Open Access Article
Open Access ArticleA first-principles investigation of a new hard multi-layered MnB2 structure†
Chunhong Xu,
Kuo Bao,
Shuailing Ma,
Yanbin Ma,
Shuli Wei,
Ziji Shao,
Xuehui Xiao,
Xiaokang Feng and
Tian Cui *
*
State Key Laboratory of Superhard Materials, College of Physics, Jilin University, Changchun, 130012, People's Republic of China. E-mail: cuitian@jlu.edu.cn
First published on 7th February 2017
Abstract
ReB2-type MnB2 has always been considered to be the ground-state structure of MnB2. However, subsequent theoretical study has revealed that this structure is easy to decompose into elemental Mn and B under ambient conditions, which motivated us to look for a stable MnB2 structure at high pressures. Using structure prediction algorithm USPEX and density functional theory calculations, we found a stable multi-layered MnB2 structure with space group Immm at high pressure. The calculated hardness of Immm-MnB2 is 22.5 GPa, which makes it a potential hard multifunctional material along with its conductive and magnetic properties. The hexagonal graphene-like boron networks of Immm-MnB2 contribute to its hardness and stability.
1 Introduction
The research of transition-metal diborides (TMB2s) never stops due to their outstanding physical and chemical properties, such as superconductivity, magnetism and superhardness, as well as being ultrahigh-temperature ceramics.1–4 The design philosophy for hard transition-metal diborides makes them very resistant to shear deformation by introducing covalent bonds into highly incompressible metals.5,6 Boron is a small and highly-bonded element,7 which provides a covalent bond network in transition metal borides.8 Recently, several transition-metal diborides, such as ReB2,9 OsB2,10 and TaB2,11 with a bulk modulus above 350 GPa, have been synthesized and greatly encouraged studies on transition metal diborides. Particularly, 3d magnetic metal manganese, with abundant valence electrons and “electron-deficient” boron, can form boron-based solids with the specific features of excellent multifunctional materials, such as high melting points, and magnetostructural and electronic behaviour.12–14 Numerous studies, theoretical and experimental, have been conducted on MnB2.15–25 Originally, Binder and Post synthesized AlB2–MnB2 (SG P6/mmm) by heating mixtures of metal Mn and B at 1400–1500 °C.19 Subsequently, in 2009, Aydin and Simsek predicted a superhard phase of MnB2 with ReB2-type structure (SG P63/mmc), which was regarded as the ground state of MnB2 at ambient conditions.16 In the same year, Jing Fan et al.15 held the view that ReB2-type MnB2 could be synthesized only below 1020 K at ambient pressure. Unfortunately, after a prolonged endeavor, ReB2-type MnB2 was never successfully synthesized to date. More recently, Haiyang Niu, et al.25 found ReB2-type MnB2 to be a metastable phase by variable-composition evolutionary algorithm calculations and first-principles calculations. Now that it is known that no stable MnB2 phase exists at ambient pressure, it should be investigated whether there are novel phases of MnB2 with new properties at high pressures. Thus, we investigated the structure, phase stability, electronic properties, and hardness of manganese diborides based on known transition-metal crystal structures and structure predictions. Through our calculations, a new high-pressure phase, Immm-MnB2, was found. It is mechanically stable and can be quenched at ambient pressure. The electronic structure results indicate that Immm-MnB2 is a metal and exhibits magnetic properties at ambient pressure, which could provide a new hard multifunctional material with a hardness of 22.5 GPa. Its hardness is mainly due to the strong covalent B–B bonds in the hexagonal graphene-like boron network.2 Computational details
We performed evolutionary variable-cell simulations on Mn–B systems (at Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratios of 2
B ratios of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with one to four formula units (FU) at moderate pressures of 0–60 GPa and zero temperature with the USPEX code.26–28 We mostly applied spin-polarized first-principles calculations with the pseudo-potential plane-wave method using the Vienna ab initio simulation program (VASP) code.29 The exchange and correlation effects were described by the generalized gradient approximation (GGA).30 The valence electron configurations of manganese and boron are 3p64s23d5 and 2s22p1, respectively. The bond lengths, cell parameters and atomic positions of any selected structure were fully optimized at different pressures with a plane-wave cut-off energy of 500 eV and dense Monkhorst–Pack k-point meshes with a reciprocal space resolution of 2π × 0.03 Å−1.31 These settings enabled excellent convergence of the energy differences, stress tensors, and structural parameters. The elastic constants were calculated using the strain–stress method. The bulk modulus B, shear modulus G, B/G ratio and Poisson's ratio (ν) were estimated using the Voigt–Reuss–Hill (VRH) approximation.32 In addition, we further derived phonon dispersions of the structures proposed by the USPEX with PHONOPY code.33 The theoretical Vickers hardness was estimated using the Chen's model.34
4) with one to four formula units (FU) at moderate pressures of 0–60 GPa and zero temperature with the USPEX code.26–28 We mostly applied spin-polarized first-principles calculations with the pseudo-potential plane-wave method using the Vienna ab initio simulation program (VASP) code.29 The exchange and correlation effects were described by the generalized gradient approximation (GGA).30 The valence electron configurations of manganese and boron are 3p64s23d5 and 2s22p1, respectively. The bond lengths, cell parameters and atomic positions of any selected structure were fully optimized at different pressures with a plane-wave cut-off energy of 500 eV and dense Monkhorst–Pack k-point meshes with a reciprocal space resolution of 2π × 0.03 Å−1.31 These settings enabled excellent convergence of the energy differences, stress tensors, and structural parameters. The elastic constants were calculated using the strain–stress method. The bulk modulus B, shear modulus G, B/G ratio and Poisson's ratio (ν) were estimated using the Voigt–Reuss–Hill (VRH) approximation.32 In addition, we further derived phonon dispersions of the structures proposed by the USPEX with PHONOPY code.33 The theoretical Vickers hardness was estimated using the Chen's model.34
3 Results and discussion
3.1 Thermodynamic stability and structural characteristics
In addition to the well-known hexagonal phase, ReB2–MnB2, we found a new high-pressure orthogonal phase, MnB2, with space group Immm at about 50 GPa, which is clearly observed in the convex hull diagram. According to the latest Mn–B phase diagram,18,24,25 the convex hull at different pressures including spin-polarization effects is shown in Fig. 1, connecting α-Mn,35 α-B (0–19 GPa), and γ-B (19–89 GPa)36 at T = 0 K. The formation enthalpy of MnxBy is defined as follows: ΔH = H(MnxBy) − xH(Mn) − yH(B). Our calculations indicate that the experimental AlB2–MnB2 and the pre-existing theoretical ReB2–MnB2 phase are metastable at ambient pressure (see Fig. 1a). Notably, when further increasing the pressure, the Immm-MnB2 phase (proposed for the first time) can be synthesized at 50 GPa because its negative formation enthalpy lies in the convex hull37,38 (Fig. 1b). Consequently, we can also obtain a phase transition of MnB2 from ReB2-type structure to Immm phase in the convex hulls.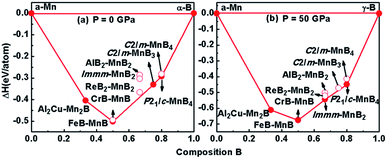 | ||
| Fig. 1 Convex hull diagram of the Mn–B systems at pressures of 0 GPa (a) and 50 GPa (b). Solid circles represent stable phases, while hollow circles denote metastable phases. | ||
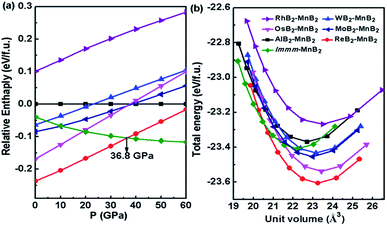
As shown in Fig. 2a, we compared the enthalpies of typical transition-metal diborides (MoB2-type MnB2,39 SG R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, WB2-type MnB2,1 SG P63/mmc, OsB2-type MnB2,40 SG Pmmn, RhB2-type MnB2,41 SG P21/m), ReB2–MnB2 and Immm-MnB2 as a function of pressure with respect to AlB2-type MnB2. As expected, the most thermodynamically stable structure is ReB2-type MnB2 at ambient pressure, which is in good agreement with previous first-principles calculations.15,24 ReB2-type MnB2 is stable in the pressure range of 0–36.8 GPa. Above 36.8 GPa, the Immm-MnB2 is the most energetically favorable up to 60 GPa. The results also show that Immm-MnB2 can be obtained from Al2Cu–Mn2B + γ-B, FeB–MnB + γ-B, Al2Cu–Mn2B + P21/c-MnB4 and FeB–MnB + P21/c-MnB4 in the convex hulls. The energy–volume curves of the known MnB2, illustrated in Fig. 2b, demonstrate a structural transition from ReB2-type MnB2 to Immm-MnB2 within the pressure range considered.
m, WB2-type MnB2,1 SG P63/mmc, OsB2-type MnB2,40 SG Pmmn, RhB2-type MnB2,41 SG P21/m), ReB2–MnB2 and Immm-MnB2 as a function of pressure with respect to AlB2-type MnB2. As expected, the most thermodynamically stable structure is ReB2-type MnB2 at ambient pressure, which is in good agreement with previous first-principles calculations.15,24 ReB2-type MnB2 is stable in the pressure range of 0–36.8 GPa. Above 36.8 GPa, the Immm-MnB2 is the most energetically favorable up to 60 GPa. The results also show that Immm-MnB2 can be obtained from Al2Cu–Mn2B + γ-B, FeB–MnB + γ-B, Al2Cu–Mn2B + P21/c-MnB4 and FeB–MnB + P21/c-MnB4 in the convex hulls. The energy–volume curves of the known MnB2, illustrated in Fig. 2b, demonstrate a structural transition from ReB2-type MnB2 to Immm-MnB2 within the pressure range considered.
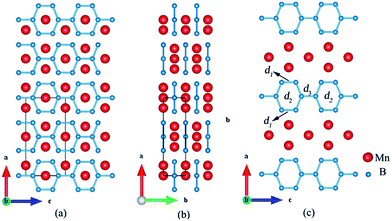
The equilibrium lattice parameters of Immm-MnB2 are a = 9.717 Å, b = 2.768 Å, c = 4.940 Å at ambient pressure. The orthogonal Immm structure contains six FUs per one unit cell with Mn atoms occupying the Wyckoff positions 2c (0.5, 0.5, 0) and 4e (0.6317, 0.5, 0.5) and B atoms occupying the 4j (0.5, 0, 0.6799) and 8m (0.6778, 0, 0.8205). From Fig. 3a and b, it can be clearly seen that Immm-MnB2 is a multi-layered structure. However, unlike the common layered structure of graphene-like boron layers that sandwich metal layers along c-axis in transitional diborides, e.g. ReB2, TaB2, our predicted Immm-MnB2 structure consists of hexagonal graphene-like boron networks formed by sp2 hybridized B–B bonds and manganese layers in each layer along the b- and c-axis. Two of the B–B bonds in the hexagonal graphene-like boron networks of Immm-MnB2, have a length of d1 = 1.773 Å and four of them have a length of d2 = 1.862 Å. The B–B bond between two adjacent six-membered boron rings has a distance of d3 = 1.778 Å (see Fig. 3c). The shortest B–B bond length, of 1.773 Å, is shorter than that of superhard ReB2 (1.820 Å),9 indicating a strong covalent bonding in Immm-MnB2 at 0 GPa. In addition, the structural characteristics of Immm-MnB2 are very similar to those of high-boron compounds, such as Immm-Mn3B4, suggesting that Immm-MnB2 may have a strong stability and good mechanical properties.
3.2 Dynamical stability, mechanical stability and hardness
Evaluating the dynamical stability is necessary for assessing whether the proposed structure is stable or not. Thus, the phonon dispersion curves of Immm-MnB2 at different pressures are displayed in Fig. 4. All the phonon modes of Immm-MnB2 are positive in the entire Brillouin zone, which confirms its dynamic stability. Immm-MnB2 was determined to be stable at 0–50 GPa, indicating that it can be obtained under ambient conditions.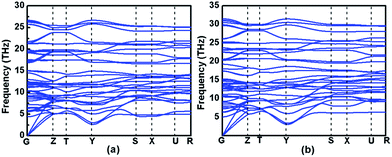 | ||
| Fig. 4 Phonon dispersion curves of Immm-MnB2 at (a) 0 GPa, and (b) 50 GPa along the high-symmetry directions of the Brillouin zone. | ||
Evaluating the mechanical properties of Immm-MnB2 is necessary for determining its mechanical stability and industrial applications. The elastic constants (Cij) for Immm-MnB2 were calculated by the strain–stress method, and the results are presented in Table 1. For comparison with the predicted Immm-MnB2 structure, we also calculated the elastic properties of some common transition-metal diborides such as TMB2 (TM = Ta, W, Re), using the same approach, as summarized in Table S1 (ESI†). The corresponding elastic modulus (B, G, Y), B/G ratio, Poisson's ratio ν and hardness Hv of all the abovementioned diborides are summarized in Table 1.
| Structure | P | B | G | Y | B/G | ν | Hv |
|---|---|---|---|---|---|---|---|
| a Ref. 42.b Ref. 43.c Ref. 44.d Ref. 16. | |||||||
| Immm-MnB2 | 0 | 308 | 195 | 483 | 1.58 | 0.24 | 22.5 |
| 50 | 449 | 274 | 684 | 1.64 | 0.25 | 27.0 | |
| TaB2 | 0 | 312 | 210 | 514 | 1.49 | 0.23 | 25.6 |
| 0 | 308a | 219a | 531a | 1.41a | 0.21a | 26.7a | |
| WB2 | 0 | 329 | 202 | 503 | 1.63 | 0.25 | 22.2 |
| 0 | 320b | 208b | 512b | 0.23b | |||
| ReB2 | 0 | 343 | 268 | 638 | 1.28 | 0.19 | 36.4 |
| 0 | 344c | 304c | 642c | 1.14c | 0.21c | ||
| 0 | 348d | 295d | 691d | 1.18d | 0.17d | 35.9d | |
From Table S1,† all the studied compounds are mechanically stable because the entire set of elastic constants Cij satisfies the Born–Huang criterion.45 Hence, the predicted Immm-MnB2 could be mechanically stable at 0 and 50 GPa. By comparing the elastic constants of Immm-MnB2, we can learn that the C11 (653 GPa) and C33 (643 GPa) are much larger than the C22 (442 GPa), implying that Immm-MnB2 is relatively strong against compression along the a- and c-axis due to the hexagonal graphene-like boron networks. The computational results for TaB2, WB2 and ReB2 are in good agreement with those of previous studies,16,42–44 which proves the reliability of our calculations. As it is known, C44 is an important parameter that indirectly controls the hardness of materials. The Immm-MnB2 phase has a C44 value of 273 GPa, which is comparable with that of ReB2, indicating a relatively strong shear strength.46 The calculated bulk modulus of Immm-MnB2 is comparable to those of TaB2,42 WB2 (ref. 43) and ReB2,44 suggesting that Immm-MnB2 has a strong ability to prevent volume decrease (see Table 1). Moreover, Immm-MnB2 has a shear modulus of 195 GPa, and hence it is reasonable to think that it could be a hard material, considering that its shear modulus is similar to that of conventional hard materials, such as TaB2 and WB2. In addition, the Poisson's ratio ν is indicative of the degree of directionality of the covalent bonding and greatly determines the hardness of materials. The Poisson's ratio of Immm-MnB2 is 0.24, which is similar to that of TaB2 and WB2. This value lies between the covalently-bound materials' value of 0.1 and the value of 0.33 for materials with typically delocalized metal–metal bonds,47 indicating a high degree of covalent and metallic bonding in the Immm-MnB2 structure. The B/G ratio of a material represents its ductility or brittleness, with 1.75 being the critical value.47 For Immm-MnB2, the calculated B/G ratio is less than 1.75, implying that its brittle nature.
We calculated the hardness of Immm-MnB2 using Chen's model: Hv = 2(k2G)0.585 − 3.34 The estimated hardness Hv of Immm-MnB2 is 22.5 GPa at ambient pressure and 27.0 GPa at P = 50 GPa. Indeed, high pressure can enhance the mechanical properties of materials by increasing the elastic constants and elastic moduli. Compared to Immm-Mn3B4, with a hardness of 8.5 GPa,25 our predicted structure is a relatively good hard material among common layered transition-metal diborides.
3.3 Electronic structure and magnetic properties
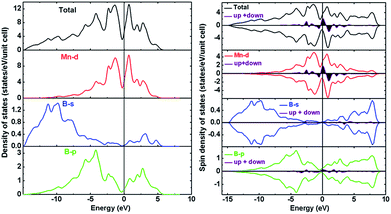
In order to explore the cause of the stability, hardness and magnetic properties of Immm-MnB2, both the non-polarized and the spin-polarized electronic density of states (DOS) of Immm-MnB2 at ambient pressure were calculated, and results are depicted in Fig. 5. As seen in Fig. 5, the calculated total density of states (TDOS) is dominated by Mn-d electrons due to their metallic character. This is the most evident feature of TM–B compounds at the Fermi level, and hence Immm-MnB2 may be a good conductor. The energy range from −15 eV to −7.5 eV is primarily composed of B-s and B-p states, which indicates a strong interaction between B atoms. The overlapping at about −4.3 eV indicates covalency between the Mn-d and B-p states. The Immm-MnB2 has a sharp peak at the Fermi level in non-polarized-DOS, which is a sign of magnetic instability (i.e., the compounds will be more stable in a magnetic state).48 According to the Stoner model, spontaneous magnetization occurs when the condition NTM(EF) × ITM > 1 is satisfied, where NTM(EF) is the non-polarized partial density of states of transition metal atoms at the Fermi level and ITM is the exchange-correlation integral. The value of ITM for Mn is 0.41 eV,49 and hence the obtained result is 13.1 and larger than 1, indicating stable ferromagnetism. We also calculated the total energy versus volume for Immm-MnB2, with and without spin-polarization, and we present the results in Fig. S1 (ESI†). The magnetic stability is confirmed once again by comparing the non-magnetic and magnetic total energy values calculated at the theoretical equilibrium volume.The calculated spin-polarized total DOS of Immm-MnB2 and partial DOS of Mn-d, B-s and B-p states are shown on the right panel in Fig. 5. According to our calculations, the results of the sum of the total spin-up and spin-down density of states (DOS) to the Fermi level reveal that Immm-MnB2 is a magnetic material in which the local spin moment of Mn is 0.185 μB per atom, which is the same as the early experimental result on AlB2–MnB2 obtained by Andersson et al. (0.19 μB per atom).50 The main ferromagnetic moment comes from the spin-polarized Mn-3d electrons. The magnetic moment in Immm-MnB2 linearly decreases with increasing pressure, as shown in Fig. S2 (ESI†), indicating that the magnetic moment is affected by pressure modulation. In summary, the observation of strong B–B interactions in the hexagonal graphene-like boron network and the relatively strong Mn–B interactions are the main cause for the stability and hardness of Immm-MnB2. Moreover, the presence of electrical conductivity and magnetic properties makes this multi-layered Immm-MnB2 structure a potential multifunctional material.
3.4 Chemical bonding
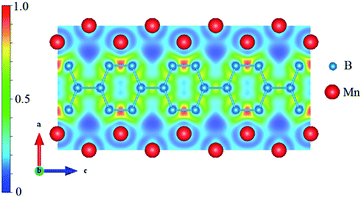
In most studies on transition-metal borides, the strong covalent B–B bonds and the TM–B bonds are always the key factor for structural stability and hardness. Therefore, we calculated the electron localization function (ELF) and Bader charge to understand the nature of the B–B and Mn–B bonding. The spin-polarized ELF for Immm-MnB2 at zero pressure is shown in Fig. 6. ELF = 1 corresponds to perfect electron-localization of covalent bonds, ELF = 0.5 corresponds to electron-gas-like pair probability, and ELF = 0 corresponds to perfect delocalization. As seen in Fig. 6, based on the ELF value, we can confirm the existence of strong covalent B–B bonding, particularly for the shortest B–B bond in the hexagonal graphene-like boron network, with a bond length of 1.773 Å, because of the high ELF value between this two boron atoms. C atoms have an electron configuration of 2s22p2. Due to short bond lengths, the 2p orbitals can form π bonds, leading to the formation of graphene-like sheets.51 Through Bader charge analysis (ESI†), it was found that there is much charge transfer from Mn to B. This promotes B− ions to be isoelectronic to C, thus leading to the existence of hexagonal graphene-like boron networks. Meanwhile, the valence electrons of Mn are mostly transferred to B, which helps the 2p orbitals of B atoms to form π bonds. For Immm-MnB2, the ELF is negligible at the Mn sites and attains maximum values between Mn and B atoms, very close to the B atoms, indicating partially covalent and metallic bonding features of the Mn–B bonds, i.e., weak interactions between the Mn and B atoms. The B–B interactions are much stronger than the Mn–B interactions. From this, combined with the abovementioned analysis of density of states, we can conclude that strong B–B bonds and relatively strong Mn–B bonds are the main cause of the hardness and stability of Immm-MnB2.4 Conclusion
In summary, we found a new multi-layered phase Immm-MnB2, with a theoretical hardness of 22.5 GPa, by structure search based on the first-principles calculations. This phase is mechanically and dynamically stable at ambient pressure. By analyzing the elastic characteristics and electronic structure, we discovered that Immm-MnB2 is a metal with magnetic properties, and has a magnetic moment of 0.185 μB/Mn, which makes it a promising hard multifunctional material.The chemical bonding analysis of Immm-MnB2 showed that the strong covalent B–B bonds in the hexagonal graphene-like boron network and the weak Mn–B interactions contribute to its hardness and stability. These results are important for understanding the structure and properties of magnetic transition-metal borides under high pressures and offer promise for the synthesis of magnetic transition-metal borides in the future.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51632002, 51572108, 11634004, 11104103), the Program for Changjiang Scholars and the Innovative Research Team in University (No. IRT_15R23), the National Fund for Fostering Talents of Basic Science (No. J1103202),Some of the calculations were performed in the High Performance Computing Center (HPCC) of the Jilin University.References
- Q. Li, D. Zhou, W. T. Zheng, Y. M. Ma and C. F. Chen, Phys. Rev. Lett., 2015, 115, 185502 CrossRef PubMed.
- J. Nagamatsu, N. Nakagawa, T. Muranaka, Y. Zenitani and J. Akimitsu, Nature, 2001, 410, 63–64 CrossRef CAS PubMed.
- M. C. Cadeville, J. Phys. Chem. Solids, 1966, 27, 667–670 CrossRef CAS.
- P. Rogl, J. Less-Common Met., 1985, 110, 283–294 CrossRef CAS.
- J. J. Gilman, R. W. Cumberland and R. B. Kaner, Int. J. Refract. Hard Met., 2006, 24, 1–5 CrossRef CAS.
- R. B. Kaner, J. J. Gilman and S. H. Tolbert, Science, 2005, 308, 1268–1269 CrossRef CAS PubMed.
- B. Albert and H. Hillebrecht, Angew. Chem., Int. Ed., 2009, 48, 8640–8668 CrossRef CAS PubMed.
- T. P. Fehlner, J. Solid State Chem., 2000, 154, 110–113 CrossRef CAS.
- H. Y. Chung, M. B. Weinberger, J. B. Levine, A. Kavner, J. M. Yang, S. H. Tolbert and R. B. Kaner, Science, 2007, 316, 436–439 CrossRef CAS PubMed.
- R. W. Cumberland, M. B. Weinberger, J. J. Gilman, S. M. Clark, S. H. Tolbert and R. B. Kaner, J. Am. Chem. Soc., 2005, 127, 7264–7265 CrossRef CAS PubMed.
- X. H. Zang, G. E. Hilmas and W. G. Fahrenholtz, Mater. Lett., 2008, 62, 4251–4253 CrossRef.
- H. Y. Gou, A. A. Tsirlin, E. Bykova, A. M. Abakumov, G. V. Tendeloo, A. Richter, S. V. Ovsyannikov, A. V. Kurnosov, D. M. Trots, Z. Konôpková, H.-P. Liermann, L. Dubrovinsky and N. Dubrovinskaia, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 064108 CrossRef.
- Y. C. Liang, X. Yuan, Y. F. Gao, W. Q. Zhang and P. H. Zhang, Phys. Rev. Lett., 2014, 113, 176401 CrossRef PubMed.
- R. O. Zaitsev and N. V. Terekhina, J. Exp. Theor. Phys., 2002, 94, 367–376 CrossRef CAS.
- J. Fan, K. Bao, X. L. Jin, X. X. Meng, D. F. Duan, B. B. Liu and T. Cui, J. Mater. Chem., 2012, 22, 17630–17635 RSC.
- S. Aydin and M. Simsek, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 134107 CrossRef.
- S. Khmelevskyi and P. Mohn, Solid State Commun., 2000, 113, 509–512 CrossRef CAS.
- H. Okamoto, J. Phase Equilib., 1993, 14, 121–122 CrossRef.
- I. Binder and B. Post, Acta Crystallogr., 1960, 13, 356 CrossRef CAS.
- X. X. Meng, K. Bao, P. W. Zhu, Z. He, Q. Tao, J. J. Li, Z. P. Mao and T. Cui, J. Appl. Phys., 2012, 111, 112616 CrossRef.
- G. Y. Gou, G. S. Neumann, E. Bykova, Y. Nakajima, N. Miyajima, Y. Li, S. V. Ovsyannikov, L. S. Dubrovinsky and N. Dubrovinskaia, Appl. Phys. Lett., 2013, 102, 061906 CrossRef.
- R. Kiessling, Acta Chem. Scand., 1950, 41, 46–159 Search PubMed.
- P. Vajeeston, P. Ravindran, C. Ravi and R. Asokamani, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 045115 CrossRef.
- B. Wang, X. Li, Y. X. Wang and Y. F. Tu, J. Phys. Chem. C, 2011, 115, 21429–21435 CAS.
- H. Y. Niu, X. Q. Chen, W. J. Ren, Q. Zhu, A. R. Oganov, D. Z. Li and Y. Y. Li, Phys. Chem. Chem. Phys., 2014, 16, 15866–15873 RSC.
- A. O. Lyakhov, A. R. Oganov, H. T. Stokes and Q. Zhu, Comput. Phys. Commun., 2013, 184, 1172–1182 CrossRef CAS.
- A. R. Oganov, A. O. Lyakhov and M. Valle, Acc. Chem. Res., 2011, 44, 227–237 CrossRef CAS PubMed.
- A. R. Oganov and C. W. Glass, J. Chem. Phys., 2006, 124, 244704 CrossRef PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- R. Hill, Proc. Phys. Soc., London, Sect. A, 1952, 65, 349–354 CrossRef.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- X. Q. Chen, H. Y. Niu, D. Z. Li and Y. Y. Li, Intermetallics, 2011, 19, 1275–1281 CrossRef CAS.
- A. C. Lawson, A. C. Larson, M. C. Aronson, S. Johnson, Z. Fisk, P. C. Canfield, J. D. Thompson and R. B. Von Dreele, J. Appl. Phys., 1994, 76, 7049–7051 CrossRef CAS.
- A. R. Oganov, J. H. Chen, C. Gatti, Y. Z. Ma, Y. M. Ma, C. W. Glass, Z. X. Liu, T. Yu, O. O. Kurakevych and V. L. Solozhenko, Nature, 2009, 457, 863–867 CrossRef CAS PubMed.
- G. Ghosh, A. van de Walle and M. Asta, Acta Mater., 2008, 56, 3202–3221 CrossRef CAS.
- X. W. Zhang, G. Trimarchi and A. Zunger, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 092102 CrossRef.
- S. Okada, T. Atoda, I. Higashi and Y. Takahashi, J. Mater. Sci., 1987, 22, 2993–2999 CrossRef CAS.
- B. Aronsson, E. Stenberg and J. Åselius, Nature, 1962, 195, 377–378 CrossRef CAS.
- B. H. Chu, D. Li, F. B. Tian, D. F. Duan, X. J. Sha, Y. Z. Lv, H. D. Zhang, B. B. Liu and T. Cui, Sci. Rep., 2015, 5, 10500 CrossRef PubMed.
- W. J. Zhao and Y. X. Wang, J. Solid State Chem., 2009, 182, 2880–2886 CrossRef CAS.
- X. F. Hao, Y. H. Xu, Z. J. Wu, D. F. Zhou, X. J. Liu, X. Q. Cao and J. Meng, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 224112 CrossRef.
- Y. X. Wang, Appl. Phys. Lett., 2007, 91, 101904 CrossRef.
- Z. J. Wu, E. J. Zhao, H. P. Xiang, X. F. Hao, X. J. Liu and J. Meng, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 054115 CrossRef.
- M. M. Zhong, X. Y. Kuang, Z. H. Wang, P. Shao, L. P. Ding and X. F. Huang, J. Phys. Chem. C, 2013, 117, 10643–10652 CAS.
- S. F. Pugh, Philos. Mag., 1954, 45, 823–843 CrossRef CAS.
- S. Kervan, J. Supercond. Novel Magn., 2011, 24, 815–818 CrossRef CAS.
- J. F. Janak, Phys. Rev. B: Solid State, 1977, 16, 255 CrossRef CAS.
- L. Andersson, B. Dellby and H. P. Myers, Solid State Commun., 1966, 4, 77–78 CrossRef CAS.
- M. J. Rice, A. R. Bishop and D. K. Campbell, Phys. Rev. Lett., 1983, 51, 2136 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra27148k |
| This journal is © The Royal Society of Chemistry 2017 |