by the minor change of particles size with time and concentration. In contrast, the particle size of Tween-80 emulsion (T4 in Table 2) is much larger than that of the Tween-80 micelle formulation (T5), and increases seriously after 1 h of the preparation, an indication of the fusion of the droplets for smaller interface area. Nevertheless, phase separation was not observed for both of the emulsified systems within 24 h where no obvious oil drops even after a dilution by 15 times. However, possible leakage of ricinine was found from the Tween-80 emulsion after holding at room temperature for 24 h. This is recognized by comparing the drug loading capacity of polymer and Tween-80 emulsions at high ricinine loading level, i.e. 10 mg mL−1. At such feeding concentration, ca. 96% of loading efficiency was achieved with the PEO–PCL as well as the Tween-80 emulsions (P6 and T7 in Table 1). But the loading efficiency is down to 88.5% in the Tween-80 emulsion after standing for 24 h, whilst the value keeps at 95% in the polymer emulsion. The instability of the Tween-80 emulsion causes the release of the pesticide probably during the fusion process of the oil droplets.
Acaricidal activity
Contact toxicity of the ricinine formulations, i.e. P2, P3, T4 and T5 (Table 1), was evaluated by dipping method, with neat ricinine water dispersion (C1), blank Tween-80 and polymer micelle dispersions and emulsions as control samples. The medium lethal concentration (LC50) values were calculated and listed in Tables 3 and 4. The ineffectiveness of the blank dispersing phases on the T. cinnabarinus (B.) was first proven, which gave IC50 values higher than 160 mg mL−1 (Table 3). Among them, it is approval that the inclusion of ethyl acetate didn't result in significant difference in toxicity to the mites, which infers the safety of the solvent in this regime. Efficient acaricidal property requires an IC50 lower than 5 mg mL−1. With such a criteria, the neat ricinine dispersion in water is also ineffective due to the IC50 value of 43.1 mg mL−1 (Table 4). In contrast, the PEO–PCL formulations, at concentration of 5 mg mL−1, show efficient contact toxicity to the T. cinnabarinus (B.), giving IC50 values of 3.0 and 2.7 mg mL−1 for the micelle and emulsion formulations respectively (Table 4). Comparably, the Tween-80 formulations achieved IC50 of 3.1 and 5.2 mg mL−1 in the two formulation forms, which have no statistic difference on the efficiency when compared to that of the polymer formulations. Besides, the toxicity data in Table 4 also indicate that the addition of ethyl acetate led to no obvious benefit to the acaricidal activity of the formulations although it is considered that the dispersity of the pesticide should be improved in the presence of organic solvent. As an alkaloid, ricinine kills T. cinnabarinus (B.) by interrupting the electron transport and the NADH respiratory chain in mitochondria37 through activating the Wnt signaling pathway.38 Because the mites has acupuncture mouthparts, to attacks T. cinnabarinus (B.), ricinine needs to travel through the integument and penetrate into the mite skin. This can explain why only the solubilized ricinine in the micelles or the oil droplets efficiently caused the death of the mites since they have smaller dispersing phase. As shown in Table 2, the particle size of the dispersing phase of the formulations is in sub-micrometer range, meaning that the formulation is easier to pass through the epicuticle of the T. cinnabarinus (B.). In addition, it should be pointed out that spreading property of the formulation is also an important factor. However, in this test, with the major aim of assessing the acaricidal activity of the active component, i.e. ricinine, the formulations were homogeneously administrated onto the surface of T. cinnabarinus (B.). Thus, the test diminished the influence of the wetting process of the formulations on the acaricidal effect. Nevertheless, the property cannot be omitted since the insecticide would be employed by spray which will be discussed below.| Sample | Regression equation | χ2 | Sig. | Correlation coefficient (R2) | LC50 (mg mL−1) |
|---|---|---|---|---|---|
| Tween-80 | Y = 0.44x − 1.05 | 0.63 | 0.98 | 0.89 | 193.78 |
| PEG–PCL | Y = 0.42x − 0.97 | 0.35 | 0.95 | 0.83 | 202.78 |
| PEG–PCL/EtAc | Y = 0.47x − 1.03 | 0.64 | 0.89 | 0.84 | 161.42 |
| Sample | Regression equation | χ2 | Sig. | Correlation coefficient (R2) | LC50 (mg mL−1) | 95% confidence limit |
|---|---|---|---|---|---|---|
| C1 | Y = 0.62x − 1.02 | 0.21 | 0.98 | 0.94 | 43.10 | — |
| P3 | Y = 1.59x − 0.76 | 0.69 | 0.88 | 0.92 | 3.01 | 1.69–15.97 |
| P6 | Y = 1.37x − 0.59 | 1.42 | 0.70 | 0.97 | 2.72 | 1.71–4.95 |
| T4 | Y = 1.60x − 0.79 | 0.75 | 0.86 | 0.95 | 3.09 | 1.72–17.29 |
| T7 | Y = 1.04x − 0.74 | 4.86 | 0.18 | 0.86 | 5.18 | 2.76–15.11 |
Surface retention on V. unguiculata (L.) leaf
The V. unguiculata (L.) leaf has a hydrophobic surface, which contains lipophilic wax, such as fatty ketone.39 And the microscopic surface morphology is highly roughness with randomly patterned topology in micrometer to sub-micrometer scale (Fig. 1a) and the structure is similar on the top and bottom sides. Obvious contamination can be seen on the leaf surface after the spray of the ricinine formulations. As shown in Fig. 1, the polymer formulations (P2, P3) when compared to Tween-80 formulations (T4, T5), and the emulsions (P2, T4) when compared to the micelle dispersions (P3, T5), resulted in larger area of taint. Meanwhile, it is also proven that the contamination could be removed by washing with water, and more important, left no remarkable damage to the surface structure of the V. unguiculata (L.) leaf. This releases the concern about the possible damage effect of the organic solvent on the wax layer of the leaf which may influence the growth of the plant.40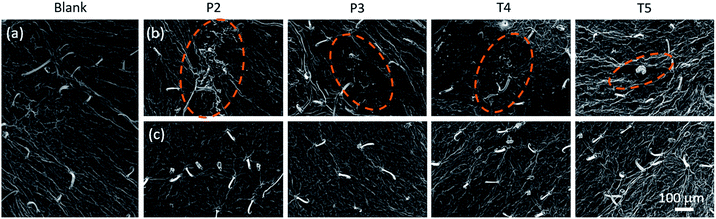 | ||
| Fig. 1 SEM image of V. unguiculata (L.) leaf (a), after being sprayed by ricinine formulations (b) and cleaned by washing with water (c). | ||
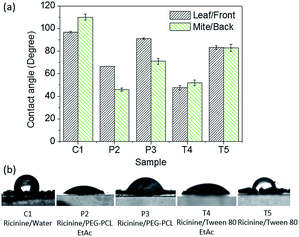
The spreading of the ricinine formulations was then quantitatively evaluated by measuring the contact angle on V. unguiculata (L.) leaf surface as well as the surface of T. cinnabarinus (B.). Fig. 2a shows that the contact angle of the micelle formulations, i.e. P3 and T5, is close to 90°, only slightly lower that that gained by the neat ricinine formulation in the absence of surfactant, i.e. C1. This is reasonable since with a continuous phase of water it hardly wets a rough hydrophobic surface, i.e. the leaf. However, after addition of ethyl acetate, the contact angle of the resultant polymer and Tween-80 emulsions, i.e. P2 and T4, significantly decreases (Fig. 2a), a clear demonstration of the organic solvent to help the spreading of the dispersed system. The mechanism is attributed to the formation of solvent layer at the interface in case the solvent content is high, i.e. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v to the aqueous phase (Fig. 2c).41,42 The contact angle data are in agreement with the SEM observation on the size of taints (Fig. 1b). Upon water evaporation, the border of a droplet will have less shrinkage when with smaller tension force at the liquid–solid interface. To further clarify the phenomenon, the contact angle of diluted formulations was measured (Fig. 3). As expected, while the contact angle of the diluted micelles has little difference compared to the concentrated samples, the angle of the emulsions increases to almost the level as the micelle formulations, and also close to that of the control sample (C1). With a dilution ratio of 15 times, the interfacial tension force is hardly reduced by the organic content.
3 v/v to the aqueous phase (Fig. 2c).41,42 The contact angle data are in agreement with the SEM observation on the size of taints (Fig. 1b). Upon water evaporation, the border of a droplet will have less shrinkage when with smaller tension force at the liquid–solid interface. To further clarify the phenomenon, the contact angle of diluted formulations was measured (Fig. 3). As expected, while the contact angle of the diluted micelles has little difference compared to the concentrated samples, the angle of the emulsions increases to almost the level as the micelle formulations, and also close to that of the control sample (C1). With a dilution ratio of 15 times, the interfacial tension force is hardly reduced by the organic content.
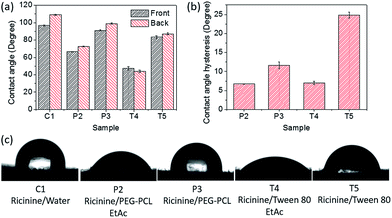 | ||
| Fig. 2 Contact angle (a) and contact angle hysteresis (b) of ricinine formulations on V. unguiculata (L.) leaf (c). The composition of the formulations can be found in Table 1. | ||
 | ||
| Fig. 3 Contact angle (a) and contact angle hysteresis (b) of ricinine formulations on V. unguiculata (L.) leaf (c) after the formulations were diluted by 15 times. | ||
Comparing the two solubilizers, it is noted that Tween-80 leads to smaller contact angle on the V. unguiculata (L.) leaf than the copolymer (Fig. 2). On the other hand, the contact angle hysteresis gives an opposite train, that is, the hysteresis value of the Tween-80 formulations is larger than that of the PEO–PCL formulations in same type, i.e. micelle or emulsion, even at the diluted state (Fig. 2b and 3b). Contact angle hysteresis is considered correlating to the roughness of the surface,43 which causes the adhesion of the hydrophilic droplet onto the V. unguiculata (L.) leaf surface although it shows hydrophobic property. No roll-off of the droplets was observed for all the formulations including the control sample, with a maximum inclination up to 80°. Through the hysteresis measurement, the influence of chemical composition on the wetting property is enlarged. It is seen that the Tween-80 formulations (T4 and T5) have larger hysteresis than the polymer formulations (P2 and P3) no matter the systems were diluted or not, and it is more obvious to the micelle dispersions where no organic solvent was involved (Fig. 2b and 3b). The results imply stronger interactions between the surfactant molecules and the leaf surface at the liquid–solid interface, which is possibly contributed by the structure similarity of the unsaturated fatty acid tail of Tween-80 and the long-chain aliphatic β-diketones on leaf surface of bean species.30,38
The retention capacity of the ricinine formulations was then tested on the V. unguiculata (L.) leaves. Fig. 4a displays that the dynamic retention of the pesticide was improved for more than three times by the formulation, with a solubilizer concentration of 5 mg mL−1, which reaches 20–23 mg per kilogram of leaf after an immersing–lifting cycle, compared to 7.5 mg kg−1 gained from the neat ricinine dispersion, demonstrating the affinity of the formulations to the leaf surface. With amphiphilic molecules, the dispersed phase, i.e. micelles or the colloid droplets, enables to adhere the leaf surface by the interaction between the solubilizer and the waxy component on the leaf surface. In contrast, the dispersed ricinine powder, in the control sample C1, would drill off with water once the V. unguiculata (L.) leaf was lifted out of the dispersion. Furthermore, Fig. 4b shows the surface retention of the pesticide against the washing using water. The test further reveals stronger interaction of Tween-80 with the leaf surface than the PEO–PCL because the Tween-80 results in much higher amount of pesticide residue (Fig. 4b). Besides, the emulsions also lead to higher ricinine residue than the micelle formulations, which is attributed to the improved wetting area of the former originated from the lower contact angle.
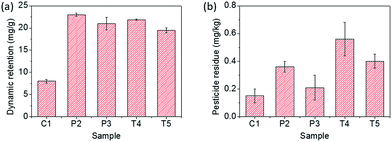 | ||
| Fig. 4 Dynamic retention capacity of the ricinine formulations on V. unguiculata (L.) leaf (a), and the resistance of the pesticide against rinsing (b). | ||
The wetting property of the formulations on T. cinnabarinus (B.) were also monitored. The back surface of the mite was selected for the test due to it is in the major pathway for pesticides to be taken up to cause an acaricidal effect.44 Compared to on the leaf surface, the apparent contact angle of the polymer formulations, i.e. micelle and emulsion, on the mite surface is significantly smaller, whereas it is similar to the Tween-80 formulations on the two surfaces (Fig. 5). For example for the PEO–PCL micelle formulation (P3), the contact angle decreases from 90° to 70° with the substrate changing from V. unguiculata (L.) leaf to T. cinnabarinus (B.). Meanwhile, no difference was got from the Tween-80 micelle formulation (T5), showing contact angles of ca. 85° on both surfaces (Fig. 5a). The results refer a favorable contacting property of the polymer formulation to the mites, attributed to an enhanced physical forces of the PCL blocks with the protein molecules on T. cinnabarinus (B.) when compared to the interaction with the V. unguiculata (L.) leaf.
Spreading and adhesive properties of pesticide are key parameters to determine the efficiency in their application.45 While an improvement of spreading is always aspired for a pesticide formulation in order to let the pesticide cover more area on the plant, the recognition on the surface adhesion behavior of pesticide is conflict because strong adhesion relates not only to long actuation time of the insecticide, but also to an enhanced difficulty on the cleaning of the pesticide for safe utilization of the plant.46 On the other hand, the increase of administration frequency, in case actuation time of the pesticide is low, could also result in accumulation of residue pesticide in the crops. Therefore, a favorable adhesion of pesticide to the mites, rather than to the plants, is ideal. In this work, our management was to achieve passive targeting effect of the pesticide to the T. cinnabarinus (B.) by selectively enhancing the interaction of the carrier with the mite. With a polyester structure, the PEO–PCL formulations indeed show increasing wetting ability to the T. cinnabarinus (B.), while the retention on the V. unguiculata (L.) leaf is less substantial against washing.
Field test
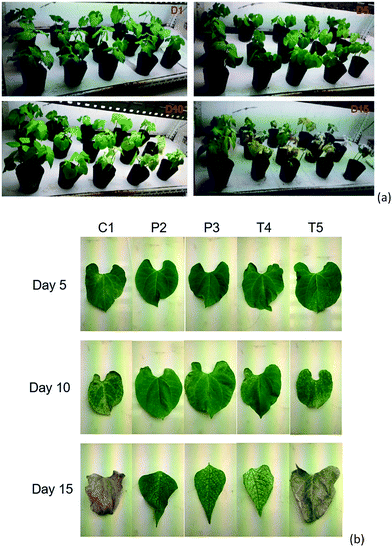
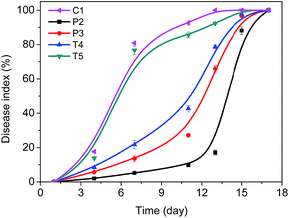
The activity of the polymer formulations on the protection of V. unguiculata (L.) was finally investigated. After the mites were infected for 3 day, the plants were sprayed by the polymer formulations, i.e. diluted form P2 and P3, containing 0.1 mg mL−1 of ricinine. The Tween-80 formulations as well as the neat ricinine dispersion at the same level of ricinine were also applied as references, i.e. the diluted samples of T4, T5 and C1. After a single administration, an evaluation on the health of V. unguiculata (L.) was recorded (Fig. 6 and 7). From the optical pictures taken on day 5 post-treatment, scabs can be distinguished from leaves treated by C1 and T5 formulations (Fig. 6a and b). And the disease became more serious after 10 days and eventually caused complete withering of the leaves within 15 days. Meanwhile, the fading of leaf in T4 treated group is also observed on the 15th day post administration. Comparably, the polymer formulations achieved much better protecting effect to the plants at this time point (Fig. 6b).Fig. 7 shows the calculated disease index (DI). It can be confirmed that although the DI value increased at the end of the test, owning to the single dose of the pesticide, the efficient inhibition time for the PEO–PCL formations is significant longer than that of the Tween-80 formulations. Especially for the emulsion formulation, i.e. P2, the DI value is below 20% for nearly two weeks (Fig. 7). Similar improvement of DI by T4, compared to T5, also implies the effect of solvent in the formulation. In agriculture application, insecticides are commonly dosed via spray route. Portion of the atomized droplets will be caught by the plants. Formulation with better spreadability enables to cover more leaf area and hence touches more mites. After that, the bioavailability of ricinine and moreover the inhibition of mite growth would be determined by the adhesion property of the formulation on the mite integument. Considering the pathway, the inclusion of ethyl acetate in the P2 sample accelerates the contact of ricinine molecules to the mite integument once the droplets favourably wet on its surface by the help of the polymer interaction.
Conclusions
In conclusion, we demonstrate a proof-of-concept work on using amphiphilic copolymer as a solubilizer for the preparation of ricinine formulation with objective not only in encapsulating the water insoluble pesticide but also for demonstrating a possibility, that is, by tuning the interface interactions a pesticide formulation could harvest increased activity in pest inhibition together with less pollution to the protecting plants. Herein, the PEO–PCL dispersions show lower contact angle on T. cinnabarinus (B.) surface than on the V. unguiculata (L.) leaf surface. The characteristic endowed the polymer formulations, especially in the emulsion form (P2), to have better acaricidal efficiency on V. unguiculata (L.) than the Tween-80 formulations which have non-specificity on their spreading ability to the plant and mite. The work suggests that amphiphilic copolymers, in particular to those with designed functional groups, are promising in the development of new insecticides and other kinds of formulations used in agriculture.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (NSFC) (51473169, 21274155, 51233007) and Beijing University of Agriculture High Quality Publication Promotion Foundation (1086716073).Notes and references
- L. Shi, J. Zhang, G. Shen, Z. Xu, P. Wei, Y. Zhang, Q. Xu and L. He, Sci. Rep., 2015, 5, 15581 CrossRef CAS PubMed.
- I. Cakmak, H. Baspinar and N. Madanlar, Turk. J. Agric. For., 2005, 29, 259 Search PubMed.
- C. C. Ho, K. C. Lo and W. H. Chen, J. Agric. Res. China, 1997, 46, 333 Search PubMed.
- J. L. Bi, Z. M. Niu, L. Yu and N. C. Toscano, Insect Sci., 2016, 23, 88 CrossRef CAS PubMed.
- F. Y. Guo and Z. M. Zhao, Acta Arachnologica Sinica, 1999, 8, 118 Search PubMed.
- T. Van Leeuwen, S. Van Pottelberge and L. Tirry, Pest Manage. Sci., 2006, 62, 425 CrossRef CAS PubMed.
- M. K. Nayak and G. J. Daglish, Pest Manage. Sci., 2007, 63, 104 CrossRef CAS PubMed.
- S. M. Upasani, H. M. Kotkar, P. S. Mendki and V. L. Maheshwari, Pest Manage. Sci., 2003, 59, 1349 CrossRef CAS PubMed.
- M. F. Bigi, V. L. Torkomian, S. T. de Groote, J. M. Hebling, O. C Bueno, F. C. Pagnocca, J. B. Fernandes, P. C. Vieira and M. F. da Silva, Pest Manage. Sci., 2004, 60, 933 CrossRef CAS PubMed.
- A. M. Elimam, K. H. Elmalik and F. S. Ali, Tropical Biomedicine, 2009, 26, 130 Search PubMed.
- Q. Y. Gao, F. L. Hu, H. H. Zhu, M. Q. Liu, H. X. Li and Hu. F, Yingyong Shengtai Xuebao, 2011, 22, 3033 CAS.
- B. R. Sampieri, A. Arnosti, K. C. Furquim, G. O. Chierice, G. H. Bechara, P. L. de Carvalho and P. H. Nunes, Vet. Parasitol., 2013, 191, 315 CrossRef CAS PubMed.
- A. C. Ferraz, M. E. Angelucci, M. L. Da Costa, I. R. Batista, B. H. De Oliveira and C. Da Cunha, Pharmacol., Biochem. Behav., 1999, 63, 367 CrossRef CAS.
- T. Fan, C. Chen, T. Fan, F. Liu and Q. Peng, J. Hazard. Mater., 2015, 297, 340 CrossRef CAS PubMed.
- L. H. Blake, C. F. Jenner, M. J. Gidley and D. Cozzolino, Carbohydr. Polym., 2015, 125, 265 CrossRef CAS PubMed.
- V. Ghormade, M. V. Deshpande and K. M. Paknikar, Biotechnol. Adv., 2011, 29, 792 CrossRef CAS PubMed.
- M. Margnia, D. Rossierb, P. Crettaza and O. Jolliet, Agric., Ecosyst. Environ., 2002, 93, 379 CrossRef.
- Y. Yi, S. Xu, H. Sun, D. Chang, Y. Yin, H. Zheng, H. Xu and Y. Lou, Carbohydr. Polym., 2011, 86, 1007 CrossRef CAS.
- C. Allen, D. Maysinger and A. Eisenberg, Colloids Surf., B, 1999, 16, 3 CrossRef CAS.
- J. Kumar, N. A. Shakil, M. K. Singh, M. K. Singh, A. Pandey and R. P. Pandey, J. Environ. Sci. Health, Part B, 2010, 45, 310 CrossRef CAS PubMed.
- L. Taborga, K. Díaz, A. F. Olea, P. Reyes-Bravo, M. E. Flores, H. Peña-Cortés and L. Espinoza, J. Agric. Food Chem., 2015, 63, 6890 CrossRef CAS PubMed.
- Z. S. Gao and A. Eisenberg, Macromolecules, 1993, 26, 7353 CrossRef CAS.
- X. Qu, V. V. Khutoryanskiy, A. Stewart, S. Rahman, B. Papahadjopoulos-Sternberg, C. Dufes, D. McCarthy, C. G. Wilson, R. Lyons, K. C. Carter, A. Schatzlein and I. F. Uchegbu, Biomacromolecules, 2006, 7, 3452 CrossRef CAS PubMed.
- J. Gu, W. P. Cheng, C. Hoskins, P. K. T. Lin, L. Zhao, L. Zhu, X. Qu and Z. Yang, J. Microencapsulation, 2011, 28, 752 CrossRef CAS PubMed.
- Y. Zhang and F. Han, J. Colloid Interface Sci., 2009, 337, 211 CrossRef CAS PubMed.
- C. P. Glagola, L. M. Miceli, M. A. Milchak, E. H. Halle and J. L. Logan, Langmuir, 2012, 28, 5048 CrossRef CAS PubMed.
- S. Yang, F. Zhu, Q. Wang, F. Liang, X. Qu, Z. Gan and Z. Yang, J. Mater. Chem. B, 2015, 3, 4043 RSC.
- B. Ramaroson-Raonizafinimanana, E. M. Gaydou and I. Bombarda, J. Agric. Food Chem., 2000, 48, 4739 CrossRef CAS PubMed.
- B. Ramaroson-Raonizafinimanana, E. M. Gaydou and I. Bombarda, J. Agric. Food Chem., 1999, 47, 3202 CrossRef CAS PubMed.
- C. Bu, J. Li, X. Q. Wang, G. Shi, B. Peng, J. Han, P. Gao and Y. Wang, BioMed Res. Int., 2015, 2015, 794718 Search PubMed.
- U. Mothes and K. A. Seitz, Cell Tissue Res., 1981, 214, 443 CrossRef CAS PubMed.
- Y. Zhang, P. Sun and Z. Gan, Sci. China: Chem., 2010, 53, 519 CrossRef CAS.
- P. Cao, C. Liu, P. Sun, X. Fu, S. Wang, F. Wu and X. Wang, Antonie van Leeuwenhoek, 2016, 109, 1573 CrossRef CAS PubMed.
- W. S. Abbott, J. Econ. Entomol., 1925, 18, 265 CrossRef CAS.
- X. G. Han and X. F. Zhang, J. Chem. Phys., 2015, 143, 214904 CrossRef PubMed.
- A. I. Zakharov, L. T. Adzhemyan and A. K. Shchekin, J. Chem. Phys., 2015, 143, 124902 CrossRef PubMed.
- A. C. Ferraz, M. E. Angelucci, M. L. Da Costa, I. R. Batista, B. H. De Oliveira and C. Da Cunha, Pharmacol., Biochem. Behav., 1999, 63, 367 CrossRef CAS.
- K. Ohishi, K. Toume, M. A. Arai, S. K. Sadhu, F. Ahmed, T. Mizoguchi, M. Itoh and M. Ishibashi, Bioorg. Med. Chem., 2014, 22, 4597 CrossRef CAS PubMed.
- S. K. Singh and K. Raja Reddy, J. Photochem. Photobiol., B, 2011, 105, 40 CrossRef CAS PubMed.
- A. Tani, M. Kiyota and I. Aiga, Biological Sciences in Space, 1995, 9, 314 CrossRef CAS PubMed.
- H. L. Nie, X. Dou, Z. Tang, H. D. Jang and J. Huang, J. Am. Chem. Soc., 2015, 137, 10683 CrossRef CAS PubMed.
- Z. Guennouni, F. Cousin, M. C. Fauré, P. Perrin, D. Limagne, O. Konovalov and M. Goldmann, Langmuir, 2016, 32, 1971 CrossRef CAS PubMed.
- N. Robert, J. Phys. Chem., 1949, 53, 1466 CrossRef.
- H. Yu, Y. Yue, X. Dong, R. Li and P. Li, Toxins, 2016, 8, 179 CrossRef PubMed.
- S. Basu, J. Luthra and K. D. Nigam, J. Environ. Sci. Health, Part B, 2002, 37, 331 CrossRef CAS PubMed.
- X. Tang, J. Dong and X. Li, J. Colloid Interface Sci., 2008, 325, 223 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |