Open Access Article
Open Access ArticleTemperature-/CO2-dual-responsiveness of a zwitterionic “schizophrenic” copolymer†
Xinde Tang*a,
Qun Zhangb and
Meishan Pei*b
aSchool of Material Science and Engineering, Shandong Jiaotong University, Jinan 250023, China. E-mail: xdtang8033@163.com
bSchool of Chemistry and Chemical Engineering, University of Jinan, Jinan 250022, China. E-mail: chm_peims@ujn.edu.cn
First published on 5th January 2017
Abstract
A zwitterionic “schizophrenic” copolymer with dual-responsiveness to temperature and carbon dioxide self-assembles to undergo a reversible phase transition in a weakly alkaline borate buffer solution. It can be switched “on” and “off” when sequentially treated with carbon dioxide/nitrogen (CO2/N2) due to the protonation–deprotonation of the tertiary amine groups along the polymer skeleton.
Due to their intriguing smart behavior, “schizophrenic” block copolymers have received considerable attention and held great promise for controlled drug release, gene delivery, and nanocatalysis, ever since the first example was reported in 1998.1–3 A schizophrenic block copolymer can self-assemble to form micelles and inverted micelles in dilute solution by simply changing external conditions such as temperature, pH, redox, photo, ionic strength,4,5 or subtle solvent changes.6 However, the previous schizophrenic block copolymers mainly combined two different response parameters such as pH and ionic strength,1 or pH and temperature.7 The schizophrenic block copolymers that can be switched to form micelles to inversed micelles only by changing temperature or pH were reported in 2002.8,9 Generally, the fully temperature-switchable schizophrenic block copolymer requires combination of lower critical solution temperature (LCST) behavior and upper critical solution temperature (UCST) behavior,10–16 and realizes switching by simply changing temperature without addition of salts, acids, or bases to the solution.14 In comparison, the pH-switchable schizophrenic block copolymer can be achieved switching through varying pH by adding acids or bases. It is possible to obtain byproducts and cause contamination of the existing system, imposing potential side effects on biological cells involving incompatibility, membrane decomposition, cytotoxicity, and gene damage.16 Therefore, the exploration of novel, mild, and environmentally friendly stimuli is emerging and promising.
Carbon oxide (CO2) is attractive as a trigger for stimuli-responsive materials due to its availability, cost-efficiency, and benign nature.17 As an endogenous metabolite, CO2 can freely diffuse through the cytomembrane without any cytotoxic effect and play a crucial role in regulating bio-aggregated constitution. The “green” stimulus only involves introduction and release of inactive gases such as CO2 and N2.17–20 Recently, Yuan's and Zhao's groups mimicked deformable behavior of vesicles in response to CO2 trigger.20–23 In addition to green chemistry, the use of CO2 as a trigger for switching property or function of materials may also hold promise for application.23 Therefore, to explore CO2 as a new trigger to drive block copolymer phase transition and to realize the “schizophrenic” behavior remains a great challenge although some nascent effort has been devoted.
In the current work, we report, for the first time, a temperature-/CO2-dual-responsive zwitterionic “schizophrenic” triblock copolymer composed of poly(methoxyethylene glycol) (MPEG), poly{[2-(methacryloyloxy)ethyl]dimethyl(3-sulfopropyl)-ammonium hydroxide} (PSBMA), and poly[2-(N,N-dimethylamino)ethyl methacrylate] (PDMAEMA). This schizophrenic block copolymer combines PSBMA with upper critical solution temperature (UCST) and PDMAEMA with lower critical solution temperature (LCST) in weakly alkaline buffer solution, which is different from most fully temperature-responsive schizophrenic block copolymers based on non-ionic/zwitterionic block self-assembly with poly(N-isopropylacylamide) (PNIPAM) as LCST polymer while polysulfobetaine as UCST polymer so far. For the triblock copolymer, the water-soluble MPEG blocks with biocompatible and anti-fouling properties endow stability to the polymer chains or the formed micelles in aqueous solution by creating a hydrophilic corona.24 PSBMA as a zwitterionic polymer is considered to be in collapsed coil in aqueous solution below UCST due to intra- and/or interchain associations, while in the form of non-associations above UCST.25 Moreover, PSBMA also owns excellent biocompatibility and biofouling-resistance. PDMAEMA is a weak base with pKa about 7.0 and is water-soluble within wide pH range,26 exhibiting a phase transfer between hydrophilic and hydrophobic characteristics below and above pKa.27–29 The chains of PDMAEMA are expanded in aqueous solution with tertiary amine groups of PDMAEMA are protonated and hydrated below the pKa. In contrast, the polymeric chains are collapsed by deprotonation and dehydration of the amine group above pKa. There are also conformational changes in PDMAEMA below and above its LCST.30–32 Beside this, tertiary-containing moiety in PDMAEMA can react with CO2 in solution to form a charged ammonium bicarbonate, which can be recovered upon CO2 removal (Scheme 1).32 Most importantly, the combination of non-ionic MPEG, cationic PDMAEMA, and zwitterionic PSBMA results in a unique molecular structure, which can be expected to create an advanced biocompatible and biofouling resistant system and to act as nanocarriers for drug uptake, delivery, and release in living organisms.
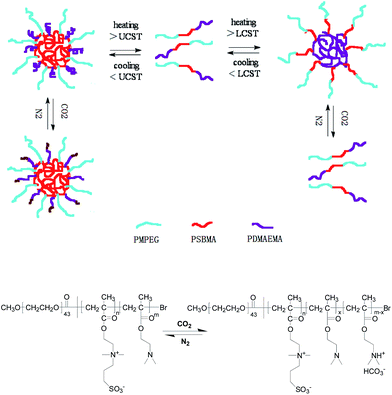 | ||
| Scheme 1 Schematic representation of the “schizophrenic” aggregation behavior for the triblock copolymer MPEG43-b-PSBMA30-b-PDMAEMA45 controlled by temperature and CO2. | ||
The triblock copolymer MPEG-b-PSBMA-b-PDMAEMA was synthesized by sequential atom transfer radical polymerization (ATRP) using an MPEG-based macroinitiator.33 The detailed synthesis and characterization are shown in the ESI.† After purification, the obtained copolymer MPEG43-b-PSBMA30-b-PDMAEMA45 was dissolved in borate buffer solution (pH 9.2) at a concentration of 10 mg mL−1.
The stimuli-responsive behavior of MPEG43-b-PSBMA30-b-PDMAEMA45 was investigated via UV-vis spectroscopy and dynamic light scattering (DLS). Measurements were conducted between 16 to 65 °C, well encompassing the UCST of PSBMA and the LCST of PDMAEMA.34,35 As shown in Fig. 1a, a sharp change of transmittance at 32 °C indicates that both PSBMA and PDMAEMA blocks are soluble. Upon decreasing the temperature from 32 to 16 °C, a decrease in transmittance was detected and shown in the black curve in Fig. 1a, accompanied by a reasonable dimensional increase as shown in the black curve in Fig. 1b. Similar results were obtained by increasing the temperature from 32 to 40 °C. An individual UCST (28 °C) or LCST (36 °C) determined at 50% transmittance was exhibited.33 The temperature-dependent assembly change behaviors can be attributed to PSBMA and PDMAEMA. Below the UCST, collapsed PSBMA forms the core of the aggregates while PDMAEMA and MPEG as the shell, which is attributed to mutual electrostatic attraction by ion pairings between ammonium cation and sulfo-anion of the zwitterionic sulfobetaine groups in PSBMA; above the LCST, PDMAEMA chains become hydrophobic and the hydrogen bond with water molecules weakens, collapsed PDMAEMA forms the core of the aggregates while MPEG-b-PSBMA as shell,33 as shown in Scheme 1.
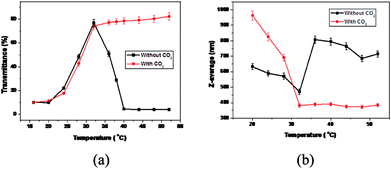 | ||
| Fig. 1 Temperature dependence of transmittance (a) and size (b) for MPEG43-b-PSBMA30-b-PDMAEMA45 in borate buffer solution (pH 9.2) before (black curve) and after (red curve) CO2 bubbling. | ||
The CO2-responsiveness was firstly confirmed by monitoring the transmittance and pH during successive CO2 and N2 bubbling cycles. Upon CO2 bubbling, no cloud point can be detected over the range of temperature for the transmittance measurement from 16 to 60 °C (Fig. 1a). This phenomenon derives from the protonation of tertiary amine groups in PDMAEMA blocks. The number of PDMAEMA units decreased due to the formation of carbonic acid upon CO2 bubbling in water, which resulted in improved solubility of the triblock copolymer. Therefore, the shell of micelles becomes looser as the PDMAEMA segments are stretched. The protonation caused by CO2 is reversible with assistance of N2. It can be observed that there is no significant difference but a slight decrease in transmittance after CO2 bubbling below 40 °C, which can be attributed to the insoluble PSBMA blocks as the core of micelles. However, the phase transitions are distinct under alternative CO2 and N2 above 32 °C, resulting in unimers with CO2 and precipitation without CO2.
The scanning electron microscopy (SEM) images of triblock copolymer aggregates at 25 °C before and after being treated with CO2 shown in Fig. 2, display quite different sizes, which is in accordance with the result determined by DLS. The formed vesicles with a diameter of over 500 nm treated with CO2, show larger size than that without CO2. In comparison with the DLS measurement, the smaller diameter by SEM can be attributed to the dry state.
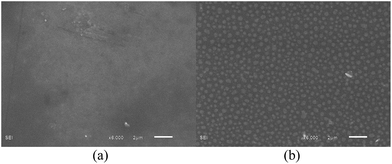 | ||
| Fig. 2 SEM images of MPEG43-b-PSBMA30-b-PDMAEMA45 self-assembling aggregates in borate buffer solution (pH 9.2) before (a) and after (b) being treated with CO2 at 25 °C. | ||
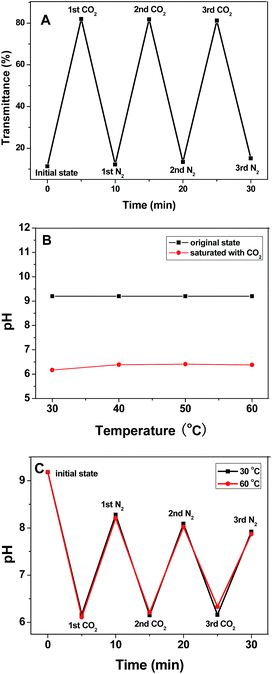
In the absence of any stimulus, hydrophobic PDMAEMA chains and weak hydrogen bonds result in poor solubility of the block copolymer above 40 °C. Upon CO2 bubbling, the transmittance of the micellar solution rapidly rises from 4.4% to 78.4% and then to the equilibrium value of ∼80% with an increase of temperature, simultaneously, the solution pH drops from 9.2 to 6.1. Upon N2 bubbling, CO2 can be released from the solution, and the transmittance decreases to 4%, while the pH recovers to 8.2. These variations are remarkably reversible and can be repeated for three cycles without alternation (Fig. 3A). The reversible transmittance “turn-on” and “turn-off” response can simulate the cellular respiration.19 Furthermore, this trigger benefits from the easy removal of the unstable bicarbonate salt36,37 produced by the reaction of CO2 with the tertiary amine groups in the PDMAEMA blocks, thus making it truly reversible, and therefore superior to the more traditional pH triggers by addition of acid or base,38 where reversibility is affected by the accumulation of by-products.39
It is worthy of note that the stimuli-responsive behavior of MPEG43-b-PSBMA30-b-PDMAEMA45 in borate buffer solution is different from in pure water, which deriving from the super buffer capacity and complexity of the buffer system. The borate buffer solution was obtained by the hydrolysis of sodium tetraborate in water, resulting in NaBO2–H3BO3 system (B4O72− + 3H2O = 2BO2− + 2H3BO3). Upon CO2 bubbling, the tertiary amine groups in PDMAEMA can be partially protonated, resulting in quaternary ammonium cations (–N(CH3)2 + CO2 + H2O = –NH+(CH3)2 + HCO3−). Concurrently, sodium tetraborate can be recovered to its initial state (4BO2 + CO2 = B4O72− + CO32−) due to the stronger acidity of carbonic acid than boric acid. Effect of temperature on the pH demonstrates that no significant difference between 30 and 60 °C can be observed in Fig. 3B. Time dependence of pH indicates that the pH gap between CO2 and N2 bubbling narrowed within an identical time interval with time extending, as shown in Fig. 3C.
To fabricate multi-responsive schizophrenic system has attracted increasing attention during recent years. Hoogenboom's group constructed a triple thermoresponsive schizophrenic diblock copolymer based on PDMAEMA and PmOEGMA, which undergoes transitions from conventional micelles via unimers to reverse micelles and finally precipitation upon heating.15 To date very few examples of such systems have been reported. In this work, a triple-responsive schizophrenic triblock copolymer based on the UCST transition of PSBMA, the LCST transition and CO2-responsiveness of PDMAEMA was developed. Following the above results, we propose the mechanism on how temperature and CO2 drive the morphological transition of the aggregate in borate buffer solution, which is schematically presented in Scheme 1. In the absence of CO2, at the turning point between the UCST and LCST, the polymer chains are totally soluble. Decreasing the temperature of the system, driven by electrostatic attraction between intra- or inter-ion pairs in PSBMA blocks, the small micelles (or vesicles) tend to fuse each other to form large fusions. Upon raising the system temperature, driven by the energy minimization between the collapsed PDMAEMA chains and water, the small micelles (or vesicles) also tends to form large fusions. Upon exposure to CO2, the PDMAEMA block can be partially protonated and hydrophilic, gradually water-soluble, leading to the unimer formation. On the other hand, protonation degree plays an important role in morphological transition. In our case, pH plays an important role in determining the protonation degree in borate buffer solution. In the absence of CO2, a low protonation degree hardly affects the hydrophobicity of the PDMAEMA block. However, the PDMAEMA block becomes hydrophilic with a high protonation degree, likely to induce substantial structural transition of the assemblies and changing of PDMAEMA copolymer structures.40
In conclusion, we have developed a new class of dual-responsive schizophrenic triblock copolymer by using temperature and CO2 to trigger morphological transition of assemblies. In borate buffer solution, the hydrophilic–hydrophobic transitions of PSBMA and PDMAEMA achieve by varying the system temperature, resulting in the core–shell exchange of assemblies. Furthermore, the aggregates can be reversibly switched “on” and “off” by alternatively bubbling and displacing CO2, in which the protonation–deprotonation mechanism of the tertiary amine groups along the PDMAEMA block is responsible for this reversible morphological transition. The temperature interval between UCST and LCST is comparatively narrow and mild, completely covering normal body temperature. CO2 offers many advantages as a trigger, in particular a lack of contaminant accumulation, and thus provides a reversible process over many cycles. To the best of our knowledge, this is the first example of temperature-/CO2-dual-responsive zwitterionic “schizophrenic” copolymer. It can be anticipated that the unique self-assembly behavior by mild and “green” stimuli my offer new opportunities for selective, well-controlled uptake, delivery and release of more drugs in one nano-vehicle. The biocompatible and bio-fouling resistant property endows the nanomaterial potential and promising application in biomedical area. The system can also provide inspiration for biomimics and simulation by synthetic polymer since the components of the block copolymer are biocompatible, and temperature and CO2 are crucial for living organisms.
Acknowledgements
The authors gratefully acknowledge financial support from Shandong Provincial Natural Science Foundation of China (Grant No. 2016ZRB01232, ZR2010BM006), Shandong Provincial Science and Technology Development Plan Project of China (2014GGX102013), and Shandong Provincial Overseas Visiting Scholar Project of China (2011).Notes and references
- A. Butun, N. Billingham and S. P. Arms, J. Am. Chem. Soc., 1998, 120, 11818 CrossRef.
- X. Ji, S. Dong, P. Wei, D. Xia and F. Huang, Adv. Mater., 2013, 25, 5725 CrossRef CAS PubMed.
- (a) Z. Tyrrell, Y. Shen and M. Radosz, Prog. Polym. Sci., 2010, 35, 1128 CrossRef CAS; (b) A. Darabi, P. G. Jessop and M. F. Cunningham, Chem. Soc. Rev., 2016, 45, 4391 RSC; (c) V. A. Vasantha, S. Jana, S. S.-C. Lee, C.-S. Lim, S. L.-M. Teo, A. Parthiban and J. G. Vancso, Polym. Chem., 2015, 6, 599 RSC.
- C. Feng, Y. Li, D. Yang, J. Hu, X. Zhang and F. Huang, Chem. Soc. Rev., 2011, 40, 1282 RSC.
- Y. Zhou, Q. Zhang and Z. Luo, Langmuir, 2014, 30, 1489 CrossRef CAS PubMed.
- R. Hoogenboom, H. M. L. Lambermont-Thijs, M. J. H. C. Jochems, S. Hoeppener, C. Guerlain, C.-A. Fustin, J.-F. Gohy and U. S. Schubert, Soft Matter, 2009, 5, 3590 RSC.
- S. Liu, N. C. Billingham and S. P. Arms, Angew. Chem., Int. Ed., 2001, 40, 2328 CrossRef CAS PubMed.
- S. Liu and S. P. Arms, Angew. Chem., Int. Ed., 2002, 41, 1413 CrossRef CAS PubMed.
- M. Arotcarena, B. Heise, S. Ishaya and A. Laschewsky, J. Am. Chem. Soc., 2002, 124, 3787 CrossRef CAS PubMed.
- F. A. Plamper, A. Schmalz and A. H. E. Muller, J. Am. Chem. Soc., 2007, 129, 14538 CrossRef CAS PubMed.
- J. Seuring and S. Agarwal, Macromolecules, 2012, 45, 3910 CrossRef CAS.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898 CrossRef CAS PubMed.
- C. Weber, R. Hoogenboom and U. S. Schubert, Prog. Polym. Sci., 2012, 37, 686 CrossRef CAS.
- Q. Zhang, J.-D. Hong and R. Hoogenboom, Polym. Chem., 2013, 4, 4322 RSC.
- R. Hoogenboom, H. M. L. Lambermont-Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, Soft Matter, 2008, 4, 103 RSC.
- Y. J. Shih, Y. Chang, A. Deratani and D. Quemener, Biomacromolecules, 2012, 13, 2849 CrossRef CAS PubMed.
- (a) A. Feng, C. Zhao, Q. Yan, B. Liu and J. Yuan, Chem. Commun., 2014, 50, 8958 RSC; (b) H. Chen and J. C. M. van Hest, J. Mater. Chem. B, 2016, 4, 4362 Search PubMed; (c) K. Jie, Y. Zhou, Y. Yao, B. Shi and F. Huang, J. Am. Chem. Soc., 2015, 137, 10472 CrossRef CAS PubMed; (d) B. A. Abel, M. B. Sims and C. L. McCormick, Macromolecules, 2015, 48, 5487 CrossRef CAS.
- X. Su, M. F. Cunningham and P. G. Jessop, Polym. Chem., 2014, 5, 940 RSC.
- Q. Yan, R. Zhou, C. Fu, H. Zhang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2011, 50, 4923 CrossRef CAS PubMed.
- (a) Q. Yan, J. Wang, Y. Yin and J. Yuan, Angew. Chem., Int. Ed., 2013, 52, 5070 CrossRef CAS PubMed; (b) H. Che, M. Huo, L. Peng, T. Fang, N. Liu, L. Feng, Y. Wei and J. Yuan, Angew. Chem., Int. Ed., 2015, 54, 8934 CrossRef CAS PubMed.
- Q. Yan and Y. Zhao, Angew. Chem., Int. Ed., 2013, 52, 9948 CrossRef CAS PubMed.
- Q. Yan and Y. Zhao, J. Am. Chem. Soc., 2013, 135, 16300 CrossRef CAS PubMed.
- Z. Li, E. Kesselman, Y. Talmon, M. A. Hillmyer and T. P. Lodge, Science, 2004, 306, 98 CrossRef CAS PubMed.
- M. Tian, J. Wang, E. Zhang, J. Li, C. Duan and F. Yao, Langmuir, 2013, 29, 8076 CrossRef CAS PubMed.
- Y. Xu, S. Bolisetty, M. Drechsler, B. Fang, Y. Yuan and M. Ballauff, Polymer, 2008, 49, 3957 CrossRef CAS.
- O. Schepelina and I. Zharov, Langmuir, 2008, 24, 14188 CrossRef CAS PubMed.
- N. Nordgren and M. W. Rutland, Nano Lett., 2009, 9, 2984 CrossRef CAS PubMed.
- J. I. Amalvy, E. J. Wanless, Y. Li, V. Michailidou and S. P. Armes, Langmuir, 2004, 20, 8992 CrossRef CAS PubMed.
- S. H. Cho, M. S. Jhon, S. H. Yuk and H. B. Lee, J. Polym. Sci., Part B: Polym. Phys., 1997, 35, 595 CrossRef CAS.
- D. Fournier, R. Hoogenboom, H. M. L. Thijs, R. M. Paulus and U. S. Schubert, Macromolecules, 2007, 40, 915 CrossRef CAS.
- F. A. Plamper, M. Ruppel, A. Schmalz, O. Borisov, M. Ballauff and A. H. E. A. Müller, Macromolecules, 2007, 40, 8361 CrossRef CAS.
- D. Han, X. Tong, O. Boissire and Y. Zhao, ACS Macro Lett., 2012, 1, 57 CrossRef CAS.
- Q. Zhang, X. Tang, T. Wang, F. Yu, W. Guo and M. Pei, RSC Adv., 2014, 4, 24240 RSC.
- S. Nayak, D. Gan, M. Serpe and L. Lyon, Small, 2005, 1, 416 CrossRef CAS PubMed.
- C. Sorrell, M. Carter and M. Serpe, Adv. Funct. Mater., 2011, 21, 425 CrossRef CAS.
- Y. Zhang, Y. Feng, J. Wang, S. He, Z. Guo, Z. Chu and C. A. Dreiss, Chem. Commun., 2013, 49, 4902 RSC.
- Y. Zhang, Z. Chu, C. A. Dreiss, Y. Wang, C. Fei and Y. Feng, Soft Matter, 2013, 9, 6217 RSC.
- S. Kumar, X. Tong, Y. Dory, M. Chaker, Y. Zhao and D. Ma, Chem. Commun., 2013, 49, 90 RSC.
- P. G. Jessop, S. M. Mercer and J. Heldebrant, Energy Environ. Sci., 2012, 5, 7240 CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis and characterization. See DOI: 10.1039/c6ra28018h |
| This journal is © The Royal Society of Chemistry 2017 |