Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Theoretical and experimental study on the degradation mechanism of atrazine in Fenton oxidation treatment†
Xue Zhaoa,
Chenxi Zhangb,
Shuguang Wang*c,
Chao Songc and
Xiang Li*d
aCollege of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University, Jinan 250014, P. R. China
bDepartment of Resources and Environment, Binzhou University, Binzhou 256600, P. R. China
cSchool of Environmental Science and Engineering, Shandong University, Jinan 250100, P. R. China. E-mail: wsg@sdu.edu.cn
dDepartment of Environmental Science & Engineering, Fudan University, Shanghai 200433, P. R. China. E-mail: lixiang@fudan.edu.cn
First published on 5th January 2017
Abstract
The residues of atrazine in surface and ground water will cause harm to human health as they are slowly biodegraded microbiologically. In this work, density functional theory (DFT) and the polarizable continuum model (PCM) were used to investigate the degradation of atrazine in an aqueous medium by Fenton oxidation technology. The results show that H atom abstraction pathways are more probable than both OH radical addition and Cl atom substitution pathways. Moreover, the H atom abstraction from the –CH– of –CH(CH3)2 group and –CH2– of –CH2CH3 group are expected to occur more easily. New dealkylation and alkyl oxidation mechanisms are proposed, in which water can act as a catalyst to reduce the reaction barrier dramatically. The stable intermediates and products: CH3COCH3, DEDIA, DIA, DEA, CAFT, CDAT, CDET, CDFT and CFIT, have been identified with LC/MS analysis. This study offers a cost-effective way to probe the degradation mechanism of atrazine in an aqueous medium by Fenton oxidation technology.
Introduction
Triazine is used worldwide to combat grassy and broadleaf weeds in many agricultural crops as well as for non-agricultural purposes, such as soil sterilization and road maintenance.1 Atrazine (ATZ), with its chemical name 2-chloro-4-ethylamino-6-isopropylamino-s-triazine (C8H14ClN5), is the main representative of the s-triazines and has been most widely used in the world in the last several decades.2–4 It was detected ubiquitously in surface and ground water in many countries. ATZ has been identified as a putative endocrine disruptor and classified as a class C carcinogen.5–8 The chromosomes of Chinese hamster egg cells will be damaged if they are exposed to 1.08–17.26 μg L−1 of ATZ within two days. The widespread use of ATZ has caused concentrations exceeding the limit of surface and ground water throughout Europe and the United States.9,10 Thus, the commercial use of ATZ has been banned in several countries.11 However, its presence in surface and ground water continues to last for several years. The search for effective methods to remove ATZ from water is of importance.Water treatment processes include physical processes,12,13 biological processes,14–16 chemical processes,17,18 catalytic oxidation19,20 and several advanced oxidation processes (AOPs).
In general, absorption and extraction are cost effective and easy to perform. However, they only transfer the pollutant to another phase, without promoting its degradation to a less harmful species.21 ATZ is toxic to microorganisms, and the triazine-ring itself is quite resistant to microbial attacks.22,23 As a result, conventional biological remediation is neither efficient and nor suitable for removing higher concentrations of ATZ from contaminated water rapidly. In the chemical process, AOPs are potentially useful to treat pesticide wastes because they generate powerful oxidizing agents. Several AOPs have been applied to ATZ degradation in the aqueous medium, such as sonolysis,24,25 electron-beam irradiation,26 TiO2-supported UV photolysis,27,28 O3/UV,29,30 UV/H2O2,31,32 O3/H2O2 (ref. 33) and Fenton oxidation technology, which includes Fenton, photo-Fenton, and electro-Fenton.34–40
Fenton oxidation technology, which generates hydroxyl radical (OH), is a promising method to treat wastewater containing ATZ. The Fenton system consists of a mixture of ferrous salt (Fe2+) and H2O2, namely Fenton's reagent. OH can be produced in the reaction, H2O2 + Fe2+ → Fe3+ + OH− + OH. Laat has compared the efficiencies of degradation of ATZ by several AOPs, and found that the photo-Fenton process was more efficient than H2O2/UV.35 Khan have compared the degradation of ATZ by photo-Fenton and photo-Fenton-like oxidation technologies. It is suggested that they are capable of removing ATZ from water efficiently, but this study did not cover the degradation mechanism.40 Balci has studied the degradation mechanism of ATZ in the aqueous medium by electro-Fenton oxidation.10 Considering all oxidation reaction intermediates and products, a general reaction mechanism for ATZ degradation by OH was proposed. But the mechanism proposed in the experiment wasn't detailed enough. Mackul'ak identified the degradation products of atrazine by HPLC after application of the Fenton reaction and modified Fenton reaction, including some small organic molecules such as oxalic acid, urea, formic acid, acetic acid, and acetone. But their attention was focused on the small fragments of the degradation process that were identified by HPLC, the intermediates were not found.41 Theoretical calculation can provide information for the reaction intermediates and pathways. Many theoretical studies on the degradation reaction by OH radical have been reported.42,43 In this work, the density functional theory (DFT) calculation and the polarized continuum model (PCM)44–46 were performed to investigate the degradation of ATZ by OH radical, and the roles of other components in Fenton's reagent have also considered. This study was helpful to further perfect the experimental mechanism, and could make up the inadequacy of experimental measurement on the short-lived substances, which provided the theoretical support for the removal of ATZ by Fenton oxidation treatment. In order to verify the theoretical results, liquid chromatography/mass spectrometry (LC/MS) analysis was used to identify the major intermediates and products.
Computational methods, experimental materials and analysis
Computational methods
Using the GAUSSIAN 09 programs,47 high-level ab initio molecular orbital calculation is carried out for the reaction of ATZ with the OH radical. The geometrical parameters of stationary points are optimized at the M05-2X/6-31+G(d,p) level. The M05-2X functional is of high nonlocality with the double amount of nonlocal exchange (2×), which is an excellent method to predict noncovalent interactions.48 The vibrational frequencies have been calculated at the same level in order to determine the nature of stationary points. Each transition state is verified to connect the designated reactants and products by performing an intrinsic reaction coordinate (IRC) analysis.49 The PCM is chosen to calculate the solution-phase energy.Materials and chemicals
Atrazine (ATZ > 98%) was purchased from Weifang Hua Yun Environmental Protection Technology Co., Ltd. Ferrous sulfate heptahydrate (FeSO4·7H2O >97%), hydrogen peroxide H2O2 (30%), HCl and sodium hydroxide NaOH were all analytical-grade and were purchased from Tianjin Kemiou Chemical Reagent Co., Ltd. All chemicals were used as being received without further purification.Experiment methods
The stock solution of ATZ was prepared at 10 mg L−1 in distilled–deionized water, and the pH values of solutions were adjusted with HCl or NaOH. Two hundred milliliters of ATZ aqueous solution was added, adjusting pH prior to 2–3. The desired dosage of FeSO4·7H2O/H2O2 was added thereafter to initiate the reaction. The mixture was magnetically stirred at 200 rpm at 25 °C. At different time intervals, 100 μL solution was taken out with an injector, and then filtered through 0.22 μm membranes before LC/MS analysis.Experimental analysis
The oxidative degradation products of ATZ were analyzed by LC/MS with a LCQ Fleet (Thermo Fisher Scientific, USA), using a Waters SunFireTM-C18 column (4.6 mm × 250 mm, 5 μm). Electron spray ionization (ESI) was used with a spray voltage set at 5000 V; sheath gas flow rate, aux gas flow rate and capillary temperature were set at 30 arb, 10 arb, and 300 °C, respectively. The mass spectra data were obtained in the positive ion mode after scanning them from m/z 50 to 350.Results and discussion
Initial reactions with OH radical
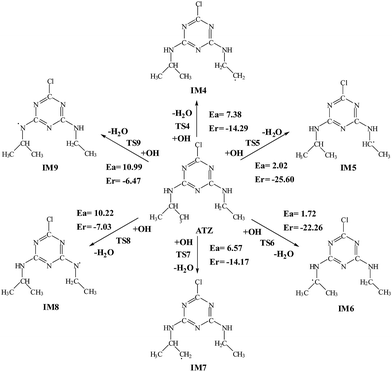
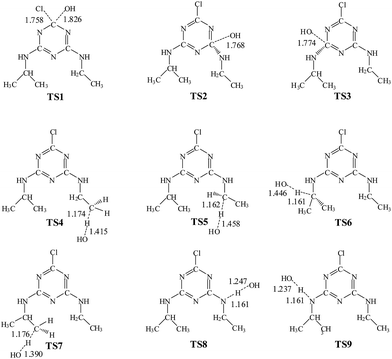
The OH radical addition to the C atom of triazine-ring, Cl atom substitution and H atom abstraction from ATZ are three possible kinds of channels for the reaction of ATZ with OH radical. The reaction pathways of OH radical addition, Cl atom substitution and H atom abstraction are depicted in Fig. 1 and 2, in which the potential barriers (Ea) and the reaction heats (Er) are marked too. The optimized structures of the transition states involved in reactions of ATZ with OH radical are shown in Fig. 3.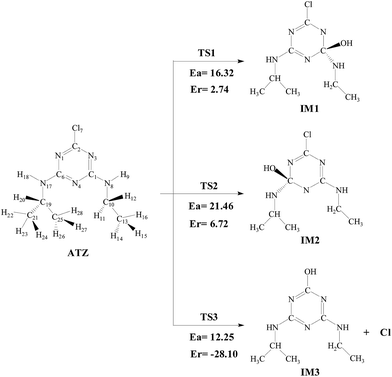 | ||
| Fig. 1 OH radical addition pathways and Cl atom substitution pathway in aqueous solution with the potential barriers Ea (kcal mol−1) and the reaction heats Er (kcal mol−1). | ||
 | ||
| Fig. 2 H atom abstraction pathways in aqueous solution with the potential barriers Ea (kcal mol−1) and the reaction heats Er (kcal mol−1). | ||
 | ||
| Fig. 3 Optimized geometries for the transition states involved in the initial reactions with OH radical. Distances are in angstroms. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. This reaction is strongly exothermic, releasing 28.10 kcal mol−1 of energy, implying that Cl atom substitution pathway is an energetically favorable reaction. HA is stable and has been detected in the experiment.10
N bond. This reaction is strongly exothermic, releasing 28.10 kcal mol−1 of energy, implying that Cl atom substitution pathway is an energetically favorable reaction. HA is stable and has been detected in the experiment.10In the ethyl group, i.e., –CH2CH3, the OH radical can abstract H atom from either –CH2– group or the –CH3 group. A transition state (TS4) was found in the abstraction of H atom in –CH3 group. This process has a potential barrier with 7.38 kcal mol−1 and is exothermic releasing 14.29 kcal mol−1 of energy. In H atom abstraction from the –CH2– group, OH radical abstracts H atom to produce IM5 via a small potential barrier with 2.02 kcal mol−1 of energy. This reaction is strongly exothermic, sending out 25.60 kcal mol−1 of energy, which shows that the H abstraction from the –CH2– group is easier than the H abstraction from the –CH3 group.
H atom is abstracted from the iso-propyl group, i.e., the –CH(CH3)2 group proceeds via either –CH– group or the –CH3 group. These reactions are required to overcome the barrier with 1.72 and 6.57 kcal mol−1 of energy, and are strongly exothermic, releasing 22.26 and 14.17 kcal mol−1 of energy, respectively. Therefore, the abstraction from the –CH– group takes place more easily than H abstraction from the –CH3 group.
As to H atom abstraction from the –NH– of ethylamino and –NH– of iso-propylamino, i.e., –NHCH2CH3 and –NHCH(CH3)2, the two reactions need to cross the barrier of 10.22 and 10.99 kcal mol−1 and are exothermic, giving out 7.03 and 6.47 kcal mol−1 of energy, respectively. Comparison of these initial reactions with OH radicals show that H atom abstraction from the –CH– of –CH(CH3)2 group and the –CH2– of –CH2CH3 group can occur more easily and are expected to play an important role in further reactions. Therefore, the ethyl group is more reactive than the isopropyl group during OH radical attack, which is consistent with the research of Acero.33
Water catalysis in subsequent reactions
From the point of thermodynamics, H atom abstraction pathways are easier to take place than OH radical addition pathways and Cl atom substitution pathway. And H atom abstraction from the –CH– of –CH(CH3)2 group and the –CH2– group of –CH2CH3 are expected to occur more easily. Thus, in this section, intermediates IM5 and IM6 are selected as reactants in the following degradation process.The production of carbon-centered radicals, IM5 and IM6, can be combined with OH radicals through barrierless reactions, generating IM10 and IM11. These processes are strongly endothermic, releasing 92.96 and 94.13 kcal mol−1 of energy. There are two ways in the following decomposition: dealkylation and alkyl oxidation with formation of formamide or acetamide. The reaction process is shown in Fig. 4 and S1.†
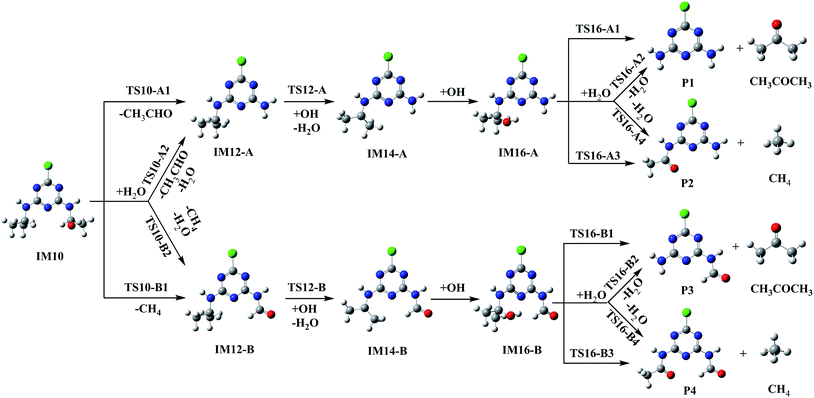 | ||
| Fig. 4 The dealkylation and alkyl oxidation process of IM10 in aqueous medium by Fenton oxidation technology. | ||
In the dealkylation reaction of IM10, the C10–N8 bond will be opened up, accompanied by the H atom migration from the O atom to the N8 atom. The C10–N8 bond in the transition state TS10-A1 is elongated to 1.747 Å as shown in Fig. S2.† Deethylatrazine (DEA, denoted as IM12-A) and acetaldehyde (CH3CHO) are produced via an apparent barrier of 54.75 kcal mol−1, and this reaction is predicted to be endothermic, giving out 13.91 kcal mol−1 of energy. Given that the barrier of 54.75 kcal mol−1 is too high for this reaction to play an important role, we took into account the possible role of a H2O. TS10-A2 shows that H2O acts as a catalyst with one H atom moving to the N8 atom simultaneously and extracting an H atom from the OH group. The C10–N8 bond will also be broken in this process via the barrier of 25.22 kcal mol−1. This process is a concerted reaction. The water serves as a catalyst to reduce the reaction barrier dramatically. Although this activation barrier is still high, it is smaller compared to the energy released from the combination of IM5 and OH radical.
Besides the dealkylation, IM10 can also ignite alkyl oxidation reaction with formamide. With a high barrier of 83.83 kcal mol−1, the C10–C13 bond will be broken along with the H migration from O to C13 via a transition state TS10-B1 (Fig. 4). 2-Chloro-4-formamido-6-isopropylamin-s-triazine (CFIT, denoted as IM12-B) and methane (CH4) will be produced in this reaction. When H2O gets involved in the reaction, it will act as a catalyst and reduce the barrier to 59.99 kcal mol−1 as shown in Fig. 4 and Table 1.
| Reactions | Potential barriers (Ea) | Reaction heats (Er) |
|---|---|---|
| IM10 → TS10-A1 → IM12-A + CH3CHO | 54.75 | 13.91 |
| IM10 + H2O → TS10-A2 → IM12-A + CH3CHO + H2O | 25.22 | 13.91 |
| IM12-A + OH → TS12A → IM14-A + H2O | 3.68 | −20.66 |
| IM14-A + OH → IM16-A | — | −96.84 |
| IM16-A → TS16-A1 → P1 + CH3COCH3 | 54.47 | 8.28 |
| IM16-A + H2O → TS16-A2 → P1 + CH3COCH3 + H2O | 31.12 | 8.28 |
| IM16-A → TS16-A3 → P2 + CH4 | 84.03 | 7.65 |
| IM16-A + H2O → TS16-A4 → P2 + CH4 + H2O | 66.79 | 7.65 |
| IM10 → TS10-B1 → IM12-B + CH3CHO | 83.83 | 8.85 |
| IM10 + H2O → TS10-B2 → IM12-B + CH3CHO + H2O | 59.99 | 8.85 |
| IM12-B + OH → TS12B → IM14-B + H2O | 6.02 | −21.04 |
| IM14-B + OH → IM16-B | — | −94.92 |
| IM16-B → TS16-B1 → P3 + CH3COCH3 | 54.65 | 7.11 |
| IM16-B + H2O → TS16-B2 → P3 + CH3COCH3 + H2O | 24.45 | 7.11 |
| IM16-B → TS16-B3 → P4 + CH4 | 84.56 | 7.84 |
| IM16-B + H2O → TS16-B4 → P4 + CH4 + H2O | 64.10 | 7.84 |
| IM11 → TS11-A1 → IM13-A + CH3COCH3 | 53.84 | 8.27 |
| IM11 + H2O → TS11-A2 → IM13-A + CH3COCH3 + H2O | 24.72 | 8.27 |
| IM13-A + OH → TS3A → IM15-A + H2O | 3.20 | −24.43 |
| IM15-A + OH → IM17-A | — | −95.04 |
| IM17-A → TS17-A1 → P1 + CH3CHO | 56.71 | 15.74 |
| IM17-A + H2O → TS17-A2 → P1 + CH3CHO + H2O | 26.70 | 15.74 |
| IM17-A → TS17-A3 → P3 + CH4 | 83.11 | 11.07 |
| IM17-A + H2O → TS17-A4 → P3 + CH4 + H2O | 60.27 | 11.07 |
| IM11 → TS11-B1 → IM13-B + CH3COCH3 | 88.41 | 6.81 |
| IM11 + H2O → TS11-B2 → IM13-B + CH3COCH3 + H2O | 66.86 | 6.81 |
| IM13-B + OH → TS3B → IM15-B + H2O | 4.64 | −23.80 |
| IM15-B + OH → IM17-B | — | −94.08 |
| IM17-B → TS17-B1 → P2 + CH3CHO | 57.95 | 14.98 |
| IM17-B + H2O → TS17-B2 → P2 + CH3CHO + H2O | 24.78 | 14.98 |
| IM17-B → TS17-B3 → P4 + CH4 | 82.38 | 11.66 |
| IM17-B + H2O → TS17-B4 → P4 + CH4 + H2O | 59.55 | 11.66 |
Then in IM12-A and IM12-B, dealkylation or alkyl oxidation can occur with three steps including H atom abstraction forming IM14-A and IM14-B, OH radical barrierless addition forming IM16-A and IM16-B, and C–N bond or C–C bond cleavage along with H atom migration. Then the stable products, deethyldeisopropylatrazine (DEDIA, denoted as P1), 6-acetamido-4-amino-2-chloro-s-triazine (CDAT, denoted as P2), 6-amino-2-chloro-4-formamido-s-triazine (CAFT, denoted as P3), 6-acetamido-2-chloro-4-formamido-s-triazine (CDFT, denoted as P4), acetone (CH3COCH3) and CH4 can come into being.
The decomposition of IM11 has undergone the similar pathways, and the schematic diagram of the reaction pathways and the optimized geometries for the transition states are drawn in Fig. S1 and S2,† respectively. However, it is worth noting that deisopropylatrazine (DIA, denoted as IM13-A) and 6-acetamido-2-chloro-4-ethylamino-s-triazine (CDET, denoted as IM13-B) will be formed.
The identification of degradation products
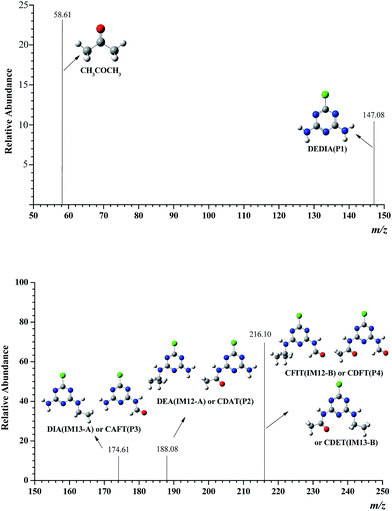
The degradation products of the ATZ were identified by LC/MS analysis and listed in Fig. 5. Several products had prominent protonated molecular ion at m/z 58.61, 147.08, 174.61, 188.08, 216.10, which were preliminarily identified as CH3COCH3, DEDIA(P1), DIA(IM13-A) or CAFT(P3), DEA(IM12-A) or CDAT(P2), CFIT(IM12-B) or CDFT(P4) or CDET(IM13-B). Products DEDIA, DIA, DEA, CDAT and CDET had been found out in earlier studies.10,33 Others were observed for the first time in the Fenton system.The toxicity of ATZ and its degradation products have been evaluated in several studies. Ralston-Hooper et al. evaluated the acute and chronic toxicity in the amphipods Hyalella azteca and Diporeia spp., and in the unicellular algae Pseudokirchneriella subcapitata, and they concluded that acute and chronic toxicities were ranked ATZ > DEA > DIA.50 Tchounwouli compared the toxicities of ATZ, DEA, DIA and DEDIA by Microtox Assay. They found that DEA and DIA are the least toxic, with EC50 81.86 and 82.68 mg L−1, followed by ATZ (EC50 = 39.87 mg L−1), which is consistent with the former.51 The EC50 of DEDIA is 12.74 mg L−1, and it suggests that the final product has more toxicity than that of ATZ.
Conclusions
The new degradation mechanism of ATZ in aqueous solutions has been investigated. From the point of thermodynamics, H atom abstraction pathways are easier to take place than those of OH radical addition and Cl atom substitution. Moreover, H atom abstraction from –CH– of –CH(CH3)2 group and –CH2– group of –CH2CH3 are the main OH-initiated reactions of ATZ.The subsequent decomposition of IM10 and IM11 involves two ways: dealkylation and alkyl oxidation with formation of formamide or acetamide. It should be pointed out that H2O can act as a catalyst to reduce the reaction barrier in these processes dramatically which is helpful to interpret the high efficiency of Fenton reagents. This mechanism can also provide a new point for the OH-initiated chemical transformation of volatile organic compounds in atmosphere.
The stable intermediates and products, CH3COCH3, DEDIA, DIA, DEA, CAFT, CDAT, CDET, CDFT and CFIT, have been observed experimentally. This study offers a cost-effective way to probe the degradation mechanism of ATZ in the aqueous medium by Fenton oxidation technology.
Acknowledgements
This work is supported by National Natural Science Foundation of China (21277082, 21337001, 21607011, 21577021, 21377028, 21177025), Natural Science Foundation of Shandong Province (No. ZR2014BP012), Program for New Century Excellent Talents in University (NCET-13-0349), Project for science and technology development of Shandong province (2014GSF117028), Beijing National Laboratory for Molecular Science (20140160), and the Fundamental Research Funds of Shandong University (2015JC020).Notes and references
- D. Barcelo, Analyst, 1991, 116, 681–689 RSC.
- R. I. Bonansea, M. V. Ame and D. A. Wunderlin, Chemosphere, 2013, 90, 1860–1869 CrossRef CAS PubMed.
- M. L. M. Tagert, J. H. Massey and D. R. Shaw, Sci. Total Environ., 2014, 481, 564–573 CrossRef CAS PubMed.
- K. B. Delwiche, J. Lehmann and M. T. Walter, Chemosphere, 2014, 95, 346–352 CrossRef CAS PubMed.
- D. C. Reid, A. C. Dwards, D. Cooper, E. Wilson and B. A. Mcgaw, Water Res., 2003, 37, 245–254 CrossRef CAS PubMed.
- G. D. Cimino-Reale, F. B. Casati, R. Brustio, C. Diodovich, A. Collotta, M. Vahter and L. Gribaldo, Toxicol. Lett., 2008, 180, 59–66 CrossRef CAS PubMed.
- M. Kucka, K. Pogrmic-Majkic, S. Fa, S. S. Stojilkovic and R. Kovacevic, Toxicol. Appl. Pharmacol., 2012, 265, 19–26 CrossRef CAS PubMed.
- S. O. Abarikwu, E. O. Farombi and A. B. Pant, Toxicol. Lett., 2013, 221, S215 CrossRef.
- S. R. Muller, M. Berg, M. M. Ulrich and R. P. Schwarzenbach, Environ. Sci. Technol., 1997, 31, 2104–2113 CrossRef.
- B. Balci, N. Oturan, R. Cherrier and M. A. Oturan, Water Res., 2009, 43, 1924–1934 CrossRef CAS PubMed.
- K. H. Chan and W. Chu, Chemosphere, 2003, 51, 305–311 CrossRef CAS PubMed.
- C. D. Adams and T. L. Watson, J. Environ. Eng., 1996, 122, 327–330 CrossRef CAS.
- P. Ghosh, L. Philip and M. Bandyopadhyay, Pract. Period. Hazard., Toxic, Radioact. Waste Manage., 2005, 9, 112–121 CrossRef CAS.
- A. Yassir, B. Lagacherie, S. Houot and G. Soulas, Pestic. Sci., 1999, 55, 799–809 CrossRef CAS.
- C. B. Shaw, C. M. Carliell and A. D. Wheately, Water Res., 2002, 36, 1193–2001 CrossRef.
- L. P. Wackett, M. J. Sadowsky, B. Martinez and N. Shapir, Appl. Microbiol. Biotechnol., 2002, 58, 39–45 CrossRef CAS PubMed.
- J. Marugán, J. Aguado, W. Gernjak and S. Malato, Catal. Today, 2007, 129, 59–68 CrossRef.
- H. L. Chen, E. Bramanti, I. Longo, M. Onor and C. Ferrari, J. Hazard. Mater., 2011, 186, 1808–1815 CrossRef CAS PubMed.
- K. H. Chan and W. Chu, Appl. Catal., B, 2005, 58, 157–163 CrossRef CAS.
- K. H. Chan and W. Chu, Water Res., 2003, 37, 3997–4003 CrossRef CAS PubMed.
- D. H. Belhateche, Chem. Eng. Prog., 1995, 91, 32–51 CAS.
- C. D. Adams and S. J. Randtke, Environ. Sci. Technol., 1992, 26, 2218–2227 CrossRef CAS.
- L. P. Wackett, M. J. Sadowsky, B. Martinez and N. Shapir, Appl. Microbiol. Biotechnol., 2002, 58, 39–45 CrossRef CAS PubMed.
- C. Petrier, B. David and S. Laguian, Chemosphere, 1996, 32, 1709–1718 CrossRef CAS.
- A. Hiskia, M. Ecke, A. Troupis, A. Kokorakis, H. Hennig and E. Papaconstantinou, Environ. Sci. Technol., 2001, 35, 2358–2364 CrossRef CAS PubMed.
- G. Xu, J. Z. Yao, L. Tang, X. Y. Yang, M. Zheng, H. Wang and M. H. Wu, Chem. Eng. J., 2015, 275, 374–380 CrossRef CAS.
- O. Sacco, V. Vaiano, C. Han, D. Sannino and D. D. Dionysiou, Appl. Catal., B, 2015, 164, 462–474 CrossRef CAS.
- J. A. Santacruz-Chávez, S. Oros-Ruiz, B. Prado and R. J. Zanella, J. Environ. Chem. Eng., 2015, 3, 3055–3061 CrossRef.
- S. Nelieu, L. Kerhoas and J. Einhorn, Environ. Sci. Technol., 2000, 34, 430–437 CrossRef CAS.
- R. C. L. Silva, R. J. Carvalho and B. A. Calfa, Rev. Ambiente Agua, 2010, 5, 9–20 CrossRef.
- G. Y. S. Chan, J. M. Hudson and N. S. J. Isaacs, Phys. Org. Chem., 1992, 5, 600–608 CrossRef CAS.
- C. W. Luo, J. Ma, J. Jiang, Y. Z. Liu, Y. Song, Y. Yang, Y. H. Guan and D. J. Wu, Water Res., 2015, 80, 99–108 CrossRef CAS PubMed.
- J. L. Acero, K. Stemmler and U. Von Gunten, Environ. Sci. Technol., 2000, 34, 591–597 CrossRef CAS.
- S. M. Arnold, W. J. Hickey and R. F. Harris, Environ. Sci. Technol., 1995, 29, 2083–2089 CrossRef CAS PubMed.
- J. De Laat, H. Gallard, A. Ancelin and B. Legube, Chemosphere, 1999, 39, 2693–2706 CrossRef CAS.
- P. L. Huston and J. J. Pignatello, Water Res., 1999, 33, 1238–1246 CrossRef CAS.
- K. Pratap and A. T. Lemley, J. Agric. Food Chem., 1998, 46, 3285–3291 CrossRef CAS.
- A. Ventura, G. Jacquet, A. Bermond and V. Camel, Water Res., 2002, 36, 3517–3522 CrossRef CAS PubMed.
- J. C. Barreiro, M. D. Capelato, L. Martin-Neto and H. C. B. Hansen, Water Res., 2007, 41, 55–62 CrossRef CAS PubMed.
- J. A. Khan, X. He, H. M. Khan, N. S. Shah and D. D. Dionysiou, Chem. Eng. J., 2013, 218, 376–383 CrossRef CAS.
- T. Mackul’ak, J. Prousek and L. ’. Švorc, Monatsh. Chem., 2011, 142, 561–567 CrossRef.
- Q. Z. Zhang, X. H. Qu and W. X. Wang, Environ. Sci. Technol., 2007, 41, 6109–6116 CrossRef CAS PubMed.
- X. M. Sun, T. L. Sun, Q. Z. Zhang and W. X. Wang, Sci. Total Environ., 2008, 402, 123–129 CrossRef CAS PubMed.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- V. Barone, M. Cossi and J. Tomasi, J. Comput. Chem., 1998, 19, 404–417 CrossRef CAS.
- B. Mennucci and J. Tomasi, J. Chem. Phys., 1996, 106, 5151–5158 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel and G. E. Scuseria, et al., Gaussian 09, revision D.01, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- Y. Zhao, N. E. Schultz and D. G. Truhlar, J. Chem. Theory Comput., 2006, 2, 364–382 CrossRef PubMed.
- K. Fukui, Acc. Chem. Res., 1981, 14, 363–368 CrossRef CAS.
- K. Ralston-Hooper, J. Hardy, L. Hahn, H. Ochoa-Acuna, L. S. Lee, R. Mollenhauer and M. S. Sepúlveda, Ecotoxicology, 2009, 18, 899–905 CrossRef CAS PubMed.
- P. B. Tchounwouli, B. Wilson, A. Ishaque, R. Ransome, M. J. Huang and J. Leszczynski, Int. J. Mol. Sci., 2000, 1, 63–74 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: The dealkylation and alkyl oxidation process of IM11 in aqueous medium by Fenton oxidation technology. See DOI: 10.1039/c6ra26918d |
| This journal is © The Royal Society of Chemistry 2017 |