Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A facilely synthesized lactam acceptor unit for high-performance polymer donors†
Han Pan‡
ab,
Zuo Xiao‡b,
Fangyuan Xieb,
Qifang Li*a and
Liming Ding *b
*b
aState Key Laboratory of Chemical Resource Engineering, College of Materials Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: qflee@mail.buct.edu.cn
bCAS Center for Excellence in Nanoscience, CAS Key Laboratory of Nanosystem and Hierarchical Fabrication, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: ding@nanoctr.cn
First published on 13th January 2017
Abstract
An aromatic lactam acceptor unit, [2,2′-bidithieno[3,2-b:2′,3′-d]pyridine]-5,5′(4H,4′H)-dione (BDTPi), was developed for making D–A copolymer donors. Two D–A copolymers, PThBDTPi and PSeBDTPi, gave power conversion efficiencies (PCEs) of 8.11% and 6.50%, respectively, when using PC71BM as the acceptor.
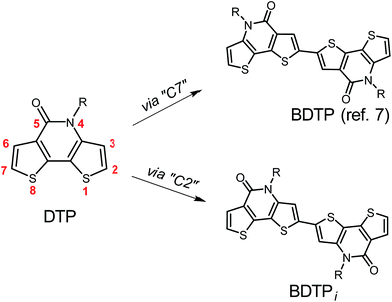
Polymer solar cells (PSCs) have attracted great interests due to the advantages of lightweight, flexibility and roll-to-roll fabrication.1 The power conversion efficiencies (PCEs) for single-junction PSCs have exceeded 12%.2 Developing donor–acceptor (D–A) copolymer donors is an effective way to obtain efficient solar cells.3 Polycyclic aromatic building blocks are favorable for constructing high-performance D–A copolymers since the extended π-conjugation of these units can improve the light-harvesting capability and the hole mobility of the materials.4 The tricyclic lactam unit, dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one (DTP), has emerged as a promising acceptor unit for D–A copolymers. Yu et al. reported the first DTP copolymer, which gave a PCE of 5.33%.5 By copolymerizing DTP with BDTT and IDT units, Yang et al. obtained wide-bandgap D–A copolymers, which afforded PCEs of 6.84% and 7.33%, respectively.6 By linking two DTPs via “C7” position, we obtained a lactam unit, BDTP (Fig. 1). The BDTP-based D–A copolymers gave decent PCEs up to 9.13%.7 These results demonstrated the potential of DTP copolymers. Developing new DTP-based D–A copolymers is highly desirable. Here, we report a new lactam acceptor unit, BDTPi, by linking two DTPs via “C2” position (Fig. 1). Different from the tedious synthesis of BDTP (8 steps from commercially available starting compounds), the synthesis of BDTPi is simplified (only 4 steps from starting compounds). Two BDTPi-based copolymers, PThBDTPi and PSeBDTPi, were prepared and used as the donor materials for PSCs. PThBDTPi:PC71BM solar cells gave a decent PCE of 8.11%.
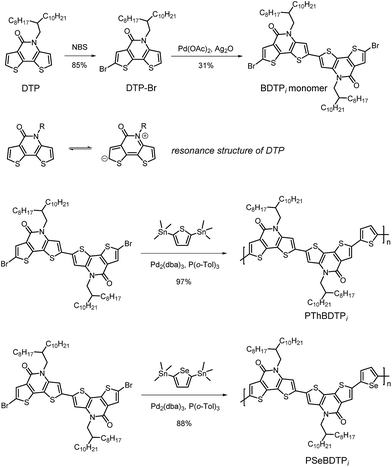
The synthetic routes for BDTPi monomer and the copolymers are shown in Scheme 1. DTP was prepared according to literature.6 The mono-bromination of DTP by NBS took place at “C7” rather than “C2”, producing DTP-Br in 85% yield. The high regioselectivity was confirmed by heteronuclear multiple bond correlation (HMBC) spectrum of DTP-Br (Fig. S3†). The proton near bromine (7.63 ppm peak) correlates with the carbonyl carbon (157.57 ppm peak), thus ruling out “C2” bromination. The regioselective bromination implies that “C7” presents higher electron density. The resonance structure of DTP could make “C7” to possess negative charge. A Pd-catalyzed dehydrogenative dimerization linked two DTP-Br via “C2”, producing BDTPi monomer in 31% yield.8 Finally, the copolymerization of BDTPi monomer with 2,5-bis(trimethylstannyl)thiophene or 2,5-bis(trimethylstannyl)selenophene via Stille coupling gave PThBDTPi or PSeBDTPi in 97% and 88% yield, respectively. The number-average molecular weights (Mn) for PThBDTPi and PSeBDTPi are 46.3 kDa and 56.7 kDa, with PDI of 2.07 and 1.74, respectively. The decomposition temperature (Td, 5% wt loss) for PThBDTPi and PSeBDTPi are 405 °C and 424 °C, respectively, indicating their good thermal stability.
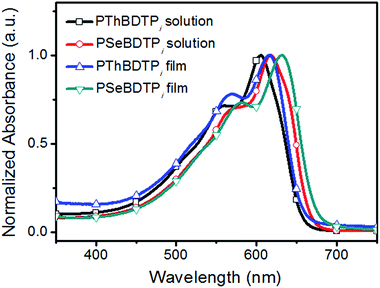
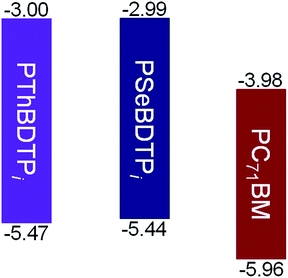
The absorption spectra for PThBDTPi and PSeBDTPi in solution and as films are shown in Fig. 2 and the optical data are listed in Table 1. In solution, PThBDTPi and PSeBDTPi show absorption peaks at 605 nm and 618 nm, respectively. For the films, the peaks shift to 616 nm and 632 nm, respectively. The optical bandgaps (Eoptg) for PThBDTPi and PSeBDTPi are 1.88 eV and 1.85 eV, respectively. The smaller bandgap of PSeBDTPi results from the reduced aromaticity of selenophene.9 The HOMO and LUMO energy levels for PThBDTPi and PSeBDTPi were calculated from the onset potentials of oxidation (Eonox) and reduction (Eonred), respectively (Fig. S9†).10 PThBDTPi and PSeBDTPi exhibit deep HOMO levels of −5.47 eV and −5.44 eV, respectively, and similar LUMO levels of −3.00 eV and −2.99 eV, respectively. Deep HOMO leads to high Voc for solar cells.11 The energy level diagram for PThBDTPi, PSeBDTPi and PC71BM is presented in Fig. 3.
| Polymers | λsol [nm] | λfilm [nm] | λon [nm] | Eoptga [eV] | Eonox [V] | Eonred [V] | HOMOb [eV] | LUMOc [eV] | Eecgd [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a Eoptg = 1240/λon.b HOMO = −(Eonox + 4.8).c LUMO = −(Eonred + 4.8).d Eecg = LUMO − HOMO. | |||||||||
| PThBDTPi | 605 | 616 | 658 | 1.88 | 0.67 | −1.80 | −5.47 | −3.00 | 2.47 |
| PSeBDTPi | 618 | 632 | 671 | 1.85 | 0.64 | −1.81 | −5.44 | −2.99 | 2.45 |
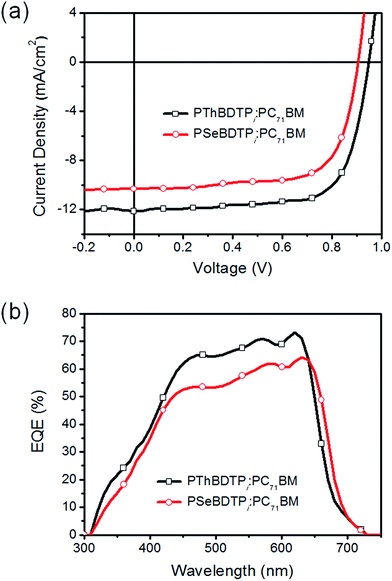
The photovoltaic performance for PThBDTPi and PSeBDTPi was evaluated by studying the inverted solar cells with a structure of ITO/ZnO/polymer:PC71BM/MoO3/Ag.12 The J–V curves and external quantum efficiency (EQE) spectra for the solar cells are shown in Fig. 4. The performance data are listed in Table 2. A mixed solvent of chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chlorobenzene (3
chlorobenzene (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was used to dissolve PThBDTPi:PC71BM blend. The best PThBDTPi:PC71BM cells gave a PCE of 8.11%, with a Voc of 0.95 V, a Jsc of 12.13 mA cm−2 and a FF of 70.5%. These cells have a D/A ratio of 1
2) was used to dissolve PThBDTPi:PC71BM blend. The best PThBDTPi:PC71BM cells gave a PCE of 8.11%, with a Voc of 0.95 V, a Jsc of 12.13 mA cm−2 and a FF of 70.5%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w), an active layer thickness of 92 nm and 0.5% (v/v) 1,8-diiodooctane (DIO) as the additive (Tables S1–S3†). PSeBDTPi:PC71BM cells were made by using chlorobenzene as the solvent and the best cells gave a PCE of 6.50%, with a Voc of 0.91 V, a Jsc of 10.32 mA cm−2 and a FF of 69.6%. These cells have a D/A ratio of 1
2 (w/w), an active layer thickness of 92 nm and 0.5% (v/v) 1,8-diiodooctane (DIO) as the additive (Tables S1–S3†). PSeBDTPi:PC71BM cells were made by using chlorobenzene as the solvent and the best cells gave a PCE of 6.50%, with a Voc of 0.91 V, a Jsc of 10.32 mA cm−2 and a FF of 69.6%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
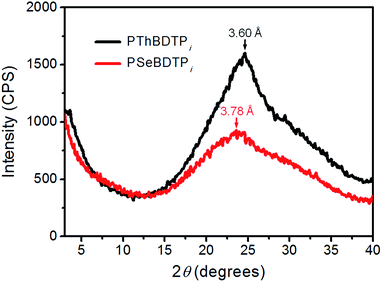
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w), an active layer thickness of 104 nm and 1% (v/v) DIO as the additive (Tables S4–S6†). Compared with PSeBDTPi cells, PThBDTPi cells gave higher Voc because of the deeper HOMO level of PThBDTPi. PThBDTPi cells produce a higher Jsc than PSeBDTPi cells, and the higher EQE of PThBDTPi cells at 310–640 nm also confirms this. The higher Jsc might result from better charge transporting capability of PThBDTPi. According to space charge limited current (SCLC) measurements, the hole mobilities for PThBDTPi and PSeBDTPi are 2.60 × 10−4 cm2 V−1 s−1 and 1.70 × 10−4 cm2 V−1 s−1, respectively (Fig. S10†). The XRD pattern for PThBDTPi film presents a stronger (010) peak, which corresponds to a π–π stacking distance of 3.60 Å, while PSeBDTPi possesses a π–π stacking distance of 3.78 Å (Fig. 5). The smaller π–π stacking d-spacing for PThBDTPi might be due to the smaller volume of sulfur atom. The smaller π–π stacking d-spacing favors hole transport. This could explain the higher hole mobility of PThBDTPi and also the higher photocurrent from PThBDTPi cells.
2 (w/w), an active layer thickness of 104 nm and 1% (v/v) DIO as the additive (Tables S4–S6†). Compared with PSeBDTPi cells, PThBDTPi cells gave higher Voc because of the deeper HOMO level of PThBDTPi. PThBDTPi cells produce a higher Jsc than PSeBDTPi cells, and the higher EQE of PThBDTPi cells at 310–640 nm also confirms this. The higher Jsc might result from better charge transporting capability of PThBDTPi. According to space charge limited current (SCLC) measurements, the hole mobilities for PThBDTPi and PSeBDTPi are 2.60 × 10−4 cm2 V−1 s−1 and 1.70 × 10−4 cm2 V−1 s−1, respectively (Fig. S10†). The XRD pattern for PThBDTPi film presents a stronger (010) peak, which corresponds to a π–π stacking distance of 3.60 Å, while PSeBDTPi possesses a π–π stacking distance of 3.78 Å (Fig. 5). The smaller π–π stacking d-spacing for PThBDTPi might be due to the smaller volume of sulfur atom. The smaller π–π stacking d-spacing favors hole transport. This could explain the higher hole mobility of PThBDTPi and also the higher photocurrent from PThBDTPi cells.
PThBDTPi:PC71BM and PSeBDTPi:PC71BM solar cells without DIO gave low PCEs of 2.55% and 2.56%, respectively, presenting low Jsc and FF. DIO can effectively dissolve fullerene aggregates and promote fullerene's mixing with polymer to form 3D charge-transport channels. More D/A interfaces could promote exciton dissociation and produce high photocurrent. PThBDTPi:PC71BM and PSeBDTPi:PC71BM blend films with DIO show fine and clear nano-structures (Fig. S11†), and this ideal morphology is always favourable for improving Jsc and FF of bulk heterojunction solar cells.
Conclusions
In summary, an aromatic lactam acceptor unit, BDTPi, was developed. By using this unit, two D–A copolymers, PThBDTPi and PSeBDTPi, were synthesized. PThBDTPi:PC71BM solar cells gave a decent PCE of 8.11%. The facile synthesis and good performance of BDTPi copolymers suggest that BDTPi is a promising building block for developing high-performance materials for polymer solar cells.Acknowledgements
We greatly appreciate National Natural Science Foundation of China (U1401244, 21374025, 21372053, 21572041 and 51503050), State Key Laboratory of Luminescent Materials and Devices (2016-skllmd-05), Youth Association for Promoting Innovation (CAS) and Center for Excellence in Nanoscience (CAS) for financial support. AFM images were taken by Xinjian Geng.Notes and references
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666 CrossRef CAS PubMed.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423 CrossRef CAS PubMed.
- H. Zhou, L. Yang and W. You, Macromolecules, 2012, 45, 607 CrossRef CAS.
- J.-S. Wu, S.-W. Cheng, Y.-J. Cheng and C.-S. Hsu, Chem. Soc. Rev., 2015, 44, 1113 RSC.
- A. M. Schneider, L. Lu, E. F. Manley, T. Zheng, V. Sharapov, T. Xu, T. J. Marks, L. X. Chen and L. Yu, Chem. Sci., 2015, 6, 4860 RSC.
- (a) M. Hao, G. Luo, K. Shi, G. Xie, K. Wu, H. Wu, G. Yu, Y. Cao and C. Yang, J. Mater. Chem. A, 2015, 3, 20516 RSC; (b) W. Gao, T. Liu, M. Hao, K. Wu, C. Zhang, Y. Sun and C. Yang, Chem. Sci., 2016, 7, 6167 RSC.
- (a) J. Cao, L. Qian, F. Lu, J. Zhang, Y. Feng, X. Qiu, H.-L. Yip and L. Ding, Chem. Commun., 2015, 51, 11830 RSC; (b) F. Lu, L. Qian, J. Cao, Y. Feng, B. Du and L. Ding, Polym. Chem., 2015, 6, 7373 RSC; (c) K. Zhang, K. Gao, R. Xia, Z. Wu, C. Sun, J. Cao, L. Qian, W. Li, S. Liu, F. Huang, X. Peng, L. Ding, H.-L. Yip and Y. Cao, Adv. Mater., 2016, 28, 4817 CrossRef CAS PubMed; (d) X. Du, O. Lytken, M. S. Killian, J. Cao, T. Stubhan, M. Turbiez, P. Schmuki, H.-P. Steinrück, L. Ding, R. H. Fink, N. Li and C. J. Brabec, Adv. Energy Mater., 2016 DOI:10.1002/aenm.201601959.
- H. Hu, K. Jiang, G. Yang, J. Liu, Z. Li, H. Lin, Y. Liu, J. Zhao, J. Zhang, F. Huang, Y. Qu, W. Ma and H. Yan, J. Am. Chem. Soc., 2015, 137, 14149 CrossRef CAS PubMed.
- A. Patra and M. Bendikov, J. Mater. Chem., 2010, 20, 422 RSC.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- B. P. Rand, D. P. Burk and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 115327 CrossRef.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials preparation and characterization, solar cell fabrication and measurements. See DOI: 10.1039/c6ra27292d |
| ‡ H. Pan and Z. Xiao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |