Open Access Article
Open Access ArticlePyridine C3-arylation of nicotinic acids accessible via a multicomponent reaction: an entry to all-substituted-3,4-diarylated pyridines†
Sankar K. Guchhait *a,
Neha Huraa,
Kanchan Sinhab and
Dulal Pandab
*a,
Neha Huraa,
Kanchan Sinhab and
Dulal Pandab
aDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research (NIPER), S. A. S. Nagar, Mohali-160062, India. E-mail: skguchhait@niper.ac.in; Fax: +91 172 2214692; Tel: +91 172 2214683
bDepartment of Biosciences and Bioengineering, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India
First published on 25th January 2017
Abstract
An efficient route for the synthesis of penta-substituted/functionalized-3,4-diarylated pyridines, biologically important templates, via pyridine C3-arylation of nicotinic acids has been developed. The poly-substituted nicotinic acid precursors were prepared by an established multicomponent condensation approach. This route shows an excellent opportunity for introducing versatile (hetero)aryls and other substituents/functionalities into the pyridine ring. Several of the synthesized compounds exhibited significant anti-proliferative properties.
Introduction
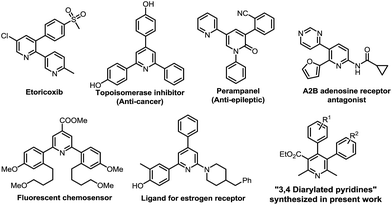
Poly-substituted/functionalized pyridines are omnipresent in natural products, bioactive compounds, and functional materials.1 In particular, aryl or poly-aryl substituted pyridine derivatives have attracted considerable synthesis attention because they are often found as a valuable structural motif in a wide range of pharmaceutically active compounds (Fig. 1). Etoricoxib that contains the 2,3-diarylpyridine motif is a COX-2 selective inhibitor.2,3 2,4,6-Triarylated pyridine compounds exhibit topoisomerase inhibition (Fig. 1).4 Perampanel is a 1,3,5-triarylated pyridine derivative that acts as an AMPA-receptor antagonist.5 5,6-Diheteroarylpyridin-2-carboxamide derivative is a selective A2B adenosine receptor antagonist.6 2-Amino-4,6-diarylpyridines have been found to be potent ligands for estrogen receptor.7 There are numerous bioactive natural products that contain polyarylated pyridines. Nosiheptide8 has 1,5,6-triarylated 3-hydroxypyridine and promothiocin A9 has 5,6-diarylated pyridine-1-carboxamide and both are potent antibiotics. 2,6-Diarylpyridine-carboxylic ester is useful as a fluorescent chemosensor.10In recent times, as a part of current momentum of exploring new useful organic functional/bioactive materials, the synthesis of compounds that possess not only important heterocyclic scaffold(s) but also structurally resembles in whole molecular skeleton to drugs/bioactive agents has become a valuable research area to organic chemists.11 In this direction, late stage functionalization of drugs has also attracted significant attention.12 The incorporation of trimethoxyphenyl moiety in target organic compounds is valuable, since it plays role as an important pharmacophoric motif in exhibiting biological properties13 and is present in natural products.14 Keeping these aspects in mind, we were interested15 in the synthesis of 3,4-diarylated pyridines which contain structural features, 3,4,5-trimethoxyphenyl as an aryl motif, and resemblance to a nifedipine drug16 and biologically important nicotinic acid.17
Over the years, numerous synthetic strategies have been developed to access poly-functionalized pyridines.18,19 They involve majorly a pyridine-forming step of appropriately functionalized precursors20 or the sequential introduction of substituents on the preformed pyridine ring.21 The first strategy is limited to accessibility of particularly-functionalized precursor, while the later strategy includes mainly aromatic substitutions,22 direct metallation, or metal halogen-exchange reactions.23 Here we report a novel route for synthesis of all-substituted/functionalized pyridines containing 3,4-diaryl moieties via one-pot pyridine-3-arylation of nicotinic acid skeleton that is easily accessible by an established multicomponent reaction approach.
Results and discussion
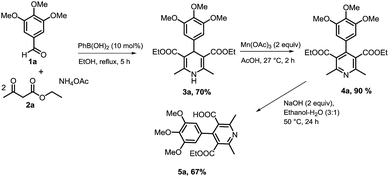
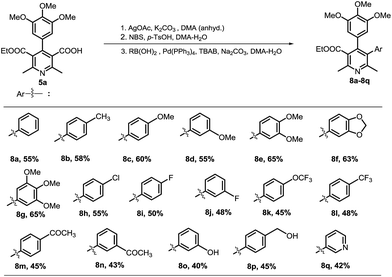
At the outset, we investigated for preparation of nicotinic acid precursor (5a) by a Hantzsch multicomponent condensation of aryl aldehyde (1a), ethyl acetoacetate (2a) and ammonium acetate to construct dihydropyridine (3a), its dehydrogenative aromatization to product 4a, and mono-hydrolysis of di-ester (Scheme 1). Hantzsch reaction by a reported method was good yielding.24 Dehydrogenative aromatization reactions utilizing different conditions25 were performed (see ESI, Table 1†). Highest yield was obtained using Mn(OAc)3 and AcOH at room temperature. Initially, the mono-hydrolysis of pyridine-3,5-diesters 4a was found to be difficult. Both the ester functionalities underwent simultaneously the hydrolysis. A survey of various conditions revealed that 2 equiv. NaOH in 2 mL of EtOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was most effective for promoting mono-hydrolysis to produce nicotinic acid 5a (see ESI, Table 2†).
1) was most effective for promoting mono-hydrolysis to produce nicotinic acid 5a (see ESI, Table 2†).
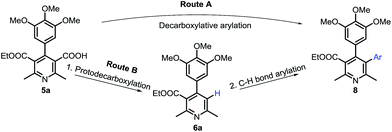
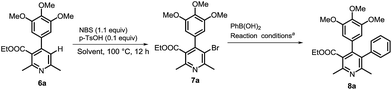
Next, we investigated to explore decarboxylative arylation of nicotinic acid 5a, with the goal of finding a convenient method for synthesis of penta-substituted/functionalized pyridines containing 3,4-diaryl moieties (Route A, Scheme 2). Several methods of decarboxylative arylation26 known for other scaffolds were investigated for the reaction of compound 5a. The desired product was obtained, but the yield more than 8% could not be accomplished by the methods as well as variation in reported conditions. We envisaged that protodecarboxylation,27 and subsequent C3–H bond arylation could afford C3-arylated pyridine (Route B, Scheme 2). The protodecarboxylation of compound 5a underwent via Ag-carboxylate on treatment with AgOAc (1 equiv.), K2CO3 (30 mol%) in DMA (anhyd.) at 140 °C for 12 h and the product 6a was obtained in 80% yield. Next, the C3–H arylation reactions of compound 6a following the reported pyridine-arylation methods were performed. Ye developed a Pd-catalyzed C3-selective arylation of unsubstituted pyridine with bromoarenes.28 Carrying out C3-arylation of substituted/functionalized pyridine 6a with bromo- or iodotoluene using Ye's conditions provided 3-arylated pyridine (8a) in poor yields (8% and 10% yields, respectively). A Pd-catalyzed 3-arylation of pyridine with aryl tosylates was reported by Dai.29 This methodology did not promote the reaction of compound 6a with p-tolyl tosylate. We explored previously a Pd-catalyzed regioselective C6–H arylation of 3-aminoimidazo[1,2-a]pyrazine that underwent via concerted metalation–deprotonation process.30 This method promoted the C3–H arylation of pyridine derivative 6a, but the method as well as the variation of its conditions could not improve the product's yield more than 20%. Majorly, the substrate remained intact. The poor conversion and yields in the C3-arylation of pyridine 6a by reported methods might be due to significant steric hindrance by 4-aryl moiety to inhibit the substrate to undergo the C3-palladation with arylpalladium complex. With aim of obtaining the C-3 arylated product, we then investigated for an alternate approach involving protodecarboxylation, C3-bromination and Suzuki coupling sequentially (Scheme 3). C3-Bromination31 of pyridine 6a leading to product 7a was found to be nearly quantitative (95%). Therefore, we were interested to explore one-pot conditions for bromination–Suzuki coupling. A Pd(OAc)2-catalyzed method reported by Liu32 for Suzuki reaction of N-heteroaryl halides was followed in the one-pot reaction of pyridine 6a with phenylboronic acid, however, the desired product was obtained in 25% yield only. Christakakou's33 reaction conditions yielded the product 8a in 40% yield only. Gratifyingly, one-pot bromination and Suzuki coupling of pyridine 6a with phenylboronic acid using Pd(PPh3)4 catalyst, Na2CO3, TBAB in DMA–H2O provided 3,4-diaryl-pyridine 8a in 70% yield (Table 1, entry 1). However, further variation in conditions did not improve yield of the product. Incomplete conversion and inferior yield (15%) were obtained for the reaction carried out in the presence of open air, indicating requirement of non-aerobic conditions for Pd(0)-catalysis. The reaction without TBAB resulted in reduced yield (40%). Other palladium sources were evaluated (Table 1, entries 2–4). Pd(PPh3)4 was found to be best. Reducing the Pd-catalyst loading (entry 5) was not beneficial. Among various bases investigated, Na2CO3 provided best result (entries 6–8). Decreasing the equivalence of sodium carbonate below to 5 equiv. resulted in reduced yield (Table 1, entry 9). DMA or EtOH was found effective solvent for bromination, however, they were inferior for promoting the Suzuki coupling in one-pot. Among various solvents and their mixture with water, DMA–H2O was found to be most effective for promoting bromination–arylation in one-pot (entries 10–12).
| Entry | Variable | Yieldb |
|---|---|---|
| a Reagents and conditions: PhB(OH)2 (1.5 equiv.), 100 °C, Ar, 2 h; 0.5 mmol scale.b Yield for maximum conversion in optimum time.c PPh3 (40 mol%).d Pd(PPh3)4 (5 mol%).e Na2CO3 (2.5 equiv.).f Reaction temperature: 80 °C. | ||
Catalyst (10 mol%), Na2CO3 (5 equiv.), TBAB (1 equiv.), DMA–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||
| 1 | Pd(PPh3)4 | 70 |
| 2 | Pd(dppf)Cl2 | 55 |
| 3 | Pd(PPh3)2Cl2 | NR |
| 4c | Pd(OAc)2 + PPh3 | NR |
| 5d | Pd(PPh3)4 | 40 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
Pd(PPh3)4 (10 mol%), base (5 equiv.), TBAB (1 equiv.), DMA–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
||
| 6 | K2CO3 | 60 |
| 7 | Cs2CO3 | 55 |
| 8 | K3PO4 | 40 |
| 9e | Na2CO3 | 45 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Pd(PPh3)4 (10 mol%), Na2CO3 (5 equiv.), TBAB (1 equiv.), solvent | ||
| 10 | EtOH–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 |
| 11 | DMA | 55 |
| 12f | EtOH | 45 |
We then investigated protodecarboxylation of nicotinic acid 5a, bromination and Suzuki coupling sequentially in one-pot. Amazingly, it worked well in one-pot with overall yield of 55% (Scheme 3). The one-pot multi-reactions synthesis of a target molecule is considered as a useful approach in synthetic organic chemistry.34 The present work illustrates an important example of one-pot three-reaction process.
With the optimized procedure, we next investigated to explore its substrate scope and synthesize various substituted pyridines. We were pleased to find that the method was found to be flexible in introducing into pyridine at C3 a variety of (hetero)aryls (Scheme 4). Aryls containing electron-withdrawing or donating functionalities were incorporated. The biologically relevant (hetero)aryl motifs were also introduced easily at C3-position of pyridine derivatives. In the established route, difficulty for pyridine C3-arylation due to steric hindrance by presence of a multi-substituted aryl (3,4,5-trimethoxyphenyl) at C4-position was circumvented.
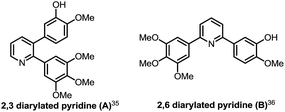
On survey of literature, we found that Simoni demonstrated an attractive profile of cytotoxicity and apoptosis-inducing activity of 2-(3,4,5-trimethoxyphenyl)-3-(3-hydroxy-4-methoxyphenyl)-pyridine (compound A, Fig. 2).35 Zheng documented that 2-(3-hydroxy-4-methoxyphenyl)-6-(3,4,5-trimethoxyphenyl)-pyridine (B) inhibited cell survival and growth, comparable to a clinical agent, combretastatin A-4 (CA-4).36 These representative classes of compounds reveal that 3,4-diarylated pyridine derivatives synthesized in the present work have potential of exhibiting versatile bioactivities especially the antiproliferative properties.
We extended our developed method applicable to the synthesis of 3,4-diarylated pyridines with relevant substitutions that are present in CA-4 and the diarylated pyridines synthesized and bio-evaluated by Simoni and Zhang.
Ethyl 5-(3-hydroxy-4-methoxyphenyl)-2,6-dimethyl-4-(3,4,5-trimethoxyphenyl)nicotinate (8r) was prepared via an approach (Scheme 5) involving C3-bromination of precursor 6a, boronation37 of 7a with bispinacolatodiborane, and Suzuki coupling with 5-bromo-2-methoxyphenol. This prompted us to synthesize compound 8t with switch in aryl substitutions of compound 8r. We began the preparation of compound 8t via similar strategy, utilizing 5-(ethoxycarbonyl)-4-(3-hydroxy-4-methoxyphenyl)-2,6-dimethylnicotinic acid as the precursor, but bromination did not take place. We anticipated that bromination was problematic due to presence of free hydroxyl group in the precursor. So, benzylation of the hydroxyl group was done to circumvent this problem, and we carried out the synthesis of compound 8t utilizing benzyloxy-aryl derivative of nicotinic acid (5b). One-pot protodecarboxylation–bromination–Suzuki coupling was done to afford compound 8s, which on debenzylation resulted in compound 8t (Scheme 6).
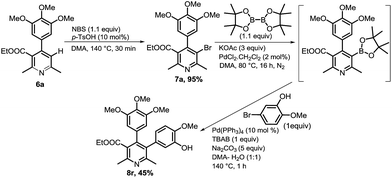 | ||
| Scheme 5 Synthesis of ethyl 5-(3-hydroxy-4-methoxyphenyl)-2,6-dimethyl-4-(3,4,5-trimethoxyphenyl)nicotinate (8r). | ||
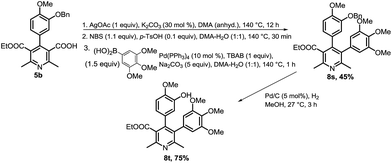 | ||
| Scheme 6 Synthesis of ethyl 4-(3-hydroxy-4-methoxyphenyl)-2,6-dimethyl-5-(3,4,5-trimethoxyphenyl)nicotinate (8t). | ||
A set of significantly varied 3,4-diarylated pyridine derivatives (8a–8t) were prepared in the developed approach. Purity (HPLC) of all the compounds were found to be >95%. Next antiproliferative activity was tested. All compounds were screened for % inhibition of cell proliferation in HeLa cells as a representative cancer cell line at 5 μM concentration (see ESI, Table 3†). Several of them were found to exhibit considerable antiproliferative activity. Four most potent compounds (pyridines 8b, 8f, 8j, 8p) were further evaluated for in vitro tubulin polymerization inhibition (see ESI, Fig. 1†). The non-activity revealed that the anti-tubulin may not be the pathway for exhibiting cytotoxicity.
Conclusions
In conclusion, we have developed an efficient route for synthesis of all-substituted/functionalized pyridines containing 3,4-diaryl moieties. It involves the preparation of nicotinic acids via multicomponent condensation and their pyridine C3-arylation via one-pot protodecarboxylation–bromination–Suzuki coupling. A wide range of (hetero)aryls, including especially the biologically important aryl-motifs can be easily introduced in this route. The structural features of the products, structural resemblance to pharmacologically important agent as well as drug and the presence of a biologically important motif 3,4,5-trimethoxyphenyl as one aryl ring, indicate that these functionalized 3,4-diarylpyridines have potential application in finding of versatile bioactive agents. Several of the synthesized compounds were found to exhibit significant anti-proliferative activity.Experimental
Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) provided the product 5a in 67% yield.
9) provided the product 5a in 67% yield.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) provided 2,6-dimethyl-3-(ethoxycarbonyl)-5-phenyl-4-(3,4,5-trimethoxyphenyl)pyridine 8a in 55% overall yield.
3) provided 2,6-dimethyl-3-(ethoxycarbonyl)-5-phenyl-4-(3,4,5-trimethoxyphenyl)pyridine 8a in 55% overall yield.Products (8b–8t) were also prepared following this representative procedure.
Biology
Acknowledgements
We gratefully acknowledge financial support from CSIR, New Delhi for this investigation. NH is thankful to DST, NewDelhi for her DST-INSPIRE fellowship. DP is thankful to DAE, Government of India for DAE-SRC fellowship.Notes and references
- (a) A. O. Plunkett, Nat. Prod. Rep., 1994, 11, 581 RSC; (b) J. Daly, H. Garaffo and T. Spande, Alkaloids, 1993, 43, 185 CAS; (c) A. R. Pinder, Nat. Prod. Rep., 1992, 9, 491 RSC; (d) G. D. Henry, Tetrahedron, 2004, 60, 6043 CrossRef CAS; (e) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC.
- D. Riendeau, M. D. Percival, C. Brideau, S. Charleson, D. Dube, D. Ethier, J.-P. Falgueyret, R. W. Friesen, R. Gordon, G. Greig, J. Guay, J. Mancini, M. Ouellet, E. Wong, L. Xu, S. Boyce, D. Visco, Y. Girard, P. Prasit, R. Zamboni, I. W. Rodger, M. Gresser, A. W. Ford-Hutchinson, R. N. Young and C.-C. Chan, J. Pharmacol. Exp. Ther., 2001, 296, 558 CAS.
- I. W. Davies, J.-F. Marcoux, E. G. Corley, M. Journet, D.-W. Cai, M. Palucki, J. Wu, R. D. Larsen, K. Rossen, P. J. Pye, L. DiMichele, P. Dormer and P. J. Reider, J. Org. Chem., 2000, 65, 8415 CrossRef CAS PubMed.
- R. Karki, P. Thapa, H. Y. Yoo, T. M. Kadayat, P.-H. Park, Y. Na, E. Lee, K.-H. Jeon, W.-J. Cho and H. Choi, Eur. J. Med. Chem., 2012, 49, 219 CrossRef CAS PubMed.
- T. Hanada, Y. Hashizume, N. Tokuhara, O. Takenaka, N. Kohmura, A. Ogasawara, S. Hatakeyama, M. Ohgoh, M. Ueno and Y. Nishizawa, Epilepsia, 2011, 52, 1331 CrossRef CAS PubMed.
- P. Eastwood, J. Gonzalez, S. Paredes, A. Nueda, T. Domenech, J. Alberti and B. Vidal, Bioorg. Med. Chem. Lett., 2010, 20, 1697 CrossRef CAS PubMed.
- R. H. Brad, H. D. David, A. J. Stacey, L. S. Eugene, L. W. Susan and W. W. Robert, Bioorg. Med. Chem. Lett., 2001, 11, 1939 CrossRef.
- C. Pascard, A. Ducruix, J. Lunel and T. Prange, J. Am. Chem. Soc., 1977, 99, 6418 CrossRef CAS PubMed.
- M. C. Bagley, K. E. Bashford, C. L. Hesketh and C. J. Moody, J. Am. Chem. Soc., 2000, 122, 3301 CrossRef CAS.
- A. G. Fang, J. V. Mello and N. S. Finney, Tetrahedron, 2004, 60, 11075 CrossRef CAS.
- (a) S. Hang, D. J. Babinski and T. Ritter, J. Am. Chem. Soc., 2015, 137, 3775 CrossRef PubMed; (b) S. K. Guchhait and G. Priyadarshani, J. Org. Chem., 2015, 80, 8482 CrossRef CAS PubMed; (c) S. K. Guchhait and G. Priyadarshani, J. Org. Chem., 2015, 80, 6342 CrossRef CAS PubMed; (d) G. Priyadarshani, S. Amrutkar, A. Nayak, U. C. Banerjee, C. N. Kundu and S. K. Guchhait, Eur. J. Med. Chem., 2016, 122, 43 CrossRef CAS PubMed.
- H. Sekizawa, K. Amaike, Y. Itoh, T. Suzuki, K. Itami and J. Yamaguchi, ACS Med. Chem. Lett., 2014, 5, 582 CrossRef CAS PubMed.
- For representative activities, e.g., antimitotic, anitproliferative, antimalarial, fungicidal: (a) C.-J. Chen, B.-A. Song, S. Yang, G.-F. Xu, P. S. Bhadury, L.-H. Jin, D.-Y. Hu, Q.-Z. Li, F. Liu and W. Xue, Bioorg. Med. Chem., 2007, 15, 3981 CrossRef CAS PubMed; (b) R. Kumar, D. Mohanakrishnan, A. Sharma, N. K. Kaushik, K. Kalia, A. K. Sinha and D. Sahal, Eur. J. Med. Chem., 2010, 45, 5292 CrossRef CAS PubMed; (c) Y.-H. Wang, M. Goto, L.-T. Wang, K.-Y. Hsieh, S. L. Morris-Natschke, G.-H. Tang, C.-L. Long and K.-H. Lee, Bioorg. Med. Chem. Lett., 2014, 24, 4818 CrossRef CAS PubMed; (d) M. Cushman, D. Nagarathnam, D. Gopal, H. M. He, C. M. Lin and E. Hamel, J. Med. Chem., 1992, 35, 2293 CrossRef CAS PubMed.
- (a) G. R. Pettit, S. B. Singh, M. R. Boyd, E. Hamel, R. K. Pettit, J. M. Schmidt and F. Hogan, J. Med. Chem., 1995, 38, 1666 CrossRef CAS PubMed; (b) C. Canela, R. M. Moraesb, F. E. Dayana and D. Ferreirab, Phytochemistry, 2000, 54, 115 CrossRef.
- (a) A. T. Baviskar, C. Madaan, R. Preet, P. Mohapatra, V. Jain, A. Agarwal, S. K. Guchhait, C. N. Kundu, U. C. Banerjee and P. V. Bharatam, J. Med. Chem., 2011, 54, 5013 CrossRef CAS PubMed; (b) V. Chaudhary, J. B. Venghateri, H. P. Dhaked, A. S. Bhoyar, S. K. Guchhait and D. Panda, J. Med. Chem., 2016, 59, 3439 CrossRef CAS PubMed; (c) G. Priyadarshani, A. Nayak, S. M. Amrutkar, S. Das, S. K. Guchhait, C. N. Kundu and U. C. Banerjee, ACS Med. Chem. Lett., 2016, 7, 1056 CrossRef CAS PubMed.
- V. Leuranguer, M. E. Mangoni, J. Nargeot and S. Richard, J. Cardiovasc. Pharmacol. Ther., 2001, 37, 649 CrossRef CAS.
- P. D. Boatman, J. G. Richman and G. Semple, J. Med. Chem., 2008, 51, 7653 CrossRef CAS PubMed.
- (a) R. Abramovitch and E. Smith, Chemistry of heterocyclic compounds: pyridine and its derivatives: part II, John Wiley & Sons, Inc, New York, 1974 Search PubMed; (b) G. Newkome, Part 5, Chemistry of Heterocyclic Compounds: Pyridine and its Derivatives, Part 5, Wiley & Sons, Hoboken, NJ, USA, 1984 Search PubMed.
- (a) E. Zhang, J. Tang, S. Li, P. Wu, J. E. Moses and K. B. Sharpless, Chem.–Eur. J., 2016, 22, 5692 CrossRef CAS PubMed; (b) Z. Song, X. Huang, W. Yi and W. Zhang, Org. Lett., 2016, 18, 5640 CrossRef CAS PubMed; (c) J. Shen, D. Cai, C. Kuai, Y. Liu, M. Wei, G. Cheng and X. Cui, J. Org. Chem., 2015, 80, 6584 CrossRef CAS PubMed; (d) R. Khajuria, P. Kannaboina, K. K. Kapoor, A. Gupta, G. Raina, A. K. Jassal, L. K. Rana, M. S. Hundald and P. Das, Org. Biomol. Chem., 2015, 13, 5944 RSC.
- (a) R. L. Frank and R. P. Seven, J. Am. Chem. Soc., 1949, 71, 2629 CrossRef CAS; (b) B. Heller and M. Hapke, Chem. Soc. Rev., 2007, 36, 1085 RSC; (c) H. Bönnemann, Angew. Chem., Int. Ed. Engl., 1978, 17, 505 CrossRef; (d) M. Ohashi, I. Takeda, M. Ikawa and S. Ogoshi, J. Am. Chem. Soc., 2011, 133, 18018 CrossRef CAS PubMed; (e) I. Linder, M. Gerhard, L. Schefzig, M. Andrä, C. Bentz, H. U. Reissig and R. Zimmer, Eur. J. Org. Chem., 2011, 2011, 6070 CrossRef CAS; (f) C. Allais, F. Liéby-Muller, T. Constantieux and J. Rodriguez, Adv. Synth. Catal., 2012, 354, 2537 CrossRef CAS; (g) S.-I. Yamamoto, K. Okamoto, M. Murakoso, Y. Kuninobu and K. Takai, Org. Lett., 2012, 14, 3182 CrossRef CAS PubMed.
- (a) K. Billingsley and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3358–3366 CrossRef CAS PubMed; (b) K. L. Billingsley, K. W. Anderson and S. L. Buchwald, Angew. Chem., Int. Ed., 2006, 45, 3484 CrossRef CAS PubMed; (c) G. M. Chapman, S. P. Stanforth, B. Tarbit and M. D. Watson, J. Chem. Soc., Perkin Trans. 1, 2002, 581 RSC; (d) S. Reimann, S. Parpart, P. Ehlers, M. Sharif, A. Spannenbergb and P. Langer, Org. Biomol. Chem., 2015, 13, 6832 RSC; (e) W. Hagui, N. Besbes, E. Srasra, J.-F. Soulé and H. Doucet, RSC Adv., 2016, 6, 17110 RSC; (f) S. Reimann, P. Ehlers, S. Parpart and A. Surkus, Tetrahedron, 2015, 71, 5371 CrossRef CAS.
- (a) C. Marti, J. Irurre, A. Alvarez-Larena, J. F. Piniella, E. Brillas, L. Fajari, C. Aleman and L. Julia, J. Org. Chem., 1994, 59, 6200 CrossRef CAS; (b) A. Schmidt, T. Mordhorst and M. Nieger, Org. Biomol. Chem., 2005, 3, 3788 RSC; (c) G. Sandford, R. Slater, D. S. Yufit, J. A. K. Howard and A. Vong, J. Org. Chem., 2005, 70, 7208 CrossRef CAS PubMed.
- (a) F. Mongin and G. Quéguiner, Tetrahedron, 2001, 57, 4059 CrossRef CAS; (b) P. C. Gros and Y. Fort, Eur. J. Org. Chem., 2009, 2009, 4199 CrossRef.
- A. Debache, R. Boulcina, A. Belfaitah, S. Rhouati and B. Carnobi, Synlett, 2008, 4, 509 CrossRef.
- (a) R. S. Varma and D. Kumar, Tetrahedron Lett., 1999, 40, 21 CrossRef CAS; (b) X. Liao, W. Lin, J. Lu and C. Wang, Tetrahedron Lett., 2010, 51, 3859 CrossRef CAS; (c) M. M. Heravi, F. K. Behbahani, H. A. Oskooie and R. H. Shoar, Tetrahedron Lett., 2005, 46, 2775 CrossRef CAS.
- (a) J.-J. Dai, J.-H. Liu, D.-F. Luo and L. Liu, Chem. Commun., 2011, 47, 677 RSC; (b) F. A. Arroyave and J. R. Reynolds, J. Org. Chem., 2011, 76, 8621 CrossRef CAS PubMed; (c) R. Shang, Z.-W. Yang, Y. Wang, S.-L. Zhang and L. Liu, J. Am. Chem. Soc., 2010, 132, 14391 CrossRef CAS PubMed; (d) P. Forgione, M.-C. Brochu, M. St-Onge, K. H. Thesen, M. D. Bailey and F. Bilodeau, J. Am. Chem. Soc., 2006, 128, 11350 CrossRef CAS PubMed.
- (a) B. Love and M. M. Goodman, J. Heterocycl. Chem., 1975, 12, 363 CrossRef; (b) L. J. Gooßen, C. Linder, N. Rodríguez, P. P. Lange and A. Fromm, Chem. Commun., 2009, 7173 RSC; (c) R. Meesala, M. N. Mordi and S. M. Mansor, Synlett, 2014, 25, 120 CrossRef CAS; (d) J. Cornella, C. Sanchez, D. Banawa and I. Larrosa, Chem. Commun., 2009, 7176 RSC; (e) P. Lu, C. Sanchez, J. Cornella and I. Larrosa, Org. Lett., 2009, 11, 5710 CrossRef CAS PubMed.
- M. Ye, G.-L. Gao, A. J. F. Edmunds, P. A. Worthington, J. A. Morris and J.-Q. Yu, J. Am. Chem. Soc., 2011, 133, 19090 CrossRef CAS PubMed.
- F. Dai, Q. Gui, J. Liu, Z. Yang, X. Chen, R. Guo and Z. Tan, Chem. Commun., 2013, 49, 4634 RSC.
- S. K. Guchhait, S. Kandekar, M. Kashyap, N. Taxak and P. V. Bharatam, J. Org. Chem., 2012, 77, 8321 CrossRef CAS PubMed.
- M. V. Adhikari and S. D. Samant, Ultrason. Sonochem., 2002, 9, 107 CrossRef CAS PubMed.
- C. Liu, N. Han, X. Song and J. Qiu, Eur. J. Org. Chem., 2010, 2010, 5548 CrossRef.
- M. Christakakou, M. Schön, M. Schnürch and M. D. Mihovilovic, Synlett, 2013, 24, 2411 CrossRef CAS.
- (a) Y. Hayashi, Chem. Sci., 2016, 7, 866 RSC; (b) C. Vaxelaire, P. Winter and M. Christmann, Angew. Chem., Int. Ed., 2011, 50, 3605 CrossRef CAS PubMed; (c) C. Liu, Z. Zeng, R. Chen, X. Jiang, Y. Wang and Y. Zhang, Org. Lett., 2016, 18, 624 CrossRef CAS PubMed; (d) A. Shavnya, S. B. Coffey, K. D. Hesp, S. C. Ross and A. S. Tsai, Org. Lett., 2016, 18, 5848 CrossRef CAS PubMed.
- D. Simoni, G. Grisolia, G. Giannini, M. Roberti, R. Rondanin, L. Piccagli, R. Baruchello, M. Rossi, R. Romagnoli, F. P. Invidiata, S. Grimaudo, M. K. Jung, E. Hamel, N. Gebbia, L. Crosta, V. Abbadessa, A. D. Cristina, L. Dusonchet, M. Meli and M. Tolomeo, J. Med. Chem., 2005, 48, 723 CrossRef CAS PubMed.
- S. Zheng, Q. Zhong, M. Mottamal, Q. Zhang, C. Zhang, E. LeMelle, H. McFerrin and G. Wang, J. Med. Chem., 2014, 57, 3369 CrossRef CAS PubMed.
- T. Ishiyama, M. Murata and N. Miyaura, J. Org. Chem., 1995, 60, 7508 CrossRef CAS.
- T. D. A. Kumar, P. Mohan, C. V. S. Subrahmanyam and K. Satyanarayana, Synth. Commun., 2014, 44, 574 CrossRef.
- P. Skehan, R. Storeng, D. Scudiero, A. Monks, J. McMahon, D. Vistica, J. T. Warren, H. Bokesch, S. Kenney and M. R. Boyd, J. Natl. Cancer Inst., 1990, 82, 1107 CrossRef CAS PubMed.
- E. Hamel and C. M. Lin, Arch. Biochem. Biophys., 1981, 209, 29 CrossRef CAS PubMed.
- D. Panda, K. Rathinasamy, M. K. Santra and L. Wilson, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9878 CrossRef CAS PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28299g |
| This journal is © The Royal Society of Chemistry 2017 |