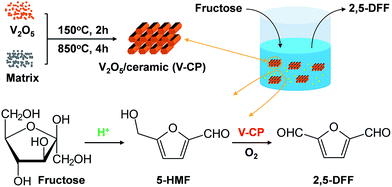
Open Access Article
Open Access ArticleCascade catalysis via dehydration and oxidation: one-pot synthesis of 2,5-diformylfuran from fructose using acid and V2O5/ceramic catalysts†
Mei Cuia,
Renliang Huang*b,
Wei Qi *acd,
Rongxin Suacd and
Zhimin Hea
*acd,
Rongxin Suacd and
Zhimin Hea
aState Key Laboratory of Chemical Engineering, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: qiwei@tju.edu.cn; Fax: +86 22 27407599; Tel: +86 22 27407799
bTianjin Engineering Center of Bio Gas/Oil Technology, School of Environmental Science and Engineering, Tianjin University, Tianjin 300072, China. E-mail: tjuhrl@tju.edu.cn
cCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China
dTianjin Key Laboratory of Membrane Science and Desalination Technology, Tianjin University, Tianjin 300072, China
First published on 23rd January 2017
Abstract
2,5-Diformylfuran (2,5-DFF) is an important biomass chemical with broad application prospects. A vanadium pentoxide (V2O5)/ceramic powder catalyst (V-CP) was designed and synthesized in this study, and its structure was characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and specific surface area analysis (BET). The catalytic properties of the V-CP catalyst were evaluated. The results show that 5-hydroxymethylfurfural (5-HMF) can be completely converted to 2,5-DFF at 140 °C under atmospheric pressure in the presence of oxygen as the oxidant in dimethyl sulfoxide (DMSO) for 5 h, and the yield of 2,5-DFF was 85.7%. After recycling the catalyst 5 times, the catalytic effect decreased slightly, and the catalyst showed good recovery performance. Furthermore, a one-pot method for the preparation of 2,5-DFF was proposed by using fructose as the raw material and V2O5/ceramic-dilute sulfuric acid as the catalyst. The fructose was dehydrated to form 5-HMF, and 2,5-DFF was generated by the V-CP catalytic oxidation of 5-HMF. The 2,5-DFF yield was 68.4%. The results of this study provide a valuable reference for the efficient one-pot synthesis of biomass-based furan chemicals.
1. Introduction
With the reduction of fossil resources and deterioration of the global environment, the development of biomass-based liquid fuels and high-value chemicals has become the focus of academic and industrial concerns. Among them, the chemical furan has become an important platform compound,1–5 including 5-hydroxymethylfurfural (5-HMF),6–11 2,5-diformylfuran (2,5-DFF),12–15 2,5-furandicarboxylic acid (FDCA),16–20 2,5-dimethylfuran (DMF),21,22 etc. These compounds can be prepared by the dehydration, oxidation, or reduction of glucose and fructose generated from the hydrolysis of biomass. There are two aldehyde groups in the 2,5-DFF, which make the chemical very active. Therefore, a variety of useful compounds and polymer materials, such as plastics, fuels, and additives, can be synthesized through the hydrogenation, oxidation, polymerization, and hydrolysis of 2,5-DFF.23–25Generally, 2,5-DFF can be prepared by the catalytic oxidation of 5-HMF. The main types of catalysts are noble metals, manganese-based catalysts, vanadium-based catalysts, and polymetallic catalysts. For noble metals, Zhu et al.26 used MnO2 microspheres as the carrier for Au catalyst, achieving a 5-HMF conversion of 82% and a 2,5-DFF selectivity of 99% using DMF as the solvent. In addition, Nie et al.27 used different carriers for Ru in the catalytic oxidation of 5-HMF. The results showed that when active carbon was used, the yield of 2,5-DFF can be as high as 96% in toluene at 110 °C and 2.0 MPa of O2. For manganese-based catalysts, Nie et al.28 investigated the effect of the existence of different forms of manganese on the catalytic oxidation of 5-HMF, and using manganese oxide octahedral molecular sieve (OMS-2) as the catalyst and DMF as the solvent after 1 h at 110 °C under 0.5 MPa O2, a 5-HMF to 2,5-DFF conversion of 100% and a selectivity of 97.2% were achieved. Furthermore, Yadav29 et al. synthesized a composite catalyst Ag-OMS-2 (silver content of 15%). This catalyst was used in isopropanol at 165 °C under an air pressure of 1.52 MPa for 4 h, giving a 5-HMF to 2,5-DFF conversion of 99% and a selectivity of 100%.
Moreover, vanadium-based catalysts are also used in the catalytic oxidation of 5-HMF for the preparation of 2,5-DFF due to their superior catalytic performance and low cost. In homogeneous catalysis, Ma et al.24 investigated the homogeneous oxidation of 5-HMF in acetonitrile using VOSO4 and Cu(NO3)2 as catalysts. It provided a 5-HMF to 2,5-DFF conversion of 99% and a selectivity of 99%. In heterogeneous catalysis, Sádaba et al.30 prepared a vanadium pentoxide (V2O5)/zeolite catalyst for 2,5-DFF preparation, and an 83.2% yield was achieved in dimethylformamide. Antonyraj et al.31 used a V2O5/active carbon catalyst for the selective oxidation of 5-HMF to 2,5-DFF, which provided a conversion of 95% and a selectivity of 96% after 4 h of reaction under 0.28 MPa of oxygen in methyl isobutyl ketone. Overall, high yields and selectivities for 2,5-DFF production were obtained in some studies, specially with high pressure of O2.
In recent years, some researchers have tried to synthesize 2,5-DFF directly from fructose or glucose. Fructose can be directly dehydrated to prepare 5-HMF, and therefore, the preparation of 2,5-DFF using fructose as raw material has been extensively studied.32–34 For example, Yang et al.32 used Fe3O4–SBA–SO3H for the catalyzed dehydration of fructose to prepare 5-HMF, followed by K-OMS-2 catalytic oxidation of 5-HMF to prepare 2,5-DFF. That study used a two-step method for the preparation of 2,5-DFF from fructose and generated a 2,5-DFF yield of 80%. Liu et al.33 synthesized a new type of molybdenum-based polyoxometalates that has dual catalytic functions of dehydration and oxidation and converted fructose to 2,5-DFF using a one-pot method. The 2,5-DFF yield reached 69.3% under the optimum conditions. In the research of glucose as raw material, Takagaki et al.35 studied the preparation of 2,5-DFF from glucose and fructose using cation exchange resin (Amberlyst-15) and ruthenium-hydrotalcite (Ru/HT) as catalysts, generating 2,5-DFF yields of 25% and 49%, respectively. Xiang et al.36 used CrCl3/NaBr and vanadate NaVO3 as the composite catalysts to prepare 2,5-DFF from glucose using a one-pot two-step method, with a 2,5-DFF yield of 55%. In general, the product yield and selectivity using catalysts with dual functionality or multiple catalysts for 2,5-DFF preparation directly from fructose or glucose, still need further improvement, and difficulty also exists in the reuse of multiple catalysts in the two-step process.
Ceramic is one of the ideal carriers for industrial catalytic reactions due to its excellent physical properties, such as a high melting point, high hardness, and high wear resistance as well as its excellent mechanical properties.37 It also has the advantages of corrosion resistance to acid and alkali, oxidation resistance, easy preparation, low cost, etc. Therefore, ceramics have been chosen as the catalyst matrix/carrier by many researchers.38,39 Previously, our group had synthesized various ceramic used as enzyme carriers, which exhibited good catalytic performance.38–40 In addition, our group has been committed to the synthesis of biomass-based furan chemicals, especially 5-HMF synthesis, regarding which much work has been carried out.8,11,14,41 The heterogeneous catalyst, which was prepared by doping ceramics with metal or metal oxide, has the catalytic properties of metal elements while retaining the advantages of ceramics. It should be an excellent catalyst for the synthesis of furan chemicals.
In this paper, a new V2O5/ceramic catalyst (V-CP) was prepared by co-sintering catalytic active V2O5 with a ceramic matrix component. The chemical composition and microstructure of the catalyst were systematically characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and specific surface area analysis (BET). 2,5-DFF was prepared from 5-HMF using V-CP as a catalyst and O2 as an oxidizing atmosphere under normal pressure. The effects of the reaction temperature, reaction time, V2O5 content, and solvent on the catalytic performance were investigated, and the recovery performance of V-CP was also evaluated. Furthermore, a one-pot method for the synthesis of 2,5-DFF from fructose was developed using V-CP and dilute sulfuric acid as the catalyst under normal pressure (Scheme 1). The changes in the contents of fructose, 5-HMF, and 2,5-DFF in the reaction system were analyzed by high performance liquid chromatography (HPLC).
2. Experimental
2.1 Materials and reagents
5-HMF (>99%), 2,5-DFF (>99%), and fructose (>99%) were obtained from the Sigma-Aldrich (Beijing, China). Dimethyl sulfoxide (DMSO, >99.8%) was purchase from the Concord Technology Co. Ltd. (Tianjing, China). V2O5 (>98%), sodium silicate (Na2SiO3, >98%), magnesium oxide (MgO, >98%), calcium oxide (CaO, >98%), silicon dioxide (SiO2, >95%), aluminum oxide (Al2O3, >98%), and concentrated sulfuric acid were purchased from the Guangfu Institute of Fine Chemicals (Tianjin, China).2.2 Preparation of the V2O5/ceramic catalyst
SiO2, Al2O3, Na2SiO3, V2O5, MgO, CaO, and water were mixed in a mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixture was then dried at 150 °C for 2 h. The temperature was increased at a heating rate of 4 °C min−1, and the mixture was calcinated at 850 °C for 4 h. After cooling down to room temperature and soaking in a 3% sulfuric acid solution for 2 h, the catalyst was washed and dried to obtain the V-CP with 10 wt% of V2O5.
1. The mixture was then dried at 150 °C for 2 h. The temperature was increased at a heating rate of 4 °C min−1, and the mixture was calcinated at 850 °C for 4 h. After cooling down to room temperature and soaking in a 3% sulfuric acid solution for 2 h, the catalyst was washed and dried to obtain the V-CP with 10 wt% of V2O5.
To compare and examine the effect of the catalyst composition on the properties of the catalysts, ceramic catalysts with the V2O5 contents of 0 (ceramic matrix, CP), 1%, 5%, 15%, and 20% were prepared using the same process as described above.
2.3 Characterization of catalysts
X-ray diffraction (XRD, D/MAX-2500, scanning range from 10–90° at a scanning speed of 6° min−1, Rigaku, Japan) was used to characterize the structure of blank ceramic matrix (CP) and V-CP. Scanning electron microscopy (SEM, S4800, Hitachi, Japan) images of the catalysts were acquired with an accelerating voltage of 5 kV to examine their morphologies. The specific surface area of the catalyst was measured using a BET nitrogen adsorption apparatus (F-sorp 2400, Beijing Jin Aipu Technology Co. Ltd.). The elemental composition and chemical valance on the surface of the catalyst were analyzed by X-ray photoelectron spectroscopy (XPS, PHI-5000 Versa Probe, ULVAC-PHI, Japan).2.4 Catalytic performance test
Route 1. 4 mL of DMSO, 90 mg of fructose, 11 μL of concentrated sulfuric acid, and 100 mg of V-CP (10 wt%) were added to the reaction vessel, which was placed in an oil bath at 140 °C to initiate a dehydration reaction. After 20 min, the oxidation reaction was conducted by passing oxygen at 40 mL min−1 into the reactor. After completion of the reaction, the catalyst was collected by centrifugation and washed three times using water. The reaction solution and washing solution were collected, and the volume was adjusted to 50 mL. The product was analyzed using HPLC.
Route 2. 4 mL of DMSO, 90 mg of fructose, and 11 μL of concentrated sulfuric acid were added to the reaction vessel, which was placed in an oil bath at 140 °C to start the dehydration reaction. After 20 min, 100 mg of V-CP (10 wt%) was added to the vessel, and the oxidation reaction was initiated by passing oxygen at 40 mL min−1 into the reactor. The following process was the same as in route 1.
3. Results and discussion
3.1 Characterization of the catalyst structure
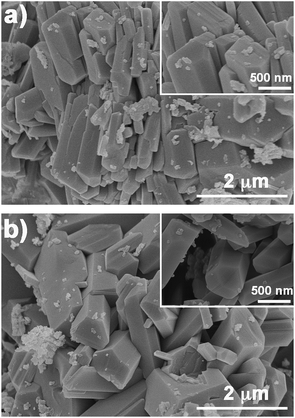
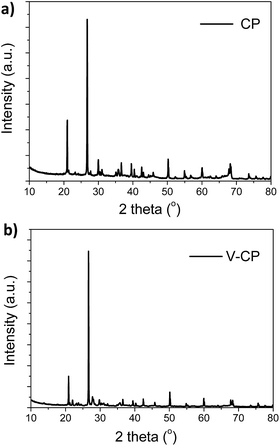
The morphologies of the ceramic matrix (CP) and V2O5/ceramic catalyst (V-CP) were observed by scanning electron microscopy (SEM), as shown in Fig. 1. SEM images showed that the CP and V-CP morphologies were similar. A large number of hexagonal prisms can be clearly seen. In addition, there were some amorphous components attached to the surfaces of the hexagonal prisms.XRD was used to verify the crystal structures of CP and V-CP. Fig. 2 shows that both CP and V-CP have obvious diffraction peaks, and particularly, there is a strong absorption peak at 27°. Analysis using MDI Jade software showed that the XRD patterns of the two were very close, and the characteristic peaks of SiO2 hexagonal crystals exist. These results indicated that the basic lattice structure of the ceramic was retained after the incorporation of V2O5. The peak intensities of MgO and CaO decreased after the incorporation of V2O5 due to their content decrease. However, SiO2 and Al2O3, as the main constituents of ceramic, still exhibit very high diffraction peak intensities in the V-CP pattern. This is consistent with other ceramic catalysts synthesized in our laboratory.39 In addition, the diffraction peaks of V2O5 were not detected in the XRD pattern of V-CP, which indicated that V2O5 existed mainly in an amorphous form.
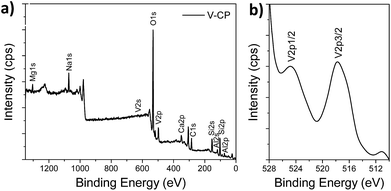
The XPS spectra of the V2O5/ceramic catalysts and the relative atomic ratios, as well as the binding energies of the major elements, are shown in Fig. 3 and Table 1. The results showed that the binding energies of the major elements, Si, Al, Mg, Ca, and Na, in CP and V-CP are basically the same, which indicated that each element has the same chemical valence in CP and V-CP. From Fig. 3b, it can be seen that there are characteristic peaks at 517.7 eV and 524.2 eV corresponding to V2p3/2 and V2p1/2 orbitals, respectively, which indicates that the vanadium exists as +5 in V2O5. This is consistent with the XPS spectra results from Antonyraj et al.,31 who used active carbon as a carrier for V2O5, and Adil et al.,42 who used a nickel manganese oxide as a carrier for V2O5 to prepare the catalyst.
| C1s | O1s | Na1s | Mg2p | Al2p | Si2p | Ca2p | V2p1/2 | V2p3/2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CP | atm% | 13.8 | 52.6 | 4.7 | 2.6 | 8.8 | 15.1 | 2.3 | — | — |
| Binding energy (eV) | 284.8 | 531.5 | 1071.9 | 49.7 | 74.2 | 102.4 | 347.1 | — | — | |
| V-CP | atm% | 16.51 | 51.4 | 3.5 | 3.1 | 4.9 | 17.2 | 2.2 | 1.2 | 1.2 |
| Binding energy (eV) | 284.8 | 531.9 | 1072.2 | 45.0 | 74.2 | 102.7 | 347.2 | 524.2 | 517.7 |
In addition, the BET specific surface areas of the blank ceramic matrix and V2O5/ceramic catalyst were also measured. The results showed that the BET specific surface area of CP is 10.8 m2 g−1 and that of V-CP is 17.9 m2 g−1. Both materials have very small specific surface areas, which is determined by the ceramic preparation process (including the temperature, heating rate, and not using a pore-forming agent). Under these conditions, the surface fluidity of the base material is significant, which results in the planarization of CP and V-CP surfaces. SEM images also showed no obvious pores for both CP and V-CP, which is consistent with the BET results.
3.2 Preparation of 2,5-DFF by the catalytic oxidation of 5-HMF using V-CP
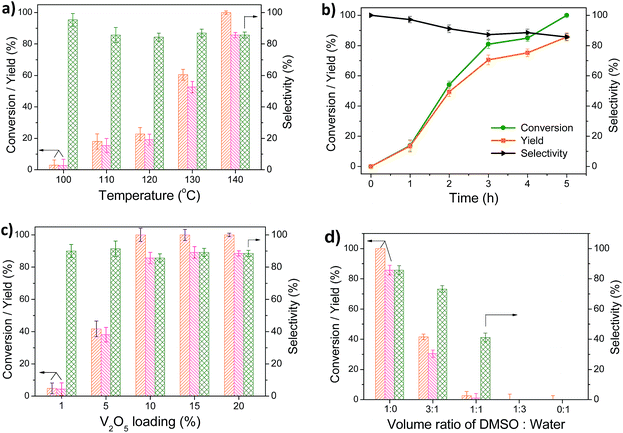
2,5-DFF was prepared by the catalytic oxidation of 5-HMF using O2 as the oxidant for the catalytic performance evaluation of V2O5/ceramic catalysts. The effects of the reaction temperature, time, V2O5 content, and solvent composition on the 5-HMF conversion and 2,5-DFF yield were systematically investigated, as shown in Fig. 4. The 2,5-DFF selectivity is 85.7–95.3% when the temperature is in the range of 100–140 °C, which indicates that the V-CP has high selectivity, especially under the lower temperature. However, only a small amount of 5-HMF (<23%) was converted to 2,5-DFF after 5 h of reaction at temperatures below 120 °C (Fig. 4a), which may due to the low reaction rate at low temperature. The conversion of 5-HMF and yield of 2,5-DFF gradually increased as the temperature increased. The 5-HMF conversion was 100% and the 2,5-DFF yield was 85.7% at 140 °C after 5 h of reaction. The selectivity gradually decreased (from 95.3% to 85.7%) with the increase of the temperature, which is likely due to formation of by-products. The HPLC results show that the main by-products are levulinic acid (LA), formic acid (FA) and 2,5-furandicarboxylic acid (FDCA). At 140 °C and 5 h, the yield of LA, FA and FDCA is 5.7%, 4.5% and 3.4%, respectively. In this case, the conversion of 5-HMF is 100% and the total yield of 2,5-DFF and by-products is 99.4%. Compared with other catalysts (see ESI Table S1†), the yield of 2,5-DFF in this paper is higher or comparable, but the value is lower than those of other homogeneous catalytic systems (e.g., VOSO4/Cu(NO3)2, ESI Table S1†).At 140 °C, the conversion of 5-HMF to 2,5-DFF versus time is shown in Fig. 4b. The conversion of 5-HMF and yield of 2,5-DFF increased rapidly in the first three hours, and then, the change in the trend became slow, reaching 100% and 87.5%, respectively, at 5 h. The selectivity of 2,5-DFF decreased as the reaction progresses, which is due to the gradually formed by-products as the reaction proceeds. For example, the generated 2,5-DFF can be further oxidized and generate FDCA.
Fig. 4c shows the effects of different V2O5 loadings on the conversion of 5-HMF and yield of 2,5-DFF. With the V2O5 loading increasing from 1% to 10%, the conversion of 5-HMF (from 4.8% to 100%) and the yield of 2,5-DFF (from 4.3% to 85.7%) both increased dramatically at 140 °C and 5 h of reaction, with an insignificant change in the 2,5-DFF yield. A further increase in the V2O5 loading to 15% also increased the 2,5-DFF yield and selectivity slightly. However, the 2,5-DFF yield and selectivity decreased slightly when the V2O5 loading increased to 20%, which indicated that the higher content of V2O5 also promoted side reactions.
We investigated the effect of the flow rate of O2 on 2,5-DFF synthesis. As shown in Fig. S1,† with increasing the flow rate of O2 from 10 to 40 mL min−1, the conversion of 5-HMF increased from 69.7% to 100% and the yield of 2,5-DFF increased form 57.9% to 85.7%. A further increase in the oxygen flow rate to 60 mL min−1, there is no significant change in the 2,5-DFF yield.
Moreover, the effects of different solvents on the efficiency of the catalytic reaction were also investigated in this paper. Fig. 4d shows that the conversion of 5-HMF and the yield/selectivity of 2,5-DFF were the highest in DMSO solvent, which indicated that DMSO can effectively inhibit the occurrence of side reactions and promote 2,5-DFF formation. Water is the greenest solvent for liquid phase reactions. In this paper, different volume ratios of DMSO–H2O mixed solutions were used as solvents, and the results showed that the 5-HMF conversion and 2,5-DFF yield decreased significantly with an increase in the water content. No oxidation of 5-HMF occurred and no 2,5-DFF was formed in pure water and DMSO/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 systems. Additionally, we also investigated the effect of the different organic solvents (acetonitrile, tetrahydrofuran, dimethylformamide) on the 2,5-DFF production. The results show that yield of 2,5-DFF is lower than 10% in these cases, which indicated that the DMSO has a significant effect on the preparation of 2,5-DFF by 5-HMF oxidation.
3 systems. Additionally, we also investigated the effect of the different organic solvents (acetonitrile, tetrahydrofuran, dimethylformamide) on the 2,5-DFF production. The results show that yield of 2,5-DFF is lower than 10% in these cases, which indicated that the DMSO has a significant effect on the preparation of 2,5-DFF by 5-HMF oxidation.
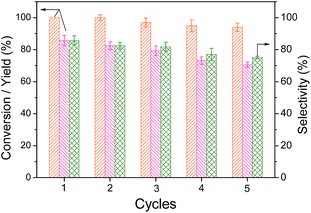
To evaluate the recovery performance of V-CP, the reaction solution was filtered and washed with double distilled water three times after the first reaction. The dried V-CP was then subjected to 5-HMF oxidation under the same conditions, and the results are shown in Fig. 5. After being recycled five times, the 5-HMF conversion decreased gradually from 100% to 94%, and the 2,5-DFF yield decreased from 85.7% to 70.6% at 140 °C and 5 h of reaction. In the recovery process, the filtration, transfer, and other experimental operations resulted in the loss of V-CP, while the active ingredient V2O5 in the catalyst may also have leaked slowly. Therefore, the 2,5-DFF yield and selectivity both decreased slightly. Overall, this catalyst showed good recovery performance.
3.3 Preparation of 2,5-DFF by the one-pot catalytic conversion of fructose
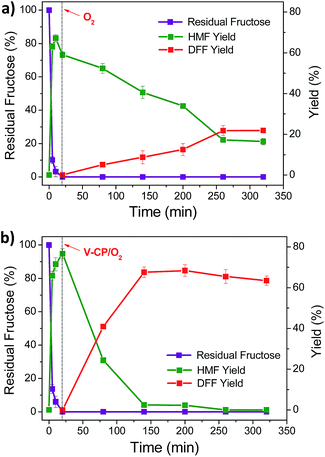
Fructose is a sustainable biomass raw material. Research has shown that 5-HMF can be prepared by acid-catalyzed dehydration using fructose. In this paper, fructose was used as the raw material to prepare 2,5-DFF by a one-pot method with diluted sulfuric acid and V2O5/ceramic catalysts to catalyze the dehydration of fructose and oxidation of 5-HMF. In the experiment, two experimental procedures were used to carry out the reaction according to the time of addition of the V2O5/ceramic catalyst.The first one included adding V-CP together with the diluted sulfuric acid into the reaction system. The oxygen was introduced after 20 min of dehydration for oxidation. Fig. 6a shows that the 5-HMF yield increased from 0 to 67.2% in the first 10 min of reaction with the rapid conversion of fructose. However, the yield decreased to 59.0% after further reaction until 20 min. The 2,5-DFF yield increased gradually from 0 to 21.8% after the introduction of oxygen, while the 5-HMF yield decreased to 16.4% due to its oxidation reaction.
The second approach included adding diluted sulfuric acid first and then adding the V-CP catalyst with oxygen for oxidation after 20 min of dehydration. Fig. 6b shows that the yield of 5-HMF reached 76.6% at 20 min of reaction time with the rapid conversion of fructose. After adding V-CP and introducing oxygen, 5-HMF was converted, and the 2,5-DFF yield increased to 67.6% rapidly from 20 min to 140 min. The 2,5-DFF yield reached the maximum of 68.4% after 200 min. Compared with another catalytic system (see ESI Table S2†), the yield of the H2SO4/V-CP system in this paper is higher or comparable. For example, in the case of molybdenum-based polyoxometalates (Cs0.5H2.5PMo12),33 the yield of 2,5-DFF is 69.3% at 433 K using air as oxidant. Formic acid and levulinic acid were detected as the main by-products; for V–g-C3N4(H+)/V–g-C3N4,43 the dehydration reaction was conducted under N2 (0.1 MPa) at 120 °C for 2 h and then the V–g-C3N4 was added under O2 (0.1 MPa) at 130 °C for 6 h, achieving the highest yield of 63%; for Fe3O4–SBA–SO3H/K-OMS-2,32 the 2,5-DFF yield was up to 80% by stepwise addition of catalysts. However, the coexistence of these two catalyst in the initial stage of reaction resulted in low 2,5-DFF yield, which is probably due to the formation of humins or the oxidation of fructose. In this study, further reactions resulted in slightly lower yields of 2,5-DFF, which was mainly due to the further reaction of 2,5-DFF.
The above experimental results showed that the second procedure is obviously superior to the first, as higher fructose conversion and 2,5-DFF yield were obtained. When V-CP and acid catalysts were added into the system at the same time, V-CP can possibly catalyze the undesired oxidation of fructose, which resulted in the lower yield of 5-HMF using the first method compared to the second one (67.2% vs. 76.6%). In addition, due to the lack of oxygen atmosphere in the system, the generated 5-HMF cannot be rapidly oxidized to 2,5-DFF, which may also generate some by-products (e.g., humins). These by-products inhibited the catalytic activity of V-CP and resulted in a lower yield of 2,5-DFF in the first method than in the second (21.8% vs. 68.4%).
4. Conclusions
A new V2O5/ceramic catalyst was synthesized and successfully applied to the efficient catalytic oxidation of 5-HMF to 2,5-DFF. The results of structural characterization showed that the doping of V2O5 did not change the crystal structure of the ceramic, and V2O5 sintered on the surface of the ceramic crystal in the amorphous form. 2,5-DFF was prepared by catalyzing 5-HMF at 140 °C under atmospheric pressure in the presence of oxygen as the oxidant in DMSO, and the yield of 2,5-DFF was 85.7%. After 5 times of recycling this catalyst, the 2,5-DFF yield was still over 70%, which showed good recovery performance. Furthermore, a new one-pot method for the preparation of 2,5-DFF from fructose was proposed by using fructose as the raw material and V2O5/ceramic-dilute sulfuric acid as the catalyst. The conversion of fructose was 100%, and the 2,5-DFF yield was 68.4%. The catalyst and catalytic process proposed in this paper provide a new method for the efficient synthesis of 2,5-DFF from fructose.Acknowledgements
This work was supported by the Natural Science Foundation of Tianjin, China (No. 16JCYBJC19600), the Natural Science Foundation of China (No. 21621004) and the Beiyang Young Scholar of Tianjin University (2012).References
- A. A. Rosatella, S. P. Simeonov, R. F. M. Frade and C. A. M. Afonso, Green Chem., 2011, 13, 754–793 RSC.
- J. P. Lange, E. van der Heide, J. van Buijtenen and R. Price, ChemSusChem, 2012, 5, 150–166 CrossRef CAS PubMed.
- T. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- J. Song, H. Fan, J. Ma and B. Han, Green Chem., 2013, 15, 2619–2635 RSC.
- X. Liu, J. Xiao, H. Ding, W. Zhong, Q. Xu, S. Su and D. Yin, Chem. Eng. J., 2016, 283, 1315–1321 CrossRef CAS.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS PubMed.
- R. Huang, W. Qi, R. Su and Z. He, Chem. Commun., 2010, 46, 1115–1117 RSC.
- A. Chinnappan, C. Baskar and H. Kim, RSC Adv., 2016, 6, 63991–64002 RSC.
- Z. M. Xue, M. G. Ma, Z. H. Li and T. C. Mu, RSC Adv., 2016, 6, 98874–98892 RSC.
- Z. Yang, W. Qi, R. Huang, J. Fang, R. Su and Z. He, Chem. Eng. J., 2016, 296, 209–216 CrossRef CAS.
- R. Fang, R. Luque and Y. Li, Green Chem., 2016, 18, 3152–3157 RSC.
- S. Wang, Z. Zhang, B. Liu and J. Li, Ind. Eng. Chem. Res., 2014, 53, 5820–5827 CrossRef CAS.
- Y. Wang, B. Liu, K. Huang and Z. Zhang, Ind. Eng. Chem. Res., 2014, 53, 1313–1319 CrossRef CAS.
- L. F. Liao, Y. Liu, Z. Y. Li, J. P. Zhuang, Y. B. Zhou and S. Z. Chen, RSC Adv., 2016, 6, 94976–94988 RSC.
- X. Wan, C. Zhou, J. Chen, W. Deng, Q. Zhang, Y. Yang and Y. Wang, ACS Catal., 2014, 4, 2175–2185 CrossRef CAS.
- H. Ait Rass, N. Essayem and M. Besson, ChemSusChem, 2015, 8, 1206–1217 CrossRef CAS PubMed.
- Z. Zhang, J. Zhen, B. Liu, K. Lv and K. Deng, Green Chem., 2015, 17, 1308–1317 RSC.
- L. Gao, K. Deng, J. Zheng, B. Liu and Z. Zhang, Chem. Eng. J., 2015, 270, 444–449 CrossRef CAS.
- Y. W. Zhang, Z. M. Xue, J. F. Wang, X. H. Zhao, Y. H. Deng, W. C. Zhao and T. C. Mu, RSC Adv., 2016, 6, 51229–51237 RSC.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS PubMed.
- X. Xiang, J. Cui, G. Ding, H. Zheng, Y. Zhu and Y. Li, ACS Sustainable Chem. Eng., 2016, 4, 4506–4510 CrossRef CAS.
- A. Gandini, Green Chem., 2011, 13, 1061–1083 RSC.
- J. Ma, Z. Du, J. Xu, Q. Chu and Y. Pang, ChemSusChem, 2011, 4, 51–54 CrossRef CAS PubMed.
- A. Gandini and M. N. Belgacem, Prog. Polym. Sci., 1997, 22, 1203–1379 CrossRef CAS.
- Y. Zhu, M. Shen, Y. Xia and M. Lu, Catal. Commun., 2015, 64, 37–43 CrossRef CAS.
- J. Nie, J. Xie and H. Liu, J. Catal., 2013, 301, 83–91 CrossRef CAS.
- J. Nie and H. Liu, J. Catal., 2014, 316, 57–66 CrossRef CAS.
- G. D. Yadav and R. V. Sharma, Appl. Catal., B, 2014, 147, 293–301 CrossRef CAS.
- I. Sádaba, Y. Y. Gorbanev, S. Kegnæs, S. S. R. Putluru, R. W. Berg and A. Riisager, ChemCatChem, 2013, 5, 284–293 CrossRef.
- C. A. Antonyraj, B. Kim, Y. Kim, S. Shin, K.-Y. Lee, I. Kim and J. K. Cho, Catal. Commun., 2014, 57, 64–68 CrossRef CAS.
- Z. Z. Yang, J. Deng, T. Pan, Q. X. Guo and Y. Fu, Green Chem., 2012, 14, 2986–2989 RSC.
- Y. Liu, L. Zhu, J. Tang, M. Liu, R. Cheng and C. Hu, ChemSusChem, 2014, 7, 3541–3547 CrossRef CAS PubMed.
- Z. Yang, W. Qi, R. Su and Z. He, Energy Fuels, 2017, 31, 533–541 CrossRef CAS.
- A. Takagaki, M. Takahashi, S. Nishimura and K. Ebitani, ACS Catal., 2011, 1, 1562–1565 CrossRef CAS.
- X. Xiang, L. He, Y. Yang, B. Guo, D. Tong and C. Hu, Catal. Lett., 2011, 141, 735–741 CrossRef CAS.
- K. Onitsuka, A. Dogan, J. F. Tressler, Q. Xu, S. Yoshikawa and R. E. Newnham, J. Intell. Mater. Syst. Struct., 1995, 6, 447–455 CrossRef CAS.
- Z. Wu, W. Qi, M. Wang, R. Su and Z. He, J. Mol. Catal. B: Enzym., 2014, 110, 32–38 CrossRef CAS.
- Z. Wu, W. Qi, M. Wang, Y. Wang, R. Su and Z. He, Biochem. Eng. J., 2013, 77, 190–197 CrossRef CAS.
- Y. Qu, Z. Wu, R. Huang, W. Qi, R. Su and Z. He, RSC Adv., 2016, 6, 64581–64588 RSC.
- N. Jiang, R. Huang, W. Qi, R. Su and Z. He, BioEnergy Res., 2012, 5, 380–386 CrossRef CAS.
- S. F. Adil, S. Alabbad, M. Kuniyil, M. Khan, A. Alwarthan, N. Mohri, W. Tremel, M. N. Tahir and M. R. H. Siddiqui, Nanoscale Res. Lett., 2015, 10, 52 CrossRef PubMed.
- J. Chen, Y. Guo, J. Chen, L. Song and L. Chen, ChemCatChem, 2014, 6, 3174–3181 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures. See DOI: 10.1039/c6ra27678d |
| This journal is © The Royal Society of Chemistry 2017 |