Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An overview of chemical constituents from Alpinia species in the last six decades†
Xiao-Ni Maab,
Chun-Lan Xieab,
Zi Miaoa,
Quan Yangb and
Xian-Wen Yang*a
aState Key Laboratory Breeding Base of Marine Genetic Resources, Key Laboratory of Marine Genetic Resources, Fujian Key Laboratory of Marine Genetic Resources, Third Institute of Oceanography, State Oceanic Administration, 184 Daxue Road, Xiamen 361005, PR China. E-mail: yangxianwen@tio,org,cn
bDepartment of Traditional Chinese Medicine, Guangdong Pharmaceutical University, Guangzhou 510006, China
First published on 2nd March 2017
Abstract
Alpinia species is one of the most important genera of the Zingiberaceae family. In Asia, they have been widely used as food and traditional medicines for centuries. This review focuses on their chemical constituents and their relevant biological activities with 252 references covering from 1955 to 2015. In total, 544 compounds were isolated from 35 Alpinia species. The major ones are terpenoids (207) and diarylheptanoids (143). The crude extracts and identified compounds exhibited a broad spectrum of bioactivities including antiemetic, antiulcer, antibacterial, anti-inflammatory, anti-amnesic, anticancer, etc.
1. Introduction
The genus Alpinia is an important member of the Zingiberaceae family. It includes ca. 230 species.1 Most of them are distributed in tropical and subtropical Asia, including India, Malaysia, China, and Japan. A few are found in Australia and the Pacific Islands.1–3 Plants of this genus have been extensively used for different purposes for centuries. For example, A. vittata, A. purpurata (Vieill.) K. Schum., A. calcarata Rosc., and A. zerumbet are cultivated as ornamental plants;3,4 A. blepharocalyx K. Schum. is a natural dye;5 A. galanga (L.) Willd is an important ingredient for curries and has been broadly utilized as a flavoring in the preparation of meats and soups in Southeast Asia6–8 and in the preparation of beverages in Europe;9 and A. officinarum Hance, listed as medicinal and edible food by the Chinese Ministry of Health, are used in medicinal diets,10–15 wines,16 sauces, and flavorings.17–19 Moreover, A. galanga (L.) Willd is also applied to preserve food and fruits.8,20 Most important of all, Alpinia plants are also broadly used as traditional medicines in India, China, and Japan to treat many diseases such as indigestion, gastralgia, vomiting, enterozoa etc.21–23 Thus, a growing investigation on the chemical constituents and bioactivities of this genus has been carried out since 1955.24 Consequently, Alpinia species were proved to have various biological activities including antiulcer,25 antiemetic,26–28 antibacterial,29–31 antitumor,32–34 hypoglycemic,35 cardioprotection,36 antifungi,37 neuroprotection,38,39 and antianxiety activities.40Up to 2015, this genus contributed about 252 papers. However, only seven review articles were published, five of which were on chemical constituents and biological activities of single plant. And the rest two were on two major components of Alpinia species. The first review came out in 2010 regarding distributions, physiological activities and 13C NMR spectroscopic data of 307 naturally occurring diarylheptanoids, which were mainly isolated from Alpinia species.41 In 2011, the pharmacological and phytochemical studies of A. galanga (L.) Willd were summarized with 30 references. Although it was claimed to concern new phytoconstituents that have appeared in recent years for A. galangal, it actually collected all reported compounds including volatile oil.42 In 2012, structural characterization and biological effects of constituents from the seeds of A. katsumadai was described. Sixty compounds were reported together with their structures and bioactivities with 18 references.43 In 2013, chemical constituents in fruits of A. oxyphylla and their pharmacological activities were summarized. Eighty-five compounds were obtained from this species between 2001 and 2012, with the major component of sesquiterpenes (61.2%). It possessed a variety of pharmacological activities, including neuroprotection, learning and memory-improving function, anticancer, anti-aging, anti-inflammation, and anti-anaphylaxis.44 In 2015, a comprehensive review on the ethnomedical uses, chemical constituents, and the pharmacological profile of A. calcarata Roscoe was published with particular attention given to the pharmacological effects of the essential oil.45 In the same year, the phytochemistry of A. purpurata with pharmacological properties of antioxidant, antibacterial, larvicidal, cytotoxic, and vasodilator activities were reported together with another ornamental ginger, Hedychium coronarium. As a matter of fact, little research was performed on A. purpurata.46 In addition, the isolation, synthesis, and characterization of dihydro-5,6-dehydrokavain, the major constituent of A. zerumbet were also reviewed.47 However, so far there has been no comprehensive review for chemical constituents of this species. Herein, we describe all isolated compounds and their relevant bioactivities of Alpinia species reported in the last six decades from 1955 to 2015.
2. Terpenoids
2.1. Monoterpenoids
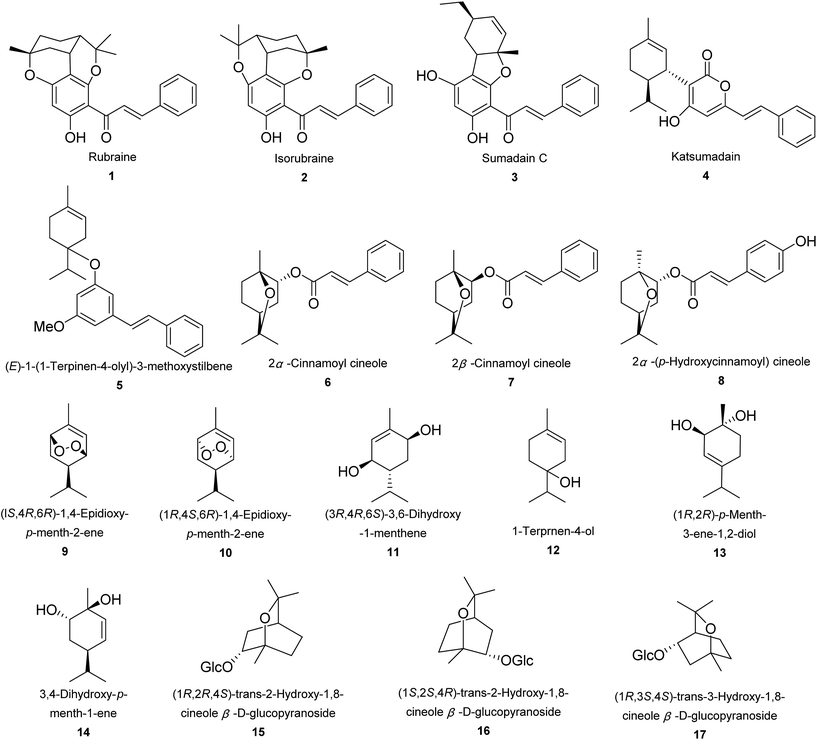
A total number of 17 monoterpenoids were obtained from Alpinia species (Fig. 1). Rubraine (1), isorubraine (2), and sumadain C (3) were three new monoterpene–chalcone conjugates obtained from A. katsumadai.48 They were tested for cytotoxic activities against three tumor cell lines of HepG2, MCF-7, and MAD-MB-435. Sumadain C (3) exhibited very weak effect with IC50 value of around 40.0 μM.48 A. katsumadai Hayata yielded a new monoterpene–kavalactone conjugate, katsumadain (4) and a new (E)-1-(1-terpinen-4-olyl)-3-methoxystilbene (5).49 While A. densibracteata T. L. Wu and Senjen yielded two diastereoisomers of cinnamate esters, 2α-cinnamoyl cineole (6) and 2β-cinnamoyl cineole (7).50 From rhizomes of A. tonkinensis Gagnep., 2α-(p-hydroxycinnamoyl) cineole (8) was isolated.50,51 Two endoperoxides, (1S,4R,6R)-1,4-epidioxy-p-menth-2-ene (9) and (1R,4S,6R)-1,4-epidioxy-p-menth-2-ene (10), were isolated from aerial parts of A. densibracteata T. L. Wu and Senjen.50 Whilst (3R,4R,6S)-3,6-dihydroxy-1-menthene (11) and 1-terpinen-4-ol (12) were obtained from A. sichuanensis Z. Y. Zhu (a synonym of A. jianganfeng T. L. Wu) and A. katsumadai Hayata, respectively.49,52 Fruit of A. oxyphylla Miq. was the source of (1R,2R)-p-menth-3-ene-1,2-diol (13).53 And aerial parts of A. densibracteata T. L. Wu and Senjen yielded 3,4-dihydroxy-p-menth-1-ene (14).50 Compounds 15–17 were three hydroxyl-1,8-cineole glucopyranosides, which were mainly isolated from rhizomes of A. galanga (L.) Willd.54,552.2. Sesquiterpenoids
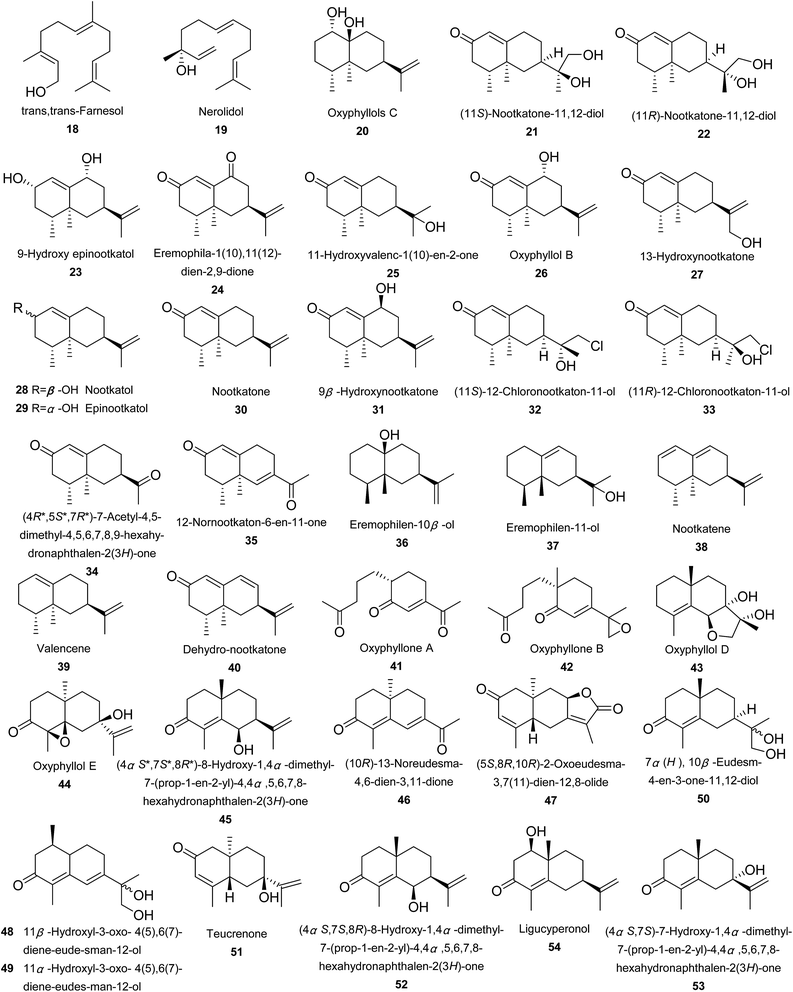
To date, 132 sesquiterpenoids were reported from Alpinia species (Fig. 2). They were divided into acyclic sesquiterpenoids (18 and 19), eremophilanes (20–40), eudesmanes (41–84), cadinanes (85–100), guaianes (101–117), caryophyllanes (118–120), bisabolanes (121–137), humulanes (138–140), drimane (141), elemane (142), carabrane (143), oplopanane (144), and others (145–149).Seeds of A. katsumadai Hayata produced an acyclic sesquiterpenoid, trans,trans-farnesol (18), which exerted weak neuraminidase inhibitory activity in vitro (IC50 = 81.4 μM).56 Nerolidol (19), another acyclic sesquiterpene, was obtained from rethizoms of A. japonica.57
Investigations on fruits of A. oxyphylla Miq. afforded 16 eremophilanes (20–35). Epinootkatol (29) and nootkatone (30) displayed insecticidal activities against larvae and adults of Drosophila melanogaster with IC50 values of 11.5 μM and 96 μg per adult, respectively.58 While 9β-hydroxynootkatone (31), (11S)-12-chloronootkaton-11-ol (32), and (11R)-12-chloronootkaton-11-ol (33) displayed anti-acetylcholinesterase (AChE) activities by TLC-bioautographic assays.59,60 12-Nornootkaton-6-en-11-one (35) was a novel nor-eremophilane. It showed potent anti-AChE bioactivity at 10 nM using the same TLC-bioautographic assay.59 The rest of five eremophilanes (36–40) were isolated from three different species. Eremophilen-10β-ol (36) and eremophilen-11-ol (37) were obtained from A. intermedia Gagnep. and A. japonica (Thunb.) Miq., respectively,61,62 whilst nootkatene (38), valencene (39), and dehydro-nootkatone (40) were all identified from A. oxyphylla Miq.59,63–65
Among 44 eudesmane sesquiterpenoids, oxyphyllones A and B (41 and 42) were isolated from A. oxyphylla. They were the first two examples of 4,5-secoeudesmanes in the Zingiberaceae family.66 Oxyphyllone A displayed moderate anti-AChE activity.59 Also obtained from A. oxyphylla Miq. were compounds 43–63.67,68 A. intermedia Gagnep. was the source of intermedeol (64) and β-selinene (65).61 Investigations of A. japonica (Thunb.) Miq. led to the identification of 66–75.21,57,69,70 Two novel trinoreudesmanes, oxyphyllanenes A (76) and B (77) were obtained from A. oxyphylla, together with four known ones (78–81).71,72 Investigation on A. oxyphylla Miq. provided three nor-eudesmane sesquiterpenoids, oxyphyllanene C (82), (5R,7S,10S)-5-hydroxy-13-noreudesma-3-en-2,11-dione (83), and 4-methoxy-oxyphyllenone A (84).67,71,73
A new 1,10-seco-15-norcadinane sesquiterpene nominated oxyphenol A (85) was isolated from A. oxyphylla.65 Fruits of A. oxyphylla Miq. also provided one tricyclic sesquiterpene, mustakone (86), nine nor-cadinanes, 87–94 and 2β-hydroxy-δ-cadinol (95).53,59,68,74 A. oxymitra K. Schum. was the source of (−)-(1R,4S)-8-hydroxy-13-calamenenoic acid (96).75 Alpiniaterpene A (97) was provided by A. officinarum Hance,76 while 4(15)-cadinene-6,10-diol (98) by A. tonkinensis Gagnep.51 Two new compounds (99 and 100) were isolated from fruits of A. oxyphylla Miq. And 100 exhibited moderate hypoglycemic activity with inhibitory rate of 11.5%, compared to 41.9% of the positive control acarbose (41.9%) at 90 μM.77
Rhizomes of A. japonica (Thunb.) Miq. produced alpinenone (101), an inhibitor of AChE.59,60 Hanamyol (102), containing a cyclic ether linkage, was also isolated from A. japonica (Thunb.) Miq.78 Rhizomes of A. intermedia Gagnep. provided hanalpinol peroxide (103), isohanalpinol (104), and aokumanol (105).61 While A. intermedia Gagnep. and A. japonica (Thunb.) Miq. produced hanalpinol (106), hanalpinone (107), and isohanalpinone (108).61,79 From A. japonica (Thunb.) Miq. and A. intermedia Gagnep., furopelargones A (109) and B (110) were obtained.61,78,80 Later on, 110 was also found from A. formossana.81 Compounds 111–114 were four secoguaiane-type sesquiterpenes with an α,β-unsaturated butenolide. A. intermedia Gagnep. produced epialpinolide (111), whilst A. japonica (Thunb.) Miq. yielded alpinolide peroxide (112), 6-hydroxy-alpinolide (113), and alpinolide (114).61,78,79 A 1,10-secoguaiane sesquiterpene, (+)-mandassidion (115), and two 1,10-seco-15-norguaiane sesquiterpenes, mandassions A (116) and B (117) were obtained from fruits of A. oxyphylla Miq.65
Caryophyllene oxide (118), caryophyllenol-I (119), and caryophyllenol-II (120) were caryophyllanes from A. galanal. In addition, caryophyllene oxide was also distributed in rhizomes of A. conchigera Griff.24,82
Investigation of the aerial parts of A. densibracteata T. L. Wu and Senjen led to the isolation of two bisabolane endoperoxides (121 and 122), three bisabolane hydroperoxides (123–125), and one 3,4-dihydroxy-bisabola-1,10-diene (126).50 Compounds 127–137 were reported from rhizomes of A. japonica (Thunb.) Miq.83
A. oxyphylla Miq. was the source of 3(12),7(13),9(E)-humulatriene-2,6-diol (138).84 While A. formossana and A. japonica produced humulene epoxideII (139).57,81 (9E)-Humulene-2,3;6,7-diepoxide (140) was reported from the fruits of A. oxyphylla Miq. However, its relative configuration remained undetermined. It exhibited moderate anti-AChE activity in bioautographic assay at 10 nM.59,84 Interestingly, the structure and molecular formula for 140 (CAS Registry Number: 21956-93-4) provided by Scifinder were not correct. It should be C15H24O2 instead of C14H21O2.
Rhizomes of A. calcarata Rosc. affored a drimane-type sesquiterpene (γ-bicyclohomofarnesal, 141),85 and an elemane one (shyobunone, 142).83 Pubescone (143) was isolated from A. oxyphylla Miq. and showed weak anti-AChE activity at the concentration of 100 μM.59 (−)-Oplopanone (144) and oxyphyllone F (145) were obtained from fruits of A. oxyphylla Miq.84 (Z)-4-(2,6-Dimethylhepta-1,5-dien-1-yl)-1-methyl-cyclobut-1-ene (146) was a novel nor-sesquiterpene incorporating cyclobutene ring from A. oxyphylla Miq.74 Seeds of A. galanga (L.) Willd produced caryolane-1,9β-diol (147), which suppressed the proliferation of four cancer cell lines of HeLa, A549, HepG2, and SMMC-7721 with IC50 values ranged from 252 to 378 μM.86 A. japonica (Thunb.) Miq. yielded alpiniol (148).87 Compound 2-ethyl-6-isopropyl-7-hydroxymethyl naphthalene (149) was a noval naphthalene from A. oxyphylla.77 It showed bioactive activity with the inhibitory rates of 10.3%, compare to 41.9% of the positive control acarbose at 0.9 mM.77
Noteworthily, compounds 22–31, 34, 48–56, 58–63, 79–82, 87–89, 117, and 129–136 exerted NO production inhibitory activities at different levels.58–60,65,67,71,73,83,88–90 While (10R)-13-noreudesma-4,6-dien-3,11-dione (46), (5S,8R,10R)-2-oxoeudesma-3,7(11)-dien-12,8-olide (47), (5R,7S,10S)-5-hydroxy-13-noreudesma-3-en-2,11-dione (83), and (4S)-10-nor-calamenen-10-one (90) showed potent auxo-action of NO production at 10 μM induced by lipopolysaccharide (LPS) in microglia.71
2.3. Diterpenoids
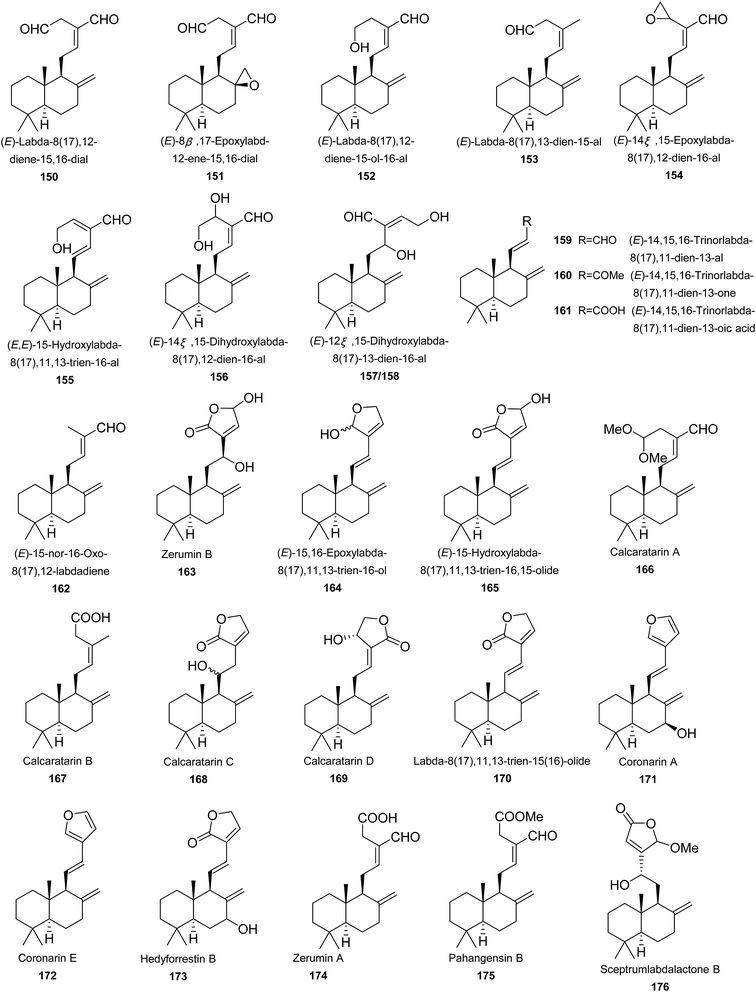
Labdane diterpenes is undoubtedly predominant in Zingiberaceae family, notably in Alpinia genus. Almost all diterpenes are labdanes (150–205). Only one grayanane diterpene was found (206) (Fig. 3).(E)-Labda-8(17),12-diene-15,16-dial (150) is widely distributed in Alpinia. It exhibited a number of bioactivities, such as antibacterial,91 α-glucosidase inhibition,92 NO production inhibition,88 antifungal,93 antiglycation,94 HIV-1 integrase, and neuraminidase inhibitory activities.95 A. katsumadai Hayata, A. galanga (L.) Willd, and A. nigra yielded (E)-8β,17-epoxylabd-12-ene-15,16-dial (151). It exhibited extensive antibacterial activities, especially against Candida guilliermondii and Candida tropicalis.49,91,93,96 Moreover, 151 also showed α-glucosidase inhibitory activity with IC50 value between 5 μM and 10 μM.70 The α-glucosidase inhibitory activity of 151 was even much higher than the positive control, acarbose (IC50 = 400 μM), indicating 151 might be a potential candidate as a future antidiabetic drug.70 A. formosana, A. calcarata Rosc., and A. pahangensis Ridley provided (E)-labda-8(17),12-diene-15-ol-16-al (152),81,85,96 while (E)-labda-8(17),13-dien-15-al (153) was only obtained from A. pahangensis Ridley.96 Flowers of A. chinensis Rosc. provided compounds 154–161.81,85,97 A. tonkinensis Gagnep. and A. speciosa K. Schum. (the accepted name is A. zerumbet (Pers.) B. L. Burtt & R. M. Sm.) were the sources of (E)-15-nor-16-oxo-8(17),12-labdadiene (162).51,98 Both A. zerumbet (Pers.) Burtt and P. M. Smith and A. pahangensis Ridley gave birth to zerumin B (163).96,99 (11E)-15,16-Epoxylabda-8(17),11,13-trien-16-ol (164) and (E)-15-hydroxylabda-8(17),11,13-trien-16,15-olide (165) were found in the flowers of A. chinensis Rosc.97 It is noteworthy that 164 was actually a mixture of two epimers. Rhizomes of A. calcarata Rosc. produced calcaratarins A–D (166–169) and labda-8(17),11,13-trien-15(16)-olide (170).85 Rhizomes of A. malaccensis yielded coronarin A (171), coronarin E (172), and hedyforrestin B (173).100 Coronarin E (172) was also isolated from A. zerumbet (Pers.) Burtt and P. M. Smith, and A. chinensis Rosc.97,99,100 Three antibacterial constituents, zerumin A (174), pahangensin B (175), and sceptrumlabdalactone B (176), were isolated from A. pahangensis Ridley.96 Interestingly, zerumin A (174) was also obtained from A. calcarata Rosc. and A. zerumbet (Pers.) Burtt and P. M. Smith.85,99 Compound 175 was also found in A. japonica (Thunb.) Miq., with NO production inhibition (IC50 = 34.3 μM) in LPS-induced RAW264.7 macrophages.101 Galanolactone (177) was isolated from A. katsumadai Hayata and A. galanga. It was reported to have moderate antifungal activity to Candida guilliermondii PW44 and Candida tropicalis PW30 with both MIC values of 25 μg mL−1.93 Isocoronarin D (178) was found in A. galanga (L.) Willd and A. calcarata Rosc., which weakly suppressed the proliferation of four cancer cells lines of HeLa, A549, HepG2, and SMMC-7721 in a concentration-dependent way with IC50 values ranging from 69.1 to 87.0 μg mL−1.64,67 Seeds of A. galanga yielded galaganin (179), which showed moderate cytotoxicity towards DU145, MCF-7, H522, and k562 cells with IC50 values of 8.2, 13.8, 17.8, and 16.1 μM, respectively.102 Rhizomes of A. pinnanensis T. L. Wu et Senjen produced labda-8(17),13(14)-di-en-15,16-olide (180) and ottensinin (181).96 A. japonica provided compounds 182–187, of which 182 and 183 were norlabdanes.101 Compounds 182, 185, and 186 exhibited significant NO production inhibitory effects in LPS-induced RAW264.7 macrophages, with respective IC50 values of 25.9, 14.6, and 25.6 μM, compare to 39.6 μM of the positive control, N-monomethyl-L-arginine (L-NMMA).101 Ethanol extract of A. oxyphylla Miq. provided 188, which showed moderate hypoglycemic effect with inhibitory rates of 10.0% at 60 μM.77 Ottensinin showed moderate antibacterial activity on the Gram-positive bacteria of Bacillus cereus with MIC value of 0.25 μg μL−1.96 Alpindenosides A–D (189–192) were four labdane glycosides from A. densespicata Hayata. They didn't show cytotoxic activities against four human tumor cell lines of Hela, KB, Doay, and WiDr at 20 μM. Instead, they all exhibited moderate NO inhibitory activities with IC50 ranging from 30 to 49 μM.103 Leaves of A. flabellate provided rel-labda-12-en-15(16)-olid-7-one-8R-spiro-1′-[2S-(2,4,5-trimethoxyphenyl)-3-cyclohexene] (193), a unique labdane diterpene coupled with a phenylbutenoid.104 Noralpindenosides A (194) and B (195) were two norditerpene glycosides from A. densespicata Hayata, both of which showed moderate inhibitory effects on NO production with IC50 values of 34.2 and 49.3 μM, respectively.103 (E,E)-15-Hydroxylabda-8(17),11,13-trien-16-al (196) and its diastereoisomer (197) from A. chinensis Rosc. may arise by direct oxygenation of (E,E)-15-hydroxylabda-8(17),11,13-trien-16-al.97 From flowers of A. chinensis Rosc., coronarin B (198) containing a seven-membered endoperoxide hemiacetal was isolated.97 It should be noted that although the structure and its NMR and MS spectroscopic data referred to coronarin B (CAS number: 119188-38-4) in the reference, the author gave a wrong name for this compound as coronarin C (CAS number: 119188-35-1) which was previously isolated from Hedychium coronarium.105 Galanals A (199) and B (200) were obtained from A. galanga (L.) Willd. Both compounds showed significant antifungal activities against Candida guilliermondii PW44 with MIC values of 12.5 μg mL−1. Furthermore, galanal A exhibited potent cytotoxic activity against KB cells (IC50 = 3.25 μg mL−1).7,93 Compound 201 was a novel metabolite conjugated of labdane diterpene with chalcone from aerial parts of A. katsumadai Hayata.49 A. pahangensis Ridley provided pahangensins A (202) and C (203) as antibacterial constituents.96,106 A. pahangensis Ridley produced calcaratarins D (204) and E (205), both of which were cytotoxic against human KB cells in vitro with IC50 value of 0.21 and 0.15 μg mL−1, respectively.107 From seeds of A. katsumadai Hayata, a grayanane diterpenoid was isolated and characterized as rhodomollein I (206).108
2.4. Triterpenoids
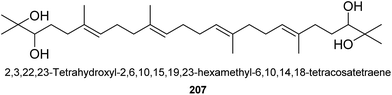
Up to now, only one triterpene was found from this genus (Fig. 4). It was named as 2,3,22,23-tetrahydroxyl-2,6,10,15,19,23-hexamethyl-6,10,14,18-tetracosatetraene (207), an acyclic triterpenoid, isolated from the seeds of A. katsumadai L.109 It showed weak cholesterol acyltransferase inhibitory activity with IC50 value of 47.9 μM.1093. Diarylheptanoids
A total of 143 diarylheptanoids (208–350, Fig. 5) were isolated from Alpinia species, including 66 acyclic diarylheptanoids (208–273), 11 cyclic diarylheptanoids (274–284), 50 diarylheptanoid and flavonoid conjugates (285–334), 10 dimeric diarylheptanoids (335–344), and six others (345–350).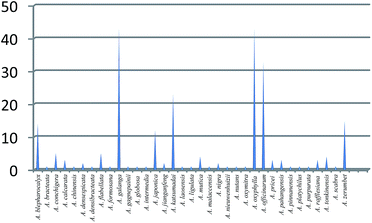 | ||
| Fig. 12 The number of published papers for each investigated Alpinia species on chemical constituents and their bioactivities over last six decades since 1955. | ||
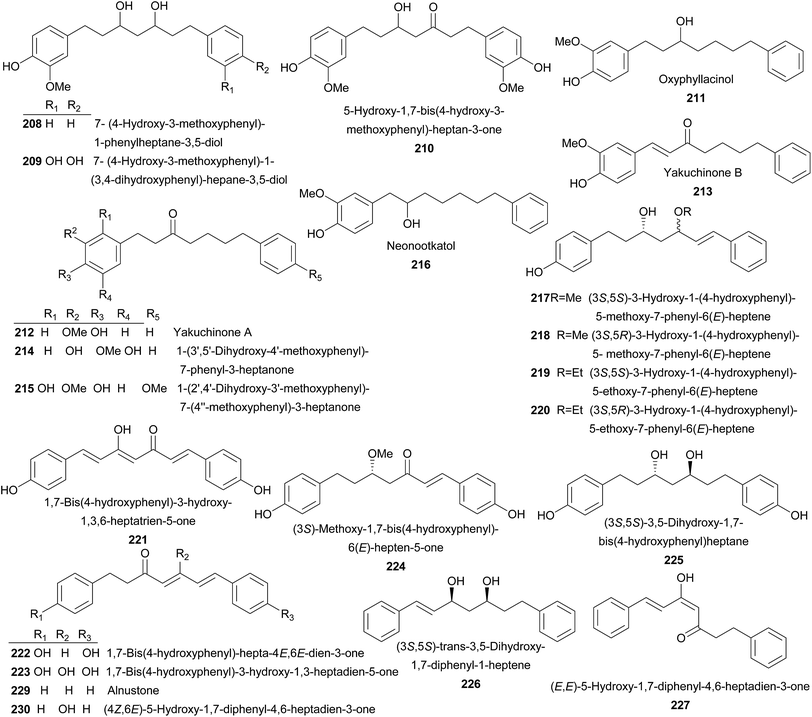
Compounds 208–210 were isolated from rhizomes of A. officinarum Hance. They were moderate or weak NO production inhibitors.110 From fruits of A. oxyphylla, oxyphyllacinol (211) and yakuchinones A–B (212–213) were isolated, of which 211 was a NO production inhibitor, while 212 and 213 exhibited anti-tumor activities to human promyelocytic leukemia (HL-60) cells in a concentration-related manner.32,67 In addition, 212 also possessed insecticidal,36 anti-adipocyte differentiation,111 NO production inhibitory,46 and cardiotonic activities.112 Compounds 213–216 were also yielded by fruits of A. oxyphylla.113,114 Seeds of A. blepharocalyx K. Schum. gave birth to 217–225.115–117 Among these compounds, 1,7-bis(4-hydroxyphenyl)-3-hydroxy-1,3-heptadien-5-one (223) significantly inhibited platelet aggregation induced by collagen with IC50 value of 14.7 μg mL−1.117 (3S,6E)-Methoxy-1,7-bis(4-hydroxyphenyl)-6-hepten-5-one (224) and (3S,5S)-3,5-dihydroxy-1,7-bis(4-hydroxyphenyl)heptane (225) showed significant antiproliferative activities against murine colon 26-L5 carcinoma and human HT-1080 fibrosarcoma with IC50 values of 5.2 and 12.8 μM, respectively.115,116 Both A. pinnanensis T. L. Wu et Senjen and A. katsumadai Hayata provided (3S,5S)-trans-3,5-dihydroxy-1,7-diphenyl-1-heptene (226).118,119 It did not showed antimycobacterial activity (MIC ≥ 64 mg L−1). Instead, it exhibited weak neuraminidase inhibitory activity (IC50 = 29.75 ± 8.15 μM) in vitro.56,120 (E,E)-5-Hydroxy-1,7-diphenyl-4,6-heptadien-3-one (227), (S)-1,7-diphenyl-6(E)-hepten-3-ol (228), and alnustone (229) were isolated from A. katsumadai Hayata with significantly neuraminidase inhibitory in vitro with IC50 values between 1.0 and 6.1 μM.56 In addition, 229 also possessed antiemetic,121 antimycobacterial activities,120 and significantly inhibited proliferation of Bel 7402 and L0-2 cells.122 Investigation of A. katsumadai Hayata also led the isolation of compounds 230–238.49,119,121,123–125 1,7-Bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one (239) and bisdemethoxycurcumin (240) were obtained from rhizomes of A. galanga (L.) Willd, both of which significantly inhibited the proliferation of melanoma cells and indistinctively inhibited cellular tyrosinase.126 A planar structure of 1,7-diphenyl-5-hydroxy-6-hepten-3-one (241) was reported from A. nutans Rosc.,127 A. rafflesiana Wall.ex.Bak.,128 and A. officinarum Hance.129 While its enantiomers, 5S (241a) and 5R (241b) counterparts, were identified from A. mutica Roxb.130 and A. katsumadai Hayata,119 respectively. It was shown that a large amount of diarylheptanoids (242–276) were obtained from the rhizomes of A. officinarum Hance.27,131–136 7-(3,4-Dihydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)-4-en-3-heptanone (257) displayed moderate cytotoxicity against human tumor cell lines of HepG2, MCF-7, and SF-268. While (4E,6E)-5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-7-phenylhepta-4,6-dien-3-one (258) showed weak cytotoxicity against two cancer cell lines of MCF-7 and T98G with IC50 values of 22.68 and 4.44 μM, respectively.135 Meanwhile, 258–267 were proved to be inhibitors of Helicobactor pylori (Hp-Sydney and Hp-F44).129 AO-5 (263) showed anti-inflammatory activity induced by 12-O-tetradecanoylphorbol-13-acetate (TPA), platelet-activating factor (PAF), and NO.110,136,137 Moreover, it exhibited very weak cytotoxic activity against human glioblastoma T98G cells (IC50 = 27 μM).138 The acetone extract of the rhizomes of A. officinarum Hance showed 5α-reductase inhibitory effect, which was superior to the drug used in the treatment of androgen-dependent disorders. Therefore, a bioactivity-guided isolation was performed and resulted in the isolation of 263–266 which exerted 5α-reductase inhibitory effect with IC50 values ranging from 220 to 390 μM, indicating potent usage in treating androgen-dependent diseases.139 Besides, AO-1 (266) also showed anti-helicobacter pylori, hypolipidemic activities, and NO production inhibitory activity.110,140,141 AO-2 (267) was identified as an inhibitor of prostaglandin (PG) biosynthesis and exerted antioxidant activity.142,143 It is interesting to note that dihydroyashabushiketol (264), AO-1 (266), and AO-2 (267) were firstly reported as planar structures, and later, their absolute configurations were established as 264a, 266a, and 267a, respectively.136,144
7-(4′′-Hydroxy-3′′-methoxyphenyl)-1-phenyl-3,5-heptadione (268) also exhibited prostaglandin biosynthesis inhibitory effect with IC50 values of 50 μM.143 AO-4 (269) was found to have marked inhibitory effect on TPA-induced inflammation and antioxidant activity.142,144 6-Hydroxy-1,7-diphenyl-4-en-3-heptanone (270) was a PAF inhibitor.137 AO-3 (271) and (5S)-5-methoxy-1,7-diphenyl-3-heptanone (272) displayed potent inhibitory effects on TPA-induced inflammation in mice with 50% of inhibition at a dose of 0.8–2.7 μmol per ear.144 (3R,5R)-1-(4-Hydroxyphenyl)-7-phenyl-3,5-heptanediol (273) showed significantly antiemetic effect induced by CuSO4 with 37.7% inhibition at a dose of 50 mg kg−1.27,145
Investigation on seeds of A. blepharocalyx K. Schum. led to the isolation of ten cyclic diarylheptanoids (274–283).115,146–148 Rhizomes of A. officinarum Hance provided 3,6-furan-7-(4′′-hydroxy-3′′-methoxyphenyl)-1-phenylheptane (284).131 From the seeds of A. katsumadai, 285–292 were obtained,149 three of which (285–287) displayed weak antiproliferative activities against four cancer cell lines of NCI-H460, HeLa, SMMC-7721, and HCT-116 with IC50 values of 15.39–42.24 mM.115,149 A. blepharocalyx K. Schum. was the source of 293–305.115,148,150,151 However, the stereochemistry at C-9′′ of six stereoisomerics (294/295, 296/297, 298/299, 300/301, 302/303, 304/305) remained unsolved. Calyxin J (298), epicalyxin J (299), calyxin K (300), and epicalyxin K (301) showed marked anti-proliferative activity against human HT-1080 fibrosarcoma cells with ED50 values from 0.3–8.2 μM.115,152 Compounds 302–305 were proved to inhibit NO production in endotoxin activated murine macrophage J774.1 with 90–94% inhibitory rate at a concentration of 100 μg mL−1.151 Seeds of A. katsumadai Hayata provided 306–318. Calyxins Q (306) and R (307) exerted potent antiproliferative activities against four cancer cell lines of NCI-H460, HeLa, SMMC-7721, and HCT-116 at the level of IC50 values of 15.3–42.2 μM.149 Calyxin B (319) and epicalyxin B (320) were obtained from A. blepharocalyx K. Schum. and A. pinnanensis as NO production inhibitiors.115,151 In addition, 319 showed potent antiproliferative activity against human HT-1080 fibrosarcoma cells with an ED50 value of 0.69 μM.148 Both A. pinnanensis T. L. Wu et Senjen and A. katsumadai Hayata yielded alpinnanin B (321).118,124 From A. katsumadai Hayata and A. blepharocalyx K. Schum., epicalyxin H (322) and calyxin H (323) were isolated.118,124,153 Epicalyxin H was identified as NO production inhibitor.115,153 Seeds of A. blepharocalyx yielded 324–330.115,152,154 It's worth mentioning that all three structures of calyxin L (325), epicalyxin F (327), and calyxins F (328) in the Scifinder were wrong. Out of a serious of diarylheptanoids bearing a chalcone or a flavanone moiety, epicalyxins I (326), F (327), and calyxin F (328) were shown to possess strong antiproliferative activities toward colon 26-L5 carcinoma and HT-1080 fibrosarcoma with IC50 values ranging from 0.5 to 10.1 μM.115,150 Meanwhile, 326 and 327 were cytotoxic against human fibrosarcoma cells with IC50 values ranging from 0.9 to 12.1 μM.152 6-Hydroxycalyxin F (329) and calyxin A (330) demonstrated NO production inhibitory activities with IC50 values of 49 and 62 μM, respectively.115,150 Rhizomes of A. pinnanensis T. L. Wu et Senjen provided deoxycalyxin A (331), alpinnanins A (332), and C (333).118 In addition, 331 was also found in A. blepharocalyx K. Schum.115 While officinin A (334) was obtained from rhizomes of A. officinarum Hance.155
Five dimeric diarylheptanoids (335–339) were obtained from rhizomes of A. officinarum Hance.135,136,138,156,157 Only alpinin C (338) displayed selective cytotoxic against MCF-7 (IC50 = 62.3 μM) and T98G cells (IC50 = 57.3 μM).135 Seeds of A. blepharocalyx K. Schum. provided 340–344 possessing two diarylheptanoid units and a chalcone moiety.115,146,153 Both blepharocalyxins A (340) and B (341) showed concentration-dependent inhibition in the range of 1–100 μg mL−1 against NO production in endotoxin-activated murine macrophages J774.1.158 Blepharocalyxins C–E (342–344) were tested for antiproliferative activities against two tested cancer cells, blepharocalyxin D (343) exhibited the strongest effect against highly liver-metastatic murine colon 26-L5 carcinoma cells (ED50 = 3.6 μM), whereas blepharocalyxin E (344) showed the strongest activity against human HT-1080 fibrosarcoma cells (ED50 = 9.02 μM).115,146,159 It is worth mentioning that the stereochemistry at C-I-5 position for 343 in Scifinder was S, which was not correct and should be revised as R. Moreover, the two diarylheptanoid moieties in 344 were wrongly connected through C-I-6 and C-II-5 by Scifinder. Instead, it should be joined through C-I-6 and C-II-7. Two unusual diarylheptanoid derivatives, neocalyxin A (345) and its epimer neocalyxin B (346), were found from the seeds of A. blepharocalyx K. Schum., with the stereochemistry at C-9′′ undetermined.115,152
Rhizomes of A. officinarum Hance produced officinaruminane B (347), a diarylheptanoid coupled with a monoterpene unit.131 Investigation on seeds of A. katsumadai Hayata identified two novel anti-emetic diaryllheptanoids, katsumadains A (348) and B (349).160 Besides, 348 also exerted promising neuraminidase inhibitory effect against human influenza virus A/PR/8/34 (IC50 = 1.05 μM).56 4-Phenethyl-1,7-diphenyl-1-heptene-3,5-dione (350) was isolated from rhizomes of A. officinarum Hance. It exhibited weak antibacterial activity against Hp-Sydney and Hp-F44 with the MIC values of 23.6–31.4 and 78.5 μM, respectively.129
4. Lignans
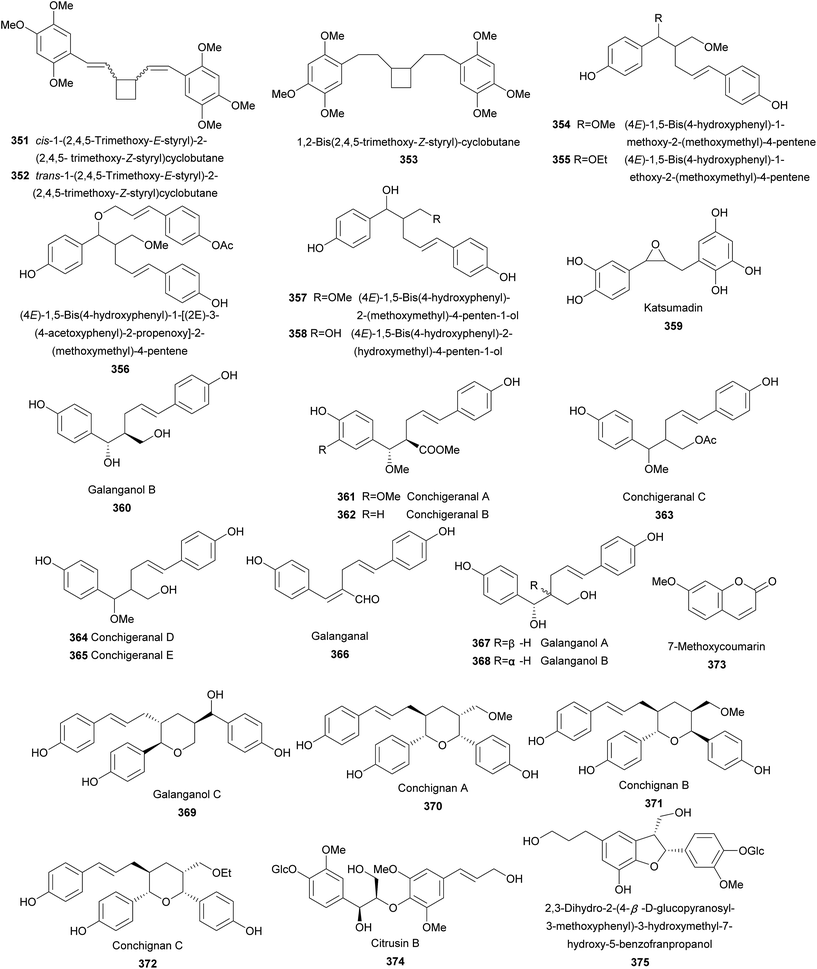
Twenty-four lignans (351–374) were reported from the genus of Alpinia (Fig. 6). Separation for leaves of A. flabellata Ridley resulted in the isolation of 351–353, three phenylbutanoid dimers bearing a novel tetracyclic moiety.161,162 cis-1-(2,4,5-Trimethoxy-E-styryl)-2-(2,4,5-trimethoxy-Z-styryl)cyclobutane (351) and trans-1-(2,4,5-trimethoxy-E-styryl)-2-(2,4,5-trimethoxy-Z-styryl)cyclobutane (352) showed weak antibacterial against Staphylococcus aureus with MIC values of 5.0 and 2.5 mM, respectively.161 Furthermore, 351 significantly decreased the ovalbumin permeability in intestinal cells.161Rhizomes of A. officinarum Hance yielded 354–358 containing a rare β–γ linkage. All five compounds exhibited weak antioxidant activities against the autoxidation of methyl linoleate in bulk phase.163 Extracts of seeds of A. katsumadai Hayata afforded antiemetic katsumadin (359) with antiemetic activity on CuSO4-induced emesis in young quail.121 Galanganol B (360) was isolated from rhizomes of A. galanga (L.) Willd.164 Investigation on the whole plant of A. conchigera afforded eight rare 8–9′ linked neolignans 361–368.165 Although conchigeranals D (364) and E (365) shared the same planar structure, their relative configurations were not be determined. Galanganal (366), galanganols A (367), and B (368) were also found from rhizomes of A. galanga (L.) Willd.166 Compounds 361–367 exhibited significant cytotoxic activity against cancer Hela cells with IC50 values ranging from 1.5 to 5.29 μg mL−1.165 Interestingly, 366 and 368 also inhibited NO production in mouse peritoneal macrophages.166 Galanganol C (369) was obtained from rhizomes of A. galanga (L.) Willd as a NO production inhibitor.166 The whole plant of A. conchigera yielded three unusual sesquineolignans, conchignans A–C (370–372) bearing a tetrahydropyrane ring.167 7-Methoxycoumarin (373) is a coumarin known from A. calcarata Rosc.85
Citrusin B (374) and 2,3-dihydro-2-(4-β-D-glucopyranosyl-3-methoxyphenyl)-3-hydroxymethyl-7-hydroxy-5-benzofranpropanol (375) were the only two lignan glycosides isolated from leaves of A. speciosa.168
5. Flavonoids
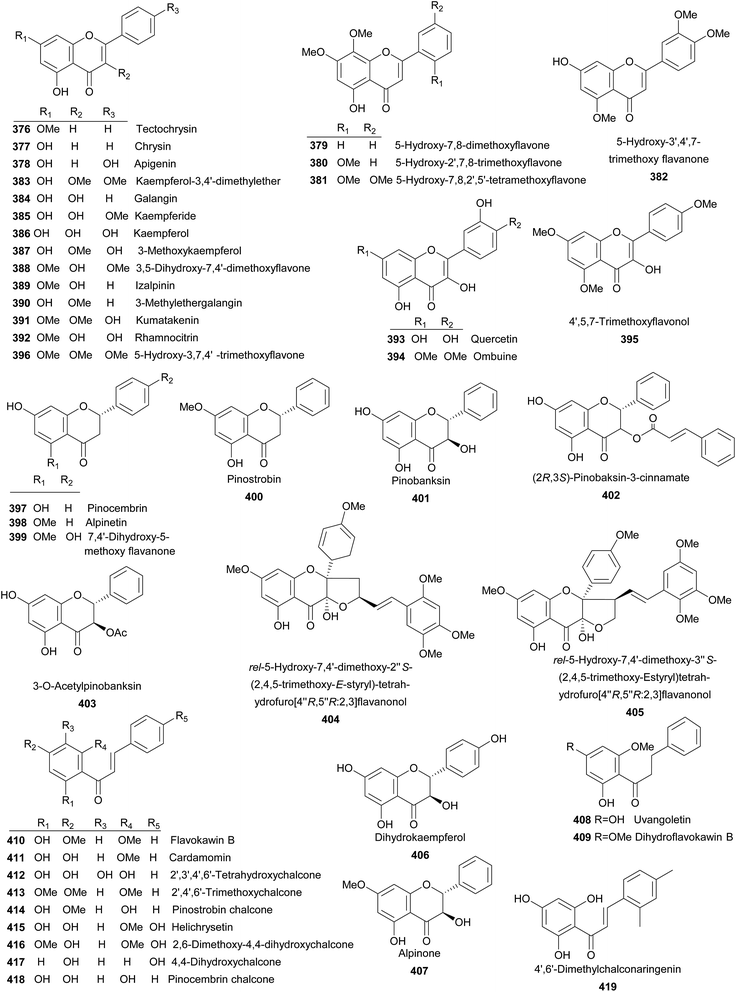
To date, 71 flavanoides (Fig. 7) were isolated from the Alpinia species, including seven flavones (376–382), 14 flavonols (383–396), four flavanones (397–400), seven flavanonols (401–407), two dihydrochalcones (408 and 409), 13 chalcones (410–422), four flavanols (423–426), and 18 flavonoid glycosides (427–444), two flavonoid oligomers (445 and 446).Tectochrysin (376) and chrysin (377) were isolated from A. oxyphylla Miq. and exhibited moderate anti-inflammatory activities against LPS-induced NO production in RAW264.7 macrophage cells.169 Both A. bracteata and A. officinarum Hance produced apigenin (378), which displayed moderate activity on scavenging DPPH free radicals (EC50 = 90 ± 1.5 μM).170 A. galanga (L.) Willd was the source of 379–381 and A. tonkinensis Gagnep. produced 5-hydroxy-3′,4′,7-trimethoxy flavanone (382).102,171 Kaempferol-3,4′-dimethylether (383) was afforded by A. sichuanensis Z. Y. Zhu.52 Galangin (384) and kaempferide (385) were the major flavonols distributed in several plants of Alpinia, both of which exhibited inhibitory against penicillinase and potent antioxidant activities.113,172 In addition, galangin effectively inhibited the TPA-induced invasion and migration of HepG2 cells at concentrations of 2.5–5 μM.173 In 2001, a review summarized anti-genotoxic activity of galangin and demonstrated that galangin was a promising candidate for cancer chemoprevention.174 Investigation on the whole plant of A. sichuanensis Z. Y. Zhu provided kaempferol (386).52 From A. speciosa, A. galanga (L.) Willd, A. katsumadai Hayata, and A. tonkinensis Gagnep., 3-methoxykaempferol (387) was isolated.175–178 While A. flabellata Ridley, A. oxyphylla, and A. tonkinensis Gagnep. yielded 3,5-dihydroxy-7,4′-dimethoxyflavone (388).113,161,171 Izalpinin (389) from different parts of A. oxyphylla Miq. was a NO production inhibitor and exhibited potent antioxidant activity.113,176 From rhizomes of A. officinarum, 3-methylethergalangin (390) was identified as an inhibitor of pancreatic lipase with an IC50 value of 1.3 mg mL−1.179 Compounds 391–395 were mainly obtained from A. tonkinensis Gagnep.171 5-Hydroxy-3,7,4′-trimethoxyflavone (396) was yielded by leaves of A. flabellata Ridley.180 Pinocembrin (397) and alpinetin (398) were distributed in several Alpinia species and both showed antiemetic activities.121,181 In addition, 397 also demonstrated several bioactivities, such as cytotoxicity (on human T4 lymphoblastoid cancer cells),182 anti-inflammation,169 and antiplatelet aggregation etc.183 While, 398 was a PAF receptor binding inhibitor.184 7,4′-Dihydroxy-5-methoxy flavanone (399), pinostrobin (400) were reported from several species.116,118,128,182 Pinobanksin (401), (2R,3S)-pinobaksin-3-cinnamate (402), and 3-O-acetylpinobanksin (403) were mainly obtained from A. galanga (L.) Willd and A. katsumadai Hayata.176,177,185 Compound 402 showed potent neuroprotective effect against PC12 cells.177,186 Leaves of A. flabellata Ridley provided 404 and 405.180 Dihydrokaempferol (406) were isolated from A. oxyphylla.169 Both A. japonica (Thunb.) Miq. and A. galanga (L.) Willd were sources for alpinone (407).176,187 From seeds of A. katsumadai Hayata, a dihydrochalcone uvangoletin (408) was isolated.108 A. speciosa K. Schum. and A. formosana afforded another dihydrochalcone, dihydroflavokawin B (409).81,188 Flavokawin B (410) was isolated from several plants and showed strong cytotoxicity against human T4 lymphoblastoid cancer cells (IC50 = 6.5 μM) and anti-inflammatory activity.182,189 Cardamomin (411) distributed in many Alpinia species100,118,123,188,190 and exhibited extensive bioactivities including death receptor 5 (DR5) promotor,175 antimicrobial,191 antiemetic,121 anticoagulation,183 and anti-inflammation.128 Interestingly, it also protected septic mice from acute lung injury by preventing endothelial barrier dysfunction.192 2′,3′,4′,6′-Tetrahydroxychalcone (412), which was obtained from A. rafflesiana Wall.ex.Bak., was potently active to DPPH free radical scavenging (IC50 = 55 μM).128 Rhizomes of A. pricei Hayata yielded 2′,4′,6′-trimethoxychalcone (413) and pinostrobin chalcone (414).189 Compounds 415–417 were isolated from the seeds of A. blepharocalyx K. Schum.,116,117 while helichrysetin (415) was also found in A. katsumadai Hayata.108 Pinocembrin chalcone (418) and 4′,6′-dimethylchalconaringenin (419) were provided by A. katsumadai Hayata and A. pinnanensis T. L. Wu et Senjen, respectively.118,181 Compound 418 was also isolated from A. platychilus.193 Galanganones A–C (420–422) were three novel chalcones bearing a long-chain alkylphenol from A. galanga.194 Whilst A. katsumadai Hayata and A. zerumbet (Pers.) B. L. Burttet Smith. provided (+)-catechin (423).195,196 Epicatechin (424) and galloepicatechin (425) were yielded by A. oxymitra K. Schum.75 (+)-Epicatechin (426) was isolated from A. speciosa K. Schum. and displayed antioxidant activity.197 Kaempferide-3-O-β-D-glucoside (427) from A. officinarum Hance had an weak inhibitory activity against penicillinase.172 Study on A. speciosa K. Schum. lead to the isolation of 428–432.198 Quercetin 3-O-robinobioside (433) and galangoflavonoside (434) were obtained from A. katsumadai Hayata and A. galanga (L.) Swartz., respectively.196,199 Compounds 435–437 from A. densespicata Hayata exhibited moderate NO inhibitory activities.103 Compounds 438–440 were obtained from the seeds of A. katsumadai Hayata and isorhamnetin-3-O-β-D-galactosyl-(6 → 1)-α-L-rhamnoside (441) was isolated from rhizomes of A. tonkinensis Gagnep.51,196 Leaves of A. zerumbet (Pers.) B. L. Burttet Smith. contained rutin (442) and kaempferol-3-O-rutinoside (443).195 The whole plant of A. sichuanensis Z. Y. Zhu yielded hesperidin (444).52 Two pairs of enantiomers of flavonoidoligomers (445a and 445b, 446a and 446b) were found from rhizomes of A. platychilus. The compounds mixture of 446a and 446b showed anticoagulant activity on the prolongation of both prothrombin times (PT) and the thrombin times (TT) with a dose-effect relationship at 6.25–100 mM.193
6. Phenolics
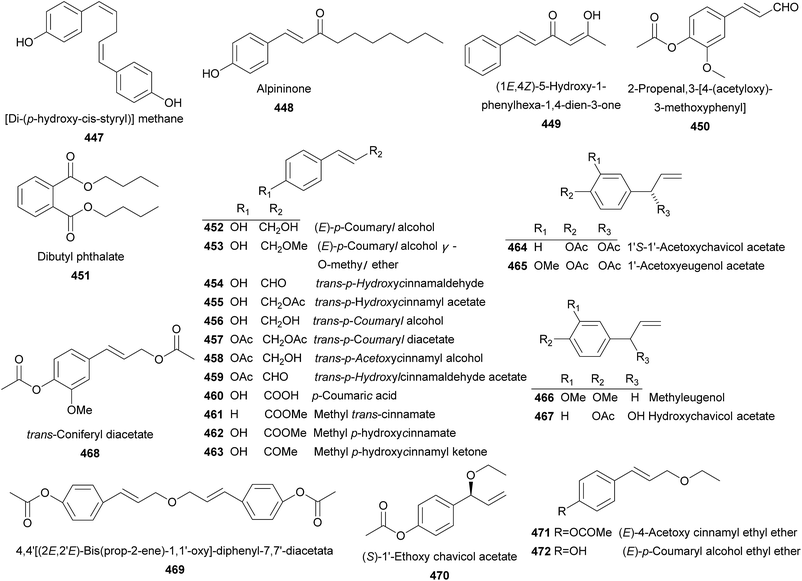
A total number of 66 phenolics (447–512) were obtained from Alpinia species (Fig. 8). [Di-(p-hydroxy-cis-styryl)]methane (447) was obtained from A. galanga (L.) Willd.200 Whist alpininone (448) was isolated from A. gagnepainii K. Schum. with antibacterial effect against E. coli, B. subtilis, and S. aureus with the same MIC value of 12.5 μg mL−1.191 (1E,4Z)-5-Hydroxy-1-phenylhexa-1,4-dien-3-one (449) and 2-propenal, 3-[4-(acetyloxy)-3-methoxyphenyl] (450) were provided by A. katsumadai Hayata and A. galanga (L.) Willd, respectively.86,108 From A. sichuanensi and A. oxyphylla, dibutyl phthalate (451) was isolated.52,201 Two compounds named as (E)-p-coumaryl alcohol (452) and (E)-p-coumaryl alcohol γ-O-methyl ether (453) exhibited potent inhibitory activities against the autoxidation of methyl linoleate in bulk phase.163 In addition, compound 453 exerted potent cytotoxic activity against the SNU638 cells with IC50 value of 1.62 μg mL−1.202A. galanga (L.) Willd and A. conchigera Griff. produced trans-p-hydroxycinnamaldehyde (454) and trans-p-hydroxycinnamyl acetate (455).170,203 Compound 454 displayed weak antiallergic effect,204 and NO production inhibitory activities (IC50 = 20 μM),166 and 455 exerted no inhibitory activity towards Staphylococcus aureus strain VISA (MIC = 203 mM).82 trans-p-Coumaryl alcohol (456) was a weak NO production inhibitor from A. galanga (L.) Willd (IC50 = 72 μM).166 trans-p-Coumaryl diacetate (457) from A. galanga showed a number of bioactivities, including anti-allergy,204 efflux pump inhibition,205 NO production inhibition,166 xanthine oxidase inhibition,206 antileishmania,164 cytotoxicity,203 and antibacteria.82 trans-p-Acetoxycinnamyl alcohol (458), trans-p-hydroxylcinnamaldehyde acetate (459), and p-coumaric acid (460) were obtained from rhizomes of A. galanga (L.) Willd.164,205 In addition, compound 460 was also distributed in A. galanga (L.) Willd,164 A. sichuanensis Z. Y. Zhu,52 A. speciosa,198 A. blepharocalyx K. Schum.,116 and A. oxyphylla.169 Both A. formosana and A. speciosa K. Schum. were sources of methyl trans-cinnamate (461).81,188 Seeds of A. blepharocaly yielded methyl p-hydroxycinnamate (462) and methyl p-hydroxycinnamyl ketone (463).116 From rhizomes of A. galanga (L.) Willd, 12 compounds (464–475) were obtained.33,82,203,207 Among them, 1S-1′-acetoxychavicol acetate (464) and 1-acetoxyeugenol acetate (465) were the most abundant phenylpropanoids presented in A. galanga (L.) Swartz., A. officinarum Hance, and A. conchigera Griff. They were reported to have anti-ulcer,24 antileishmanial,164 and antitumor bioactivities,33,202,208 Furthermore, 464 also showed antiallergic,204 efflux pump inhibitory,205 NO production inhibitory,166 xanthine oxidase inhibitory,206 gastroprotective,209 anti-HIV,210 anti-cancer,86 antibacterial,30,211 plant growth-inhibitory and fungal growth-inhibitory activities.212 Two compounds, methyleugenol (466) and hydroxychavicol acetate (467), were isolated from A. galanga (L.) Willd.82,164,166,204,211 It was demonstrated that 467, a chavicol acetate analogue, suppressed T-bet expression in Th cells.211 Besides, 467 also showed weak antibacterial activity against Staphylococcus aureus strain VISA (MIC = 0.8 mM).82 trans-Coniferyl diacetate (468) was proved to be a xanthine oxidase inhibitor.206 Three new phenolics 469, 470, and 471, along with four known ones 472–475 were also yielded by A. galang.33,203,207 Chavicol acetate (476) and 1′S-acetoxyeugenol acetate (477) were two known phenolics found from A. conchigera Griff.82 Compound 477 possessed antibacterial,82 xanthine oxidase inhibitory,206 gastroprotective,209 and anti-cancer activities.86 Investigation on leaves of A. flabellata Ridley provided 478–480, with strong antibacterial activities against Staphylococcus aureus.161,162,180 Compounds 481–489 were nine phenolic acids isolated from several Alpinia species.52,89,122,180,198,213,214 Protocatechuic acid (489) showed potent neuroprotective effect on MPP+-induced neurotoxicity and H2O2-induced oxidative damage in PC12 cells.215–219 In addition, it also exerted anti-aging effect on spleen and liver antioxidative system of senescent mice.31 4-Hydroxybenzaldehyde (490), isolated from A. sichuanensis Z. Y. Zhu, A. blepharocalyx K. Schum., A. bracteata, and A. galanga (L.) Willd,52,116,117,166,170 didn't show any DPPH radical-scavenging activity. Instead, it exhibited inhibitory activity on xanthine oxidase (IC50 = 19.6 μM).170,206 Compounds 491–496 were provided by several Alpinia plants.52,84,137,167,220
Ethyl 4-O-feruloyl-β-glucopyranoside (497) and 4-hydroxy-3-methoxyphenyl 4-O-feruloyl-β-glucopyranoside (498) were two new glucoside esters of ferulic acid from rhizomes of A. speciosa, both of which showed antioxidant activities.197 Investigation on rhizomes of A. officinarum Hance yielded 499–506.55 While from rhizomes of A. bracteata, a new phenolic glycoside (507) was isolated and showed moderate antioxidant activity on scavenging DPPH free radicals (EC50 = 169 ± 4.8 μM).170 Leaves of A. speciosa K. Schum. provided coniferin (508) and syringin (509).168
Dihydro-5,6-dehydrokawain (510) and 5,6-dehydrokawain (511) were major chemical constituents in several Alpinia species.81,100,128,175,188,221 They showed antiulcerogenic, antithrombotic,195 antifungal,191 anti-obesity,222 and plant growth inhibitory activities.223 Recently, it was reported that they could strongly inhibit HIV-1 integrase with respective IC50 values of 4.4 and 3.6 μg mL−1. In addition, they exhibited mixed type of inhibition against neuraminidase with both IC50 values of 25 μM.95 Furthermore, 511 was also reported as a slow and time-dependent reversible inhibitor of neuraminidase, a moderated antioxidant, a strong inhibitor of skin diseases-related enzymes, and strong antiplatelet inhibitor.95,127,224 Interestingly, a dimer of 5,6-dehydrokawain, AS-II (511a), was an artifact formed by photo-irradiation during the isolation procedure of A. speciosa K. Schum. leaves.223 4-Hydroxy-5,6-dehydrokawain (512) was an α-pyrone isolated from A. blepharocalyx K. Schum. It displayed antiproliferative activity against murine colon 26-L5 carcinoma and human HT-1080 fibrosarcoma with ED50 20.7 and 20.1 μM, respectively.116,117 It also showed inhibitory effect on platelet aggregation induced by collagen, arachidonic acid (AA), adenosine diphosphate, and ristocetin.96
7. Steroids
Seven steroids (Fig. 9) were isolated from Alpinia species including four cholestanes (513–516) and three sitosterol glycosides (517–519).27,52,89,116,118,225 As it is the same in plants of the other genera, β-sitosterol (513) and stigmasterol (514) were also widely distributed in Alpinia species.52,82,89,118,178,191,226–228 β-Sitosterol-3-O-β-D-6-palmitoylglucoside (518) showed potent antiemetic activity induced by CuSO4.278. Alkaloids
Officinaruminane A (520) and officinine B (521), two alkaloids of bi-diarylheptanoid connecting by a pyridine ring were contributed by rhizomes of A. officinarum Hance.131,157 A study on seeds of A. katsumadai Hayata afforded another six alkaloids (522–527) (Fig. 9).108,1969. Stilbenes
Six stilbenes, 528–533 (Fig. 9), were all isolated from aerial parts of A. katsumadai Hayata.29,12110. Others
One esters (534) and three fatty acids, 535–537, were isolated from several Alpinia species.64,227–229 (S)-2-Pentanol-2-O-β-D-glucopyranoside (538), which showed inhibitory effect on NO production from LPS-activated RAW264.7 macrophage cells, was obtained from fruits of A. oxyphylla.89 Two glycosides known as 3-methyl-but-2-en-1-yl-β-D-glucopyranoside (539) and n-butyl-β-D-fructopyranoside (540) were isolated from A. officinarum Hance.55,230 While 541–544 were found in different Alpinia species (Fig. 9).24,51,108,196,201 Interestingly, 5-hydroxymethylfurfural (544) exerted memory improvement activity against Alzheimer's disease (AD) by mitigating the degree of neuronal damage.23111. Conclusions
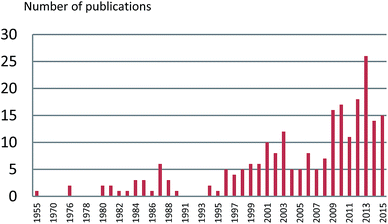
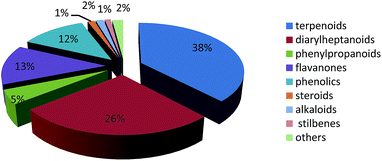
The number of publications on the chemical constituents and their bioactivities for Alpinia species from 1955 to 2015 are shown in Fig. 10. Before 1999, fewer investigations (less than five per year, except six in 1987) were performed on this genus. However, after 2009, there were more than 10 papers published for each year. In 2013, the number of published articles reached 26, indicating a growing interest in the genus of Alpinia.Till 2015, investigations on chemical constitutes of the Alpinia species afforded a total of 544 compounds, including 207 terpenoids, 143 diarylheptanoids, 25 phenylpropanoids, 71 flavanones, 66 phenolics, seven steroids, eight alkaloids, six stilbenes, and 11 others (Fig. 11). Among 207 terpenoids, 17 are monoterpenoids, 132 are sesquiterpenoids, 57 are diterpenoids, and the rest one is a triterpenoid. For sesquiterpenoids, eudesmanes and eremophilanes are undoubtedly predominant with 44 and 21 components, respectively. While for diterpenoids, almost all are labdanes.
Amongst 544 isolated compounds from the genus of Alpinia, 247 are new ones (Table 1), including 96 diarylheptanoids and 106 terpenoids. Obviously, diarylheptanoids, especially diarylheptane–flavonoids conjugates, are characteristic components for the genus of Alpinia.149
| Sources of new compounds | Terpenoids | Diarylheptanoids | Lignans | Flavanoids | Phenolics | Sum |
|---|---|---|---|---|---|---|
| A. blepharocalyx | — | 45 | — | — | — | 45 |
| A. bracteata | — | — | — | — | 1 | 1 |
| A. conchigera | — | — | 11 | — | — | 11 |
| A. calcarata | 6 | — | 1 | — | — | 7 |
| A. chinensis | 10 | — | — | — | — | 10 |
| A. densibracteata | 1 | — | — | — | — | 1 |
| A. densespicata | 6 | — | — | — | — | 6 |
| A. flabellata | 1 | — | 3 | 2 | 1 | 7 |
| A. formosana | 1 | — | — | — | — | 1 |
| A. galanga | 4 | — | — | 5 | 4 | 13 |
| A. gagnepainii | — | — | — | — | 1 | 1 |
| A. intermedia | 8 | — | — | — | — | 8 |
| A. japonica | 14 | — | — | — | — | 14 |
| A. katsumadai | 2 | 29 | 1 | — | 1 | 33 |
| A. nigra | 1 | — | — | — | — | 1 |
| A. oxymitra | 1 | — | — | — | — | 1 |
| A. officinarum | 1 | 18 | 5 | — | 6 | 30 |
| A. oxyphylla | 41 | 2 | — | — | — | 43 |
| A. pahangensis | 5 | — | — | — | — | 5 |
| A. pinnanensis | — | 2 | — | — | — | 2 |
| A. rafflesiana | — | — | — | 1 | — | 1 |
| A. tonkinensis | 2 | — | — | — | — | 2 |
| A. zerumbet (A. speciosa) | 2 | — | — | — | 2 | 4 |
| Sum | 106 | 96 | 21 | 8 | 16 | 247 |
The crude extracts of Alpinia species and their chemical constituents were found to possess various biological activities. Mainly reported were antiemetic,26,27 antibacterial,29–31,37,82,232–236 antioxidant,127,237–239 anticancer,32–34,240–245 anti-inflammatory,189,246,247 insecticidal,36,164 and neuroprotective bioactivities.38,39,231,248–250
In addition, they also showed antiulcer,25 antiplatelet,117,183 hepatoprotective,251 and hypolipidemic effects.252 Meanwhile, evidences showed that ethanol extract of A. galangal can retard lipid oxidation for minced beef, indicating a great potential utility for food storage.8 What should be aroused considerable interest was the promising anticancer and hepatoprotective properties, which could be a great potential to be developed as herbal medicines.
Although there are about 230 species for the Alpinia genus, only 35 were investigated for their chemical constituents and bioactivities (Fig. 12), because A. jianganfeng T. L. Wu includes Alpinia sichuanensis Z. Y. Zhu, and A. zerumbet (Pers.) B. L. Burtt & R. M. Sm. includes A. speciosa K. Schum. according to The Plant List. Among these species, A. galanga, A. oxyphylla, A. officinarum, and A. katsumadai are four most studied plants with referenced papers of 43, 40, 32, and 23, respectively. While for the rest of 31 species, only very fewer articles were published, most of which were less than five. As a matter of fact, there was even only one paper published for 18 species. Although this genus contributed a diverse array of bioactive compounds, the potential of Alpinia species remains virtually untapped. Thus, much attention should be paid to Alpinia species on further phytochemical and pharmacological studies, which would produce structurally interesting and biologically active compounds with potential use in agricultural and medicinal applications. In addition, although most of Alpinia species were also used as edible plants, the nutritious components and their effects were seldom investigated, which could be a hotspot in the near future.
Acknowledgements
The project was supported by National Natural Science Foundation of China (41176148, 21372233, 21202080).Notes and references
- D. Wu and L. Kai, in Flora of China, ed. Z. Y. Wu, Science Press, Beijing, 2000, vol. 24, p. 333 Search PubMed.
- T. Wu, Redai Yaredai Zhiwu Xuebao, 1994, 2, 1 Search PubMed.
- J. Jonczyk, Acta Pol. Pharm., 1970, 27, 155 CAS.
- K. D. Kobayashi, J. McEwen and A. J. Kaufman, Ornamentals and Flowers, 2007, 37 Search PubMed.
- C. Wang, C. Xu, A. Tian, S. Fu and C. Wang, Color. Technol., 2013, 129, 32 CAS.
- X. Yang and R. G. Eilerman, J. Agric. Food Chem., 1999, 47, 1657 CrossRef CAS PubMed.
- H. Morita and H. Itokawa, Chem. Lett., 1986, 15, 1205 CrossRef.
- P. B. Cheah and N. H. Abu Hasim, J. Sci. Food Agric., 2000, 80, 1565 CrossRef CAS.
- P. Pripdeevech, N. Nuntawong and S. Wongpornchai, Chem. Nat. Compd., 2009, 45, 562 CrossRef CAS.
- J. Qiu, CN. Pat., CN101711590A, 2010.
- Z. Wei, CN. Pat., CN1385106A, 2002.
- L. Xu and X. Gao, CN. Pat., CN1481713A, 2004.
- H. Yan, CN. Pat., CN102084999A, 2011.
- H. Yang, CN. Pat., CN103156116A, 2013.
- B. Zhu, CN. Pat., CN101380121A, 2009.
- J. Wang, W. Hou and Z. Zhang, CN. Pat., CN104893910A, 2015.
- Z. Cheng, CN. Pat., CN102429267A, 2012.
- Y. Dou, CN. Pat., CN101088380A, 2007.
- H. Zhao, CN. Pat., CN1138960A, 1997.
- L. X. Du, Z. T. Jiang and R. Li, Zhongguo Tiaoweipin, 2012, 37, 22 CAS.
- H. Itokawa, H. Morita, K. Watanabe, S. Mihashi and Y. Iitaka, Chem. Pharm. Bull., 1985, 33, 1148 CrossRef CAS.
- H. Itokawa, H. Morita, I. Midorikawa, R. Aiyama and M. Morita, Chem. Pharm. Bull., 1985, 33, 4889 CrossRef CAS.
- B. Roy and A. Swargiary, J. Parasit. Dis., 2009, 33, 48 CrossRef PubMed.
- S. Mitsui, S. Kobayashi, H. Nagahori and A. Ogiso, Chem. Pharm. Bull., 1976, 24, 2377 CrossRef CAS PubMed.
- M. A. Al-Yahya, S. Rafatullah, J. S. Mossa, A. M. Ageel, M. S. Al-Said and M. Tariq, Phytother. Res., 1990, 4, 112 CrossRef.
- Y. Yang, K. Kinoshita, K. Koyama, K. Takahashi, T. Tai, Y. Nunoura and K. Watanabe, Nat. Prod. Sci., 1999, 5, 20 CAS.
- D. Shin, K. Kinoshita, K. Koyama and K. Takahashi, J. Nat. Prod., 2002, 65, 1315 CrossRef CAS.
- Y. Yang, K. Kinoshita, K. Koyama, K. Takahashi, S. Kondo and K. Watanabe, Phytomedicine, 2002, 9, 146 CrossRef CAS PubMed.
- K. Rao, B. Ch, L. Narasu and A. Giri, Appl. Biochem. Biotechnol., 2010, 162, 871 CrossRef CAS PubMed.
- P. Niyomkam, S. Kaewbumrung, S. Kaewnpparat and P. Panichayupakaranant, Pharm. Biol., 2010, 48, 375 CrossRef CAS PubMed.
- X. Zhang, G. F. Shi, X. Z. Liu, L. J. An and S. Guan, Cell Biochem. Funct., 2011, 29, 342 CrossRef CAS PubMed.
- K. S. Chun, Y. Sohn, H. S. Kim, O. H. Kim, K. K. Park, J. M. Lee, J. Lee, J. Y. Lee, A. Moon, S. S. Lee and Y. J. Surh, Mutat. Res., 1999, 428, 49 CrossRef CAS PubMed.
- H. Itokawa, H. Morita, T. Sumitomo, N. Totsuka and K. Takeya, Planta Med., 1987, 53, 32 CrossRef CAS PubMed.
- S. Samarghandian, M. A. R. Hadjzadeh, J. Afshari and M. Hosseini, BMC Complementary Altern. Med., 2014, 14, 1 CrossRef PubMed.
- R. Rajasekar, K. Manokaran, N. Rajasekaran, G. Duraisamy and D. Kanakasabapathi, J. Diabetes Metab. Disord., 2014, 13, 1 CrossRef PubMed.
- Y. M. Chang, C. T. Tsai, C. C. R. Wang, Y. S. Chen, Y. M. Lin, C. H. Kuo, B. S. Tzang, R. J. Chen, F. J. Tsai and C. Y. Huang, Biosci., Biotechnol., Biochem., 2013, 77, 229 CrossRef CAS PubMed.
- K. Klahan, N. Nantapong and N. Chudapongse, Planta Med., 2011, 77, PM27 CrossRef PubMed.
- S. H. Shi, X. Zhao, A. J. Liu, B. Liu, H. Li, B. Wu, K. S. Bi and Y. Jia, Physiol. Behav., 2015, 139, 13 CrossRef CAS PubMed.
- X. Z. Li, S. N. Zhang, S. M. Liu and F. Lu, Fitoterapia, 2013, 84, 273 CrossRef CAS PubMed.
- D. P. de Sousa, P. de Almeida Soares Hocayen, L. N. Andrade and R. Andreatini, Molecules, 2015, 20, 18620 CrossRef PubMed.
- H. Lv and G. She, Nat. Prod. Commun., 2010, 5, 1687 CAS.
- D. Kaushik, J. Yadav, P. Kaushik, D. Sacher and R. Rani, Zhongxiyi Jiehe Xuebao, 2011, 9, 1061 CAS.
- J. W. Nam and E. K. Seo, Nat. Prod. Commun., 2012, 7, 795 CAS.
- P. Chen, P. P. Wang, Z. Z. Jiao and L. Xiang, Xiandai Yaowu Yu Linchuang, 2013, 28, 617 CAS.
- M. A. Rahman and M. S. Islam, Pharmacogn. Rev., 2015, 9, 55 CrossRef CAS PubMed.
- E. W. Chan and S. K. Wong, J. Integr. Med., 2015, 13, 368 CrossRef PubMed.
- T. D. Xuan and R. Teschke, Molecules, 2015, 20, 16306 CrossRef CAS PubMed.
- S. Z. Hua, J. G. Luo, X. B. Wang, J. S. Wang and L. Y. Kong, Bioorg. Med. Chem. Lett., 2009, 19, 2728 CrossRef CAS PubMed.
- K. S. Ngo and G. D. Brown, Phytochemistry, 1998, 47, 1117 CrossRef CAS.
- L. K. Sy and G. D. Brown, Phytochemistry, 1997, 45, 537 CrossRef CAS.
- J. Zhang and L. Y. Kong, J. Asian Nat. Prod. Res., 2004, 6, 199 CrossRef CAS PubMed.
- D. Liu, W. Qu and J. Y. Liang, Biochem. Syst. Ecol., 2013, 46, 127 CrossRef CAS.
- J. J. Xu, N. H. Tan, Y. S. Chen, X. L. Pan, G. Z. Zeng, H. J. Han, C. J. Ji and M. J. Zhu, Helv. Chim. Acta, 2009, 92, 1621 CrossRef CAS.
- Y. Someya, A. Kobayashi and K. Kubota, Biosci., Biotechnol., Biochem., 2001, 65, 950 CrossRef CAS PubMed.
- T. N. Ly, R. Yamauchi, M. Shimoyamada and K. Kato, J. Agric. Food Chem., 2002, 50, 4919 CrossRef CAS PubMed.
- U. Grienke, M. Schmidtke, J. Kirchmair, K. Pfarr, P. Wutzler, R. Dürrwald, G. Wolber, K. R. Liedl, H. Stuppner and J. M. Rollinger, J. Nat. Med., 2010, 53, 778 CAS.
- M. Morita, H. Nakanishi, H. Morita, S. Mihashi and H. Itokawa, Chem. Pharm. Bull., 1996, 44, 1603 CrossRef CAS.
- M. Miyazawa, Y. Nakamura and Y. Ishikawa, J. Agric. Food Chem., 2000, 48, 3639 CrossRef CAS PubMed.
- P. Chen, P. P. Wang, Z. Z. Jiao and L. Xiang, Helv. Chim. Acta, 2014, 97, 388 CrossRef CAS.
- P. Chen, L. Qu, L. Tian, P. P. Wang and L. Xiang, Helv. Chim. Acta, 2013, 96, 1163 CrossRef CAS.
- H. Itokawa, H. Morita, T. Kobayashi, K. Watanabe and Y. Iitaka, Chem. Pharm. Bull., 1987, 35, 2860 CrossRef CAS.
- S. Ando, H. Matsuda, T. Morikawa and M. Yoshikawa, Bioorg. Med. Chem., 2005, 13, 3289 CrossRef CAS PubMed.
- X. Luo, J. Yu, L. Xu, K. Li, P. Tan and J. Feng, Yaoxue Xuebao, 2000, 35, 204 CAS.
- L. Hou, X. X. Lu, B. B. Xie, W. H. Huang, J. G. Yu and B. L. Guo, Tianran Chanwu Yanjiu Yu Kaifa, 2013, 25, 878 CAS.
- J. Luo, X. Lv, X. Wang and L. Kong, Phytochem. Lett., 2012, 5, 134 CrossRef CAS.
- J. J. Xu, N. H. Tan, J. Xiong, A. H. Adebayo, H. J. Han, G. Z. Zeng, C. J. Ji, Y. M. Zhang and M. J. Zhu, Chin. Chem. Lett., 2009, 20, 945 CrossRef CAS.
- D. H. Park, J. W. Lee, Q. Jin, W. K. Jeon, M. K. Lee and B. Y. Hwang, Bull. Korean Chem. Soc., 2014, 35, 1565 CrossRef CAS.
- X. Q. Lv, J. G. Luo, X. B. Wang, J. S. Wang, J. Luo and L. Y. Kong, Chem. Pharm. Bull., 2011, 59, 402 CrossRef CAS PubMed.
- H. Itokawa, H. Morita and K. Watanabe, Chem. Pharm. Bull., 1987, 35, 1460 CrossRef CAS.
- H. Itokawa, K. Watanabe, S. Mihashi and Y. Iitaka, Chem. Pharm. Bull., 1980, 28, 681 CrossRef CAS.
- B. Jiang, W. J. Wang, M. P. Li, X. J. Huang, F. Huang, H. Gao, P. H. Sun, M. F. He, Z. J. Jiang, X. Q. Zhang and W. C. Ye, Bioorg. Med. Chem. Lett., 2013, 23, 3879 CrossRef CAS PubMed.
- O. Muraoka, M. Fujimoto, G. Tanabe, M. Kubo, T. Minematsu, H. Matsuda, T. Morikawa, I. Toguchida and M. Yoshikawa, Bioorg. Med. Chem. Lett., 2001, 11, 2217 CrossRef CAS PubMed.
- J. Xu, C. Ji, Y. Zhang, J. Su, Y. Li and N. Tan, Bioorg. Med. Chem. Lett., 2012, 22, 1660 CrossRef CAS PubMed.
- J. J. Xu, N. H. Tan, G. Z. Zeng, H. J. Han and Y. F. Peng, Chin. J. Nat. Med., 2010, 8, 6 CrossRef CAS.
- K. Jitsaeng, W. De-Eknamkul and B. Schneider, Rec. Nat. Prod., 2009, 3, 110 CAS.
- S. M. Xu, X. J. Huang, Y. Wang and W. C. Ye, Zhongguo Tianran Yaowu, 2012, 10, 374 CAS.
- L. Hou, G. Ding, B. L. Guo, W. H. Huang, X. J. Zhang, Z. Y. Sun and X. F. Shi, Molecules, 2015, 20, 1551 CrossRef PubMed.
- H. Itokawa, H. Morita, K. Watanabe, A. Takase and Y. Iitaka, Chem. Lett., 1984, 13, 1687 CrossRef.
- H. Itokawa, H. Morita, K. Osawa, K. Watanabe and Y. Iitaka, Chem. Pharm. Bull., 1987, 35, 2849 CrossRef CAS.
- H. Itokawa, K. Watanabe, H. Morita, S. Mihashi and Y. Iitaka, Chem. Pharm. Bull., 1985, 33, 2023 CrossRef CAS.
- H. Itokawa, S. Yoshimoto and H. Morita, Phytochemistry, 1988, 27, 435 CrossRef CAS.
- A. N. Aziz, H. Ibrahim, D. Rosmy Syamsir, M. Mohtar, J. Vejayan and K. Awang, J. Ethnopharmacol., 2013, 145, 798 CrossRef CAS PubMed.
- Q. M. Li, J. G. Luo, X. B. Wang, M. H. Yang and L. Y. Kong, Fitoterapia, 2013, 86, 29 CrossRef CAS PubMed.
- J. Xu, N. Tan, G. Zeng, H. Han, H. Huang, C. Ji, M. Zhu and Y. Zhang, Zhongguo Zhongyao Zazhi, 2009, 34, 990 CAS.
- L. Y. Kong, M. J. Qin and M. Niwa, J. Nat. Prod., 2000, 63, 939 CrossRef CAS.
- Q. H. Zeng, C. L. Lu, X. W. Zhang and J. G. Jiang, Food Funct., 2015, 6, 431 CAS.
- H. Itokawa, H. Morita, K. Watanabe and Y. Iitaka, Chem. Lett., 1984, 13, 451 CrossRef.
- T. Morikawa, H. Matsuda, I. Toguchida, K. Ueda and M. Yoshikawa, J. Nat. Prod., 2002, 65, 1468 CrossRef CAS.
- Z. J. Qing, Y. Wang, L. Y. Hui, L. W. Yong, L. H. Long, D. J. Ao and P. L. Xia, Arch. Pharmacal Res., 2012, 35, 2143 CrossRef CAS PubMed.
- J. Xu, J. Su, Y. Li and N. Tan, Chem. Nat. Compd., 2013, 49, 457 CrossRef CAS.
- S. Ghosh, K. Indukuri, S. Bondalapati, A. K. Saikia and L. Rangan, Eur. J. Med. Chem., 2013, 66, 101 CrossRef CAS PubMed.
- S. Ghosh and L. Rangan, Appl. Biochem. Biotechnol., 2015, 175, 1477 CrossRef CAS PubMed.
- H. Morita and H. Itokawa, Planta Med., 1988, 54, 117 CrossRef CAS PubMed.
- J. Chompoo, A. Upadhyay, W. Kishimoto, T. Makise and S. Tawata, Food Chem., 2011, 129, 709 CrossRef CAS PubMed.
- A. Upadhyay, J. Chompoo, W. Kishimoto, T. Makise and S. Tawata, J. Agric. Food Chem., 2011, 59, 2857 CrossRef CAS PubMed.
- Y. Sivasothy, H. Ibrahim, A. S. Paliany, S. A. Alias, N. R. Md Nor and K. Awang, Planta Med., 2013, 79, 1775 CrossRef CAS PubMed.
- L. K. Sy and G. D. Brown, J. Nat. Prod., 1997, 60, 904 CrossRef CAS.
- H. Itokawa, M. Morita and S. Mihashi, Chem. Pharm. Bull., 1980, 28, 3452 CrossRef CAS.
- H. X. Xu, H. Dong and K. Y. Sim, Phytochemistry, 1996, 42, 149 CrossRef CAS.
- N. Nuntawong and A. Suksamrarn, Biochem. Syst. Ecol., 2008, 36, 661 CrossRef CAS.
- Q. M. Li, J. G. Luo, M. H. Yang and L. Y. Kong, Chem. Biodiversity, 2015, 12, 388 CAS.
- V. S. Chauhan, M. Swapna and A. Singh, Int. J. Appl. Biol. Pharm. Technol., 2014, 5, 186 Search PubMed.
- Y. J. Kuo, P. C. Hsiao, L. J. Zhang, M. D. Wu, Y. H. Liang, H. O. Ho and Y. H. Kuo, J. Nat. Prod., 2009, 72, 1097 CrossRef CAS PubMed.
- S. Tesaki, H. Kikuzaki, S. Yonemori and N. Nakatani, J. Nat. Prod., 2001, 64, 515 CrossRef CAS.
- H. Itokawa, H. Morita, I. Katou, K. Takeya, A. J. Cavalheiro, R. C. B. de Oliveira, M. Ishige and M. Motidome, Planta Med., 1988, 54, 311 CrossRef CAS PubMed.
- Y. Sivasothy, H. Ibrahim, A. S. Paliany, S. A. Alias and K. Awang, Bioorg. Med. Chem. Lett., 2013, 23, 6280 CrossRef CAS PubMed.
- L. Y. Kong, M. J. Qin and M. Niwa, Planta Med., 2002, 68, 813 CrossRef CAS PubMed.
- X. B. Wang, C. S. Yang, S. Z. Hua and L. Y. Kong, Zhongguo Tianran Yaowu, 2010, 8, 419 CAS.
- S. Y. Choi, M. H. Lee, J. H. Choi and Y. K. Kim, Biol. Pharm. Bull., 2012, 35, 2092 CAS.
- H. J. Lee, J. S. Kim and J. H. Ryu, Planta Med., 2006, 72, 68 CrossRef CAS PubMed.
- R. J. Lin, C. M. Yen, T. H. Chou, F. Y. Chiang, G. H. Wang, Y. P. Tseng, L. Wang, T. W. Huang, H. C. Wang, L. P. Chan, H. Y. Ding and C. H. Liang, BMC Complementary Altern. Med., 2013, 13, 1 CrossRef PubMed.
- N. Shoji, A. Umeyama, T. Takemoto and Y. Ohizumi, Planta Med., 1984, 50, 186 CrossRef CAS PubMed.
- Q. Y. Bian, S. Y. Wang, L. J. Xu, C. O. Chan, D. K. W. Mok and S. B. Chen, J. Asian Nat. Prod. Res., 2013, 15, 1094 CrossRef CAS PubMed.
- Q. Zhang, S. Luo, H. Wang and D. Fan, Zhongcaoyao, 1997, 28, 131 CAS.
- S. Kadota, Y. Tezuka, J. K. Prasain, M. S. Ali and A. H. Banskota, Curr. Top. Med. Chem., 2003, 3, 203 CrossRef CAS PubMed.
- M. S. Ali, Y. Tezuka, S. Awale, A. H. Banskota and S. Kadota, J. Nat. Prod., 2001, 64, 289 CrossRef CAS.
- H. Dong, S. X. Chen, H. X. Xu, S. Kadota and T. Namba, J. Nat. Prod., 1998, 61, 142 CrossRef CAS PubMed.
- P. M. Giang, P. T. Son, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2005, 53, 1335 CrossRef CAS PubMed.
- M. Kuroyanagi, T. Noro, S. Fukushima, R. Aiyama, A. Ikuta, H. Itokawa and M. Morita, Chem. Pharm. Bull., 1983, 31, 1544 CrossRef CAS.
- B. Groeblacher, O. Kunert and F. Bucar, Bioorg. Med. Chem., 2012, 20, 2701 CrossRef CAS PubMed.
- W. Z. Huang, C. F. Zhang, M. Zhang and Z. T. Wang, J. Chin. Chem. Soc., 2007, 54, 1553 CrossRef CAS.
- Y. Li, L. Yang, C. Wang, G. Chou and Z. Wang, Shanghai Zhongyiyao Daxue Xuebao, 2010, 24, 72 CAS.
- Y. Y. Li, G. X. Chou and Z. T. Wang, Helv. Chim. Acta, 2010, 93, 382 CrossRef CAS.
- J. W. Nam, G. Y. Kang, A. R. Han, D. Lee, Y. S. Lee and E. K. Seo, J. Nat. Prod., 2011, 74, 2109 CrossRef CAS PubMed.
- J. W. Nam and E. K. Seo, Helv. Chim. Acta, 2013, 96, 1670 CrossRef CAS.
- C. Y. Lo, P. L. Liu, L. C. Lin, Y. T. Chen, Y. C. Hseu, Z. H. Wen and H. M. Wang, Sci. World J., 2013, 186505, DOI:10.1155/2013/186505.
- M. Habsah, N. H. Lajis, A. M. Ali, M. A. Sukari, Y. Y. Hin, H. Kikuzaki and N. Nakatani, Pharm. Biol., 2003, 41, 7 CrossRef CAS.
- H. Mohamad, F. Abas, D. Permana, N. H. Lajis, A. M. Ali, M. A. Sukari, T. Y. Y. Hin, H. Kikuzaki and N. Nakatani, Z. Naturforsch., C: J. Biosci., 2004, 59, 811 CAS.
- B. B. Zhang, Y. Dai, Z. X. Liao and L. S. Ding, Fitoterapia, 2010, 81, 948 CrossRef CAS PubMed.
- H. M. Sirat, A. A. Rahman, H. Itokawa and H. Morita, Planta Med., 1996, 62, 188 CrossRef CAS PubMed.
- N. An, H. W. Zhang, L. Z. Xu, S. L. Yang and Z. M. Zou, Food Chem., 2010, 119, 513 CrossRef CAS.
- L. Zhao, W. Qu, J. Q. Fu and J. Y. Liang, Chin. J. Nat. Med., 2010, 8, 241 CrossRef CAS.
- N. An, Z. M. Zou, Z. Tian, X. Z. Luo, S. L. Yang and L. Z. Xu, Fitoterapia, 2007, 79, 27 CrossRef PubMed.
- Y. Sun, K. Tabata, H. Matsubara, S. Kitanaka, T. Suzuki and K. Yasukawa, Planta Med., 2008, 74, 427 CrossRef CAS PubMed.
- D. Liu, Y. W. Liu, F. Q. Guan and J. Y. Liang, Fitoterapia, 2014, 96, 76 CrossRef CAS PubMed.
- Y. Sun, H. Matsubara, S. Kitanaka and K. Yasukawa, Helv. Chim. Acta, 2008, 91, 118 CrossRef CAS.
- G. J. Fan, Y. H. Kang, Y. N. Han and B. H. Han, Bioorg. Med. Chem. Lett., 2007, 17, 6720 CrossRef CAS PubMed.
- D. Liu, W. Qu, L. Zhao, F. Q. Guan and J. Y. Liang, Chin. J. Nat. Med., 2014, 12, 139 CAS.
- Y. U. Kim, H. K. Son, H. K. Song, M. J. Ahn, S. S. Lee and S. K. Lee, Planta Med., 2003, 69, 72 CrossRef CAS PubMed.
- H. B. Lee, H. K. Lee, J. R. Kim and Y. J. Ahn, J. Korean Soc. Appl. Biol. Chem., 2009, 52, 367 CrossRef CAS.
- J. E. Shin, M. J. Han, M. C. Song, N. I. Baek and D. H. Kim, Biol. Pharm. Bull., 2004, 27, 138 CAS.
- Z. Liu, M. M. Rafi, N. Zhu, K. Ryu, S. Sang, C. T. Ho and R. T. Rosen, ACS Symp. Ser., 2003, 851, 369 CrossRef CAS.
- F. Kiuchi, M. Shibuya and U. Sankawa, Chem. Pharm. Bull., 1982, 30, 2279 CrossRef CAS.
- K. Yasukawa, Y. Sun, S. Kitanaka, N. Tomizawa, M. Miura and S. Motohashi, J. Nat. Med., 2008, 62, 374 CrossRef CAS PubMed.
- S. I. Uehara, I. Yasuda, K. Akiyama, H. Morita, K. Takeya and H. Itokawa, Chem. Pharm. Bull., 1987, 35, 3298 CrossRef CAS.
- M. S. Ali, Y. Tezuka, A. H. Banskota and S. Kadota, J. Nat. Prod., 2001, 64, 491 CrossRef CAS.
- J. K. Prasain, Y. Tezuka, J. X. Li, K. Tanaka, P. Basnet, H. Dong, T. Namba and S. Kadota, Planta Med., 1999, 65, 196 CrossRef CAS PubMed.
- M. S. Ali, A. H. Banskota, Y. Tezuka, I. Saiki and S. Kadota, Biol. Pharm. Bull., 2001, 24, 525 CAS.
- X. B. Wang, C. S. Yang, C. Zhang, J. Luo, M. H. Yang, J. G. Luo, W. Y. Yu and L. Y. Kong, Tetrahedron, 2014, 70, 8714 CrossRef CAS.
- J. Kumar Prasain, Y. Tezuka, J.-X. Li, K. Tanaka, P. Basnet, H. Dong, T. Namba and S. Kadota, J. Chem. Res., Synop., 1998, 22, 10.1039/A706250H.
- J. Kumar Prasain, Y. Tezuka, J. Xin Li, K. Tanaka, P. Basnet, H. Dong, T. Namba and S. Kadota, Tetrahedron, 1997, 53, 7833 CrossRef.
- Y. Tezuka, M. B. Gewali, M. S. Ali, A. H. Banskota and S. Kadota, J. Nat. Prod., 2001, 64, 208 CrossRef CAS.
- J. K. Prasain, J. X. Li, Y. Tezuka, K. Tanaka, P. Basnet, H. Dong, T. Namba and S. Kadota, J. Nat. Prod., 1998, 61, 212 CrossRef CAS PubMed.
- M. B. Gewali, Y. Tezuka, A. H. Banskota, M. S. Ali, I. Saiki, H. Dong and S. Kadota, Org. Lett., 1999, 1, 1733 CrossRef CAS PubMed.
- L. Zhao, J. Y. Liang, J. Y. Zhang and Y. Chen, Chin. Chem. Lett., 2010, 21, 194 CrossRef CAS.
- D. Liu, W. Qu, L. Zhao and J. Y. Liang, Chin. Chem. Lett., 2012, 23, 189 CrossRef CAS.
- L. Zhao, J. Y. Liang and W. Qu, Chem. Nat. Compd., 2012, 48, 836 CrossRef CAS.
- S. Kadota, J. K. Prasain, J. X. Li, P. Basnet, H. Dong, T. Tani and T. Namba, Tetrahedron Lett., 1996, 37, 7283 CrossRef CAS.
- Y. Tezuka, M. S. Ali, A. H. Banskota and S. Kadota, Tetrahedron Lett., 2000, 41, 5903 CrossRef CAS.
- Y. Yang, K. Kinoshita, K. Koyama, K. Takahashi, T. Tai, Y. Nunoura and K. Watanabe, J. Nat. Prod., 1999, 62, 1672 CrossRef CAS.
- S. Tesaki, H. Kikuzaki, S. Tanabe, M. Watanabe and N. Nakatani, ITE Lett. Batteries, New Technol. Med., 2001, 2, 106 CAS.
- H. Kikuzaki, S. Tesaki, S. Yonemori and N. Nakatani, Phytochemistry, 2001, 56, 109 CrossRef CAS PubMed.
- T. N. Ly, M. Shimoyamada, K. Kato and R. Yamauchi, J. Agric. Food Chem., 2003, 51, 4924 CrossRef CAS PubMed.
- A. Kaur, R. Singh, C. S. Dey, S. S. Sharma, K. K. Bhutani and I. P. Singh, Indian J. Exp. Biol., 2010, 48, 314 CAS.
- J. J. Xu, G. Z. Zeng, S. C. Yang, Y. Shen and N. H. Tan, Fitoterapia, 2013, 91, 82 CrossRef CAS PubMed.
- T. Morikawa, S. Ando, H. Matsuda, S. Kataoka, O. Muraoka and M. Yoshikawa, Chem. Pharm. Bull., 2005, 53, 625 CrossRef CAS PubMed.
- J. J. Xu, H. M. Zhao, Y. Shen, J. H. Chen, Y. Li, N. H. Tan and S. C. Yang, J. Asian Nat. Prod. Res., 2013, 15, 833 CrossRef CAS PubMed.
- T. Obata, A. Sawabe, M. Morita, N. Yamashita and Y. Matsubara, J. Jpn. Oil Chem. Soc., 1995, 44, 1012 CrossRef CAS.
- N. Wei, Y. Wang, H. F. Li, J. Q. Zhang and Y. H. Li, Chem. Nat. Compd., 2013, 49, 934 CrossRef CAS.
- L. Liu, J. G. Luo and L. Y. Kong, Chem. Nat. Compd., 2012, 48, 785 CrossRef CAS.
- J. Zhang, Q. H. Guo and L. Y. Kong, Zhongguo Zhongyao Zazhi, 2003, 28, 41 CAS.
- G. Eumkeb, S. Siriwong, S. Phitaktim, N. Rojtinnakorn and S. Sakdarat, J. Appl. Microbiol., 2012, 112, 55 CrossRef CAS PubMed.
- S. T. Chien, M. D. Shi, Y. C. Lee, C. C. Te and Y. W. Shih, Cancer Cell Int., 2015, 15, 1 CrossRef PubMed.
- M. Y. Heo, S. J. Sohn and W. W. Au, Mutat. Res., 2001, 488, 135 CAS.
- T. Ohtsuki, H. Kikuchi, T. Koyano, T. Kowithayakorn, T. Sakai and M. Ishibashi, Bioorg. Med. Chem., 2009, 17, 6748 CrossRef CAS PubMed.
- M. Q. Bian, H. Q. Wang, J. Kang, R. Y. Chen, Y. F. Yang and H. Z. Wu, Yaoxue Xuebao, 2014, 49, 359 CAS.
- B. R. Xin, S. J. Ren and J. Li, Zhongguo Zhongyao Zazhi, 2014, 39, 2674 CAS.
- J. Zhang, X. He, J. Gao and L. Kong, Zhongguo Yaoxue Zazhi, 2003, 38, 502 CAS.
- J. E. Shin, M. J. Han and D. H. Kim, Biol. Pharm. Bull., 2003, 26, 854 CAS.
- H. Kikuzaki and S. Tesaki, J. Nat. Prod., 2002, 65, 389 CrossRef CAS.
- X. Xiao, X. Si, X. Tong and G. Li, Sep. Purif. Technol., 2011, 81, 265 CrossRef CAS.
- N. A. Mustahil, M. A. Sukari, A. B. Abdul, N. A. Ali and G. E. C. Lian, Pak. J. Pharm. Sci., 2013, 26, 391 CAS.
- I. Jantan, S. M. Raweh, H. M. Sirat, S. Jamil, Y. H. Mohd Yasin, J. Jalil and J. A. Jamal, Phytomedicine, 2008, 15, 306 CrossRef CAS PubMed.
- I. Jantan, M. Pisar, H. M. Sirat, N. Basar, S. Jamil, R. M. Ali and J. Jalil, Phytother. Res., 2004, 18, 1005 CrossRef CAS PubMed.
- H. Zhang, L. X. Xu, P. Wu and X. Y. Wei, Redai Yaredai Zhiwu Xuebao, 2014, 22, 89 CAS.
- B. R. Xin, J. F. Liu, J. Kang and W. P. Chan, Mol. Cell. Toxicol., 2014, 10, 165 CrossRef CAS.
- J. Gripenberg, E. Honkanen and K. Silander, Acta Chem. Scand., 1956, 10, 393 CrossRef CAS.
- H. Itokawa, M. Morita and S. Mihashi, Phytochemistry, 1981, 20, 2503 CrossRef CAS.
- C. T. Lin, K. J. Senthil Kumar, Y. H. Tseng, Z. J. Wang, M. Y. Pan, J. H. Xiao, S. C. Chien and S. Y. Wang, J. Agric. Food Chem., 2009, 57, 6060 CrossRef CAS PubMed.
- S. Ahmad, D. A. Israf, N. H. Lajis, K. Shaari, H. Mohamed, A. A. Wahab, K. T. Ariffin, W. Y. Hoo, N. A. Aziz, A. A. Kadir, M. R. Sulaiman and M. N. Somchit, Eur. J. Pharmacol., 2006, 538, 188 CrossRef CAS PubMed.
- H. T. Le, M. G. Phan and T. S. Phan, Tap Chi Hoa Hoc, 2007, 45, 126 CAS.
- Z. Wei, J. Yang, Y. F. Xia, W. Z. Huang, Z. T. Wang and Y. Dai, J. Biochem. Mol. Toxicol., 2012, 26, 282 CrossRef CAS PubMed.
- C. P. Shen, J. G. Luo, M. H. Yang and L. Y. Kong, Fitoterapia, 2015, 106, 153 CrossRef CAS PubMed.
- W. Q. Yang, Y. Gao, M. Li, D. R. Miao and F. Wang, J. Asian Nat. Prod. Res., 2015, 17, 783 CrossRef CAS PubMed.
- M. A. Mpalantinos, R. S. De Moura, J. P. Parente and R. M. Kuster, Phytother. Res., 1998, 12, 442 CrossRef CAS.
- Y. Y. Li, G. X. Chou and Z. T. Wang, Zhongguo Tianran Yaowu, 2009, 7, 417 CAS.
- T. Masuda, S. Mizuguchi, T. Tanaka, K. Iritani, Y. Takeda and S. Yonemori, J. Agric. Food Chem., 2000, 48, 1479 CrossRef CAS PubMed.
- N. Nakatani, Abstracts of Papers, 223rd ACS National Meeting, AGFD, Orlando, FL, United States, April 7-11, 2002, p. 2002 Search PubMed.
- S. B. Jaju, N. H. Indurwade, D. M. Sakarkar, N. K. Fuloria, M. D. Ali, S. Das and S. P. Basu, Trop. J. Pharm. Res., 2009, 8, 545 CAS.
- B. R. Barik, A. B. Kundu and A. K. Dey, Phytochemistry, 1987, 26, 2126 CrossRef CAS.
- S. H. Shi, C. N. Zhang, A. J. Liu, H. Li, K. S. Bi and Y. Jia, Zhongguo Shiyan Fangjixue Zazhi, 2013, 19, 97 CAS.
- J. W. Nam, S. J. Kim, A. R. Han, S. K. Lee and E. K. Seo, J. Appl. Pharmacol., 2005, 13, 263 CAS.
- L. Zhao, L. Y. Chen and J. Y. Liang, Chin. J. Nat. Med., 2012, 10, 370 CAS.
- H. Matsuda, T. Morikawa, H. Managi and M. Yoshikawa, Bioorg. Med. Chem. Lett., 2003, 13, 3197 CrossRef CAS PubMed.
- S. K. Roy, S. Pahwa, H. Nandanwar and S. M. Jachak, Fitoterapia, 2012, 83, 1248 CrossRef CAS PubMed.
- T. Noro, T. Sekiya, M. Katoh, Y. Oda, T. Miyase, M. Kuroyanagi, A. Ueno and S. Fukushima, Chem. Pharm. Bull., 1988, 36, 244 CrossRef CAS.
- N. Sukhirun, W. Pluempanupat, V. Bullangpoti and O. Koul, J. Econ. Entomol., 2011, 104, 1534 CrossRef CAS PubMed.
- N. Hasima, L. I. L. Aun, M. N. Azmi, A. N. Aziz, E. Thirthagiri, H. Ibrahim and K. Awang, Phytomedicine, 2010, 17, 935 CrossRef CAS PubMed.
- H. Matsuda, Y. Pongpiriyadacha, T. Morikawa, M. Ochi and M. Yoshikawa, Eur. J. Pharmacol., 2003, 471, 59 CrossRef CAS PubMed.
- Y. Ye and B. Li, J. Gen. Virol., 2006, 87, 2047 CrossRef CAS PubMed.
- H. J. Min, J. W. Nam, E. S. Yu, J. H. Hong, E. K. Seo and E. S. Hwang, Int. Immunopharmacol., 2009, 9, 448 CrossRef CAS PubMed.
- R. Mongkol, W. Chavasiri, M. Ishida, K. Matsuda and M. Morimoto, Weed Biol. Manage., 2015, 15, 87 CrossRef CAS.
- H. C. Wang, J. X. Li, H. Li, L. W. Yang, M. H. Gao and H. A. Chen, Yaoxue Yanjiu, 2013, 32, 559 CAS.
- W. Wang, S. Qi, H. Zhong and Q. Yao, Shipin Yu Yaopin, 2012, 14, 88 CAS.
- S. Guan, Y.-M. Bao, B. Jiang and L.-J. An, Eur. J. Pharmacol., 2006, 538, 73 CrossRef CAS PubMed.
- S. Guan, B. Jiang, Y. M. Bao and L. J. An, Food Chem. Toxicol., 2006, 44, 1659 CrossRef CAS PubMed.
- L. J. An, S. Guan, G. F. Shi, Y. M. Bao, Y. L. Duan and B. Jiang, Food Chem. Toxicol., 2006, 44, 436 CrossRef CAS PubMed.
- G. F. Shi, L. J. An, B. Jiang, S. Guan and Y. M. Bao, Neurosci. Lett., 2006, 403, 206 CrossRef CAS PubMed.
- Y. M. Liu, B. Jiang, Y. M. Bao and L. J. An, Toxicol. in Vitro, 2008, 22, 430 CrossRef PubMed.
- H. Zhao, J. Xu and S. Yang, Yunnan Nongye Daxue Xuebao, 2014, 29, 468 CAS.
- M. G. Phan and T. S. Phan, Tap Chi Hoa Hoc, 2004, 42, 376 CAS.
- P. T. B. Tu and S. Tawata, Molecules, 2014, 19, 16656 CrossRef PubMed.
- T. Fujita, H. Nishimura, K. Kaburagi and J. Mizutani, Phytochemistry, 1994, 36, 23 CrossRef CAS.
- J. Chompoo, A. Upadhyay, M. Fukuta and S. Tawata, BMC Complementary Altern. Med., 2012, 12, 106 CrossRef.
- S. B. Jaju, N. H. Indurwade, D. M. Sakarkar, N. K. Fuloria, M. D. Ali and S. P. Basu, Pharmacogn. Res., 2010, 2, 264 CrossRef CAS PubMed.
- X. Wang, X. Yang and J. Li, Zhongyaocai, 2008, 31, 853 CAS.
- C. Qiao, X. Hao, Z. Wang and L. Xu, Zhongguo Zhongyao Zazhi, 2002, 27, 130 CAS.
- L. Di, Z. Wang, Z. Wang, N. Li and K. Wang, Zhiwu Ziyuan Yu Huanjing Xuebao, 2011, 20, 94 CAS.
- S. Qi, F. Ji and Q. Yao, Shipin Yu Yaopin, 2010, 12, 39 CAS.
- N. An, J. Lin, S. Yang, Z. Zou and L. Xu, Yaoxue Xuebao, 2006, 41, 233 CAS.
- A. Liu, X. Zhao, H. Li, Z. Liu, B. Liu, X. Mao, L. Guo, K. Bi and Y. Jia, Int. Immunopharmacol., 2014, 23, 719 CrossRef CAS PubMed.
- P. G. Ray and S. K. Majumdar, Indian J. Exp. Biol., 1976, 14, 712 CAS.
- J. J. C. Scheffer, A. Gani and A. Baerheim Svendsen, Planta Med., 1981, 42, 140 CrossRef CAS PubMed.
- H. Haraguchi, Y. Kuwata, K. Inada, K. Shingu, K. Miyahara, M. Nagao and A. Yagi, Planta Med., 1996, 62, 308 CrossRef CAS PubMed.
- K. Chukanhom, P. Borisuthpeth and K. Hatai, Biocontrol Sci., 2005, 10, 105 CrossRef CAS.
- M. M. Yusoff, H. Ibrahim and N. A. Hamid, Chem. Biodiversity, 2011, 8, 916 CAS.
- S. E. Lee, H. T. Shin, H. J. Hwang and J. H. Kim, Phytother. Res., 2003, 17, 1041 CrossRef PubMed.
- W. Y. Hsu, A. Simonne, A. Weissman and J. M. Kim, Food Sci. Biotechnol., 2010, 19, 873 CrossRef.
- C. A. Raj, P. Ragavendran, D. Sophia, T. Starlin, M. A. Rathi and V. K. Gopalakrishnan, Chin. J. Integr. Med., 2014, 1, DOI:10.1007/s11655-014-1762-1.
- P. Muangnoi, M. Lu, J. Lee, A. Thepouyporn, R. Mirzayans, X. C. Le, M. Weinfeld and S. Changbumrung, Planta Med., 2007, 73, 748 CrossRef CAS PubMed.
- C. W. Phang, S. Malek and H. Ibrahim, BMC Complementary Altern. Med., 2013, 13, 1 CrossRef PubMed.
- C. L. Hsu, Y. S. Yu and G. C. Yen, J. Agric. Food Chem., 2009, 58, 2201 CrossRef PubMed.
- A. Reddy, S. Malek, H. Ibrahim and K. Sim, BMC Complementary Altern. Med., 2013, 13, 1 CrossRef PubMed.
- T. P. T. Be, J. Chompoo and S. Tawata, Drug Discoveries Ther., 2015, 9, 197 CrossRef PubMed.
- W. Fan, C. Z. Wang, X. L. Bao, H. H. Yuan and M. B. Lan, Asian J. Chem., 2015, 27, 532 CAS.
- M. Y. Lee, C. S. Seo, J. A. Lee, I. S. Shin, S. J. Kim, H. K. Ha and H. K. Shin, Inflammation, 2012, 35, 746 CrossRef CAS PubMed.
- Y. S. Yu, C. L. Hsu and G. C. Yen, J. Agric. Food Chem., 2009, 57, 7673 CrossRef CAS PubMed.
- X. Yu, Y. W. Ceballos, H. Zhao and Z. Xu, Neurosci. Res. Commun., 2003, 33, 105 CrossRef.
- J. C. Hanish Singh, V. Alagarsamy, P. V. Diwan, S. Sathesh Kumar, J. C. Nisha and Y. Narsimha Reddy, J. Ethnopharmacol., 2011, 138, 85 CrossRef CAS PubMed.
- Z. J. Zhang, L. C. V. Cheang, M. W. Wang, G. H. Li, I. Chu, Z. X. Lin and S. M. Y. Lee, Cell. Mol. Neurobiol., 2012, 32, 27 CrossRef PubMed.
- F. M. Hammouda, S. S. El-Hawary, H. A. Kassem, W. A. Tawfik, A. A. Abdel Motaal, N. M. Nazif and S. S. El-Shamy, Res. J. Pharm., Biol. Chem. Sci., 2015, 6, 448 CAS.
- C. R. Achuthan and J. Padikkala, Indian J. Clin. Biochem., 1997, 12, 55 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The name, source, plant part, and reference for each compound. A comparison of Alpinia species names from the references and the accepted name in The Plant List. See DOI: 10.1039/c6ra27830b |
| This journal is © The Royal Society of Chemistry 2017 |