Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sub-micron silk fibroin film with high humidity sensibility through color changing
Qingsong Lia,
Ning Qia,
Yu Peng a,
Yafeng Zhange,
Lei Shib,
Xiaohua Zhang*c,
Yuekun Lai
a,
Yafeng Zhange,
Lei Shib,
Xiaohua Zhang*c,
Yuekun Lai a,
Kai Weia,
Ick Soo Kimd and
Ke-Qin Zhang
a,
Kai Weia,
Ick Soo Kimd and
Ke-Qin Zhang *a
*a
aNational Engineering Laboratory for Modern Silk, College of Textile and Clothing Engineering, Soochow University, Suzhou 215123, China. E-mail: kqzhang@suda.edu.cn
bDepartment of Physics, Key Laboratory of Micro and Nano Photonic Structures (MOE), Key Laboratory of Surface Physics, Fudan University, Shanghai 200433, China
cSuzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Ruoshui Road 398, Suzhou 215123, China. E-mail: xhzhang2009@sinano.ac.cn
dNano Fusion Technology Research Lab, Interdisciplinary Cluster for Cutting Edge Research (ICCER), Division of Frontier Fibers, Institute for Fiber Engineering (IFES), Shinshu University, Ueda, Nagano 386 8567, Japan
eNational Laboratory for Infrared Physics, Shanghai Institute of Technical Physics, Chinese Academy of Sciences, Shanghai, 200083, China
First published on 23rd March 2017
Abstract
Use of structural colors for humidity sensors has great potential owing to their not being power driven and having distinct stimulus/color variation properties, but unfortunately most 1D, 2D or 3D photonic crystals have subtle nanostructures which are difficult to fabricate. Here we report a one-layer sub-micron thin film with bright color and high sensitivity to humidity, by spin coating of silk fibroin (SF) solution. The optical properties of the SF film caused by thin film interference can be easily tuned by the coating rates. Due to the high hydrophilicity of SF, the film exhibits fast responses with evident color variation in 5 s. And combined with the large peak red-shifts for more than 130 nm, such a thin film is superior to many other multilayered or photonic crystal based humidity sensors. Considering the good reversibility and durability, these low-cost but highly efficient SF spin coating sensors can realize colorimetric detection of humidity like pH indicator papers, and may have great potential in applications for anti-counterfeit labeling.
1 Introduction
The phenomenon of structural color was first observed on peacock tail feathers, by Robert Hooke in the 17th century.1 Since then, structural color has attracted considerable attention due to its advantageous dye-free coloration and resilience to fading. Structural color widely exists in nature as a result of light interaction with various periodically patterned nanostructures, including diffraction grating, photonic crystals, and multilayer interference.2–8 For example, the spectacular structural coloration of the male Sapphirinid copepods is generated via the regularly alternating layers of hexagonally-shaped guanine crystals and cytoplasm found on their bodies.9 The color varies when viewed from different angles and extends to the ultraviolet region, making their bodies virtually invisible during spiral swimming. The elytra of longhorn beetles Tmesisternus isabellae can change color from golden in a dry environment to red when wet. This is due to the optical interference caused by the swelling of their long, flat, multilayered scales after water infiltration.10 The most well-known display of the structural color effect is the active color change of chameleons, which is produced by tuning a lattice of guanine nanocrystals in their skin in response to the external environment.11 The unique structural colors of these aforementioned organisms provide them with visually striking appearances, allowing them to protect themselves from predators or making them better suited to environmental changes.Optical multilayer structures are notable as a simple, yet important technique for achieving structural coloration. This structure consists of multiple layers of periodically stacked materials with varying refractive indices. Periodic variation-induced interference can even produce results for one-layer structures consisting of simple thin films such as soap bubbles and oil film on water; varying characteristics such as thickness can produce rainbow-like iridescence.12 Compared to photonic crystals, one-layer or multilayer thin films can easily be fabricated via various comparatively simpler methods.13 As the optical properties of these films highly depend on refractive index and layer thickness, they can be customized for specific device applications via variation of film thickness and introduction of other materials. Functional devices can be fabricated using bottom-up approaches based on dip- or spin-coating techniques.14,15 Such tunable coloration opens up applications in controllable release,16 displays,17–19 stimuli responsive sensors,20,21 optical coatings for lenses22–24 and so on. Generally, multilayer thin films are composed of inorganic materials with high refractive indices such as TiO2 or SiO2. However, while these materials usually possess good optical properties, they are difficult to tune, as they show physicochemistry stability, and have limited variation in film thickness or period in response to external stimuli.25 Incorporation of organic, structurally flexible materials can greatly improve the responsivity, but the fabrication of these hybrid films still requires laborious stacking steps. Therefore, it is necessary to develop facile organic polymers with simpler preparation, such that straightforward fabrication of thin films with tunable colors and highly sensitive response to external stimuli can be achieved.
As a natural biomaterial, silk fibroin is environment-friendly and totally biodegradable. Owing to its favourable material properties, SF has been used for many high performance optical devices.26–29 Its easy self-crosslinking and controllable stability make it programmable with desired properties under certain conditions.30 In this paper, we report a novel SF-based sub-micron thin film that exhibits superior humidity-responsive coloration. The film can be rapidly prepared by spin-coating the SF solution, and film interference can be controlled via spin-coating rate. In wet environments, the SF film can quickly absorb water and expand. As a result, there is a remarkable red shift of the reflection spectrum: greater than 130 nm upon achieving over 90% humidity. The color change simultaneously increases with humidity in only several seconds, and the film blue shifts back to its original hue immediately after being placed in a dry environment. Compared with many other silk optical sensors which are complicated either with multicomponent or hierarchical structures,31–33 such simple and low-cost humidity sensors not only show good reversibility and durability, but also can realize fast colorimetric detection of humidity, similarly to pH indicator paper, as well as applications in anti-counterfeit labelling.
2 Experimental details
2.1 Preparation of silk fibroin solution
Cocoons of Bombyx mori silkworm were cut into 10 mm × 10 mm pieces and boiled in an aqueous solution of 0.5% (w/v) Na2CO3 for 40 min, then rinsed thoroughly with distilled water to remove the glue-like sericin proteins. The extracted fibroin bundles were dried in an oven overnight and then dissolved in 9.3 mol L−1 LiBr solution at 60 °C for 1 h. The obtained solution was dialyzed in deionized water using a cellulose dialysis membrane (MWCO 6000–8000 Da, Spectra/Por, USA) at room temperature for 3 days to remove LiBr. The dialyzed silk solution was then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min. Finally, the supernatant with a concentration of 7 wt% was collected and stored at 4 °C for later usage.
000 rpm for 20 min. Finally, the supernatant with a concentration of 7 wt% was collected and stored at 4 °C for later usage.
2.2 Preparation of thin films
The SF films were prepared by spin coating the 7 wt% aqueous solution at 1500, 2000, 2500, 3000, and 3500 rpm for 40 s, onto 20 mm × 20 mm silicon wafers, using a WS-650-23NPP Spin Coater (Laurell Technologies Corp. USA). The film thickness was adjusted based on the coating rate, as discussed in the main text. After spin-coating, all films were solidified in air at room temperature without any heating treatment.2.3 Characterization
A Canon EOS 700D digital camera was used to photograph the films. FTIR spectra were recorded in the range 2000–4000 cm−1 with a Thermo Nicolet 5700 FTIR spectrometer (Thermo Fisher Scientific Inc., USA) to analyse the fibroin conformation. The crystalline structure of the samples was examined using X-ray diffraction (XRD, PANalytical X'pert-PRO, Netherland) in a 2θ range from 4° to 60°. A PG2000-Pro spectrometer (Idea Optics Co., Ltd., China) equipped with a UV-VIS-NIR light source was used to detect the reflectance spectrum within 200–1100 nm at normal incidence. The film thickness and refractive index was measured using an alpha-SE spectroscopic ellipsometer (J. A. Woollam Co., Inc., USA) under various humidities between ∼25% and ∼80% by blowing wet air (by pumping wet air, water molecules taken from water easily condensate on the film surface when humidity is higher than ∼80%). To obtain surface information, atomic force microscopy (AFM) characterization was applied in tapping mode with a Dimension Icon AFM system (Bruker Nano Inc., USA). Scanning electron microscopy (SEM) images were obtained by an S-4800 field emission microscope (Hitachi Ltd., Japan). The contact angle of water droplets on the SF film was measured by a DSA100 drop shape analysis system (Krüss GmbH, Germany). For the anti-counterfeiting label, the Au film approximately 2–3 nm in thickness was sputtered using an E-1045 ion sputter and carbon coating unit for 15 s with a rate of 10 nm min−1 (Hitachi Ltd., Japan).For the reflection measurement, different levels of humidity inside the small chamber was obtained using different saturated salt solutions at about 21 °C, i.e., LiBr, MgCl2, NH4NO3, KCl, or Na2CO3. Relative humidity levels of 7%, 33%, 67%, 85%, and 92% were studied. The SF films were placed inside the chamber, at 3–5 mm above the liquid surface. For the reproducibility test, the humidity inside the 20 cm × 20 cm × 20 cm glass box was controlled by pumping dry nitrogen gas or wet air, set to vary between ∼10% and ∼90%. The humidity was calibrated by a DSR-TH hygrometer (Zoglab Microsystem Co., Ltd, China). All the experiments were carried out at normal atmosphere.
To obtain the water-uptake property, the SF film was prepared at first by evaporating 7 wt% SF solution in fume hood at about 25 °C for more than 3 days. The relative humidities (RH) of 7, 33, 67, 85, 92% was provided by storing saturated solutions as mentioned above in a closed chamber. All the samples were encapsulated in the chamber above the solution for more than 12 h to make sure they absorb water totally. After that the samples were taken out to weigh the mass within 10 s. In order to get the dry weight of all the samples, they were dried in vacuum oven at 140 °C for more than 90 min to remove the water.
3 Results and discussion
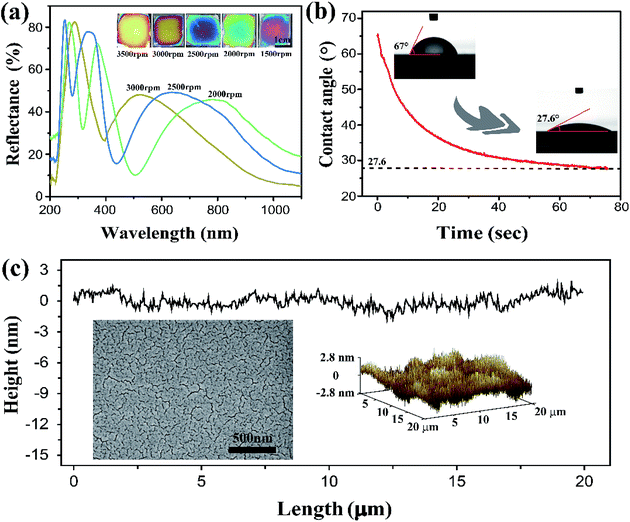
The SF solution was obtained from cocoons of the Bombyx mori silkworm, which was processed through degumming, dissolution and dialysis. By spin-coating the 7 wt% aqueous SF solution at various rates of 1500, 2000, 2500, 3000, and 3500 rpm, sub-micron one-layer films were obtained with thicknesses of ∼414, 312, 258, 207, and 187 nm respectively. These films exhibited bright colors from violet to light orange (Fig. 1a, inset) under ambient conditions, and the reflection spectra collected on the centre of the thin films displayed multiple peaks, owing to the constructive interference over the wide spectrum of incident light ranging from 200 to 1100 nm (Fig. 1a). The Si wafer is smooth and high light-reflecting, it is favourable for SF film to display vivid interference color. In principle, the film color can be adjusted across entire visible spectrum just by carefully tuning the coating rate. However, the films suffered from non-uniformity at the film edge, or for coating rates below 2000 rpm. In the following discussion, most measurements were performed on the samples obtained at 3000 rpm, corresponding with a film color of bright yellow, which allowed for obvious visible detection of red and blue shifting.The hydrophilic property of SF can affect the films' ability to absorb water. As shown in Fig. 1b, the contact angle of a water droplet on the SF film is only ∼65°, and it decreased quickly to ∼27° within 50–70 s, corresponding to good hydrophilicity and high water absorbability. This indicates that the SF films have rapid response to changes in humidity. Furthermore, the SF films have an extremely flat surface, and thus, uniform film thickness that is favourable for displaying bright colors. Fig. 1c shows images from SEM and surface mapping by AFM. Within a length scale of 20 μm, the surface corrugation was measured to be within ∼2.8 nm and the root mean square roughness (Rq) was just 0.79 nm; the SF film is much flatter than some other interference films (for example, roughness was approximately ∼3.7 nm in ref. 34 and ∼20 nm in ref. 35). The flatness is a result of the SF molecules forming a tightly crosslinked network with a nanometer scale pore size.
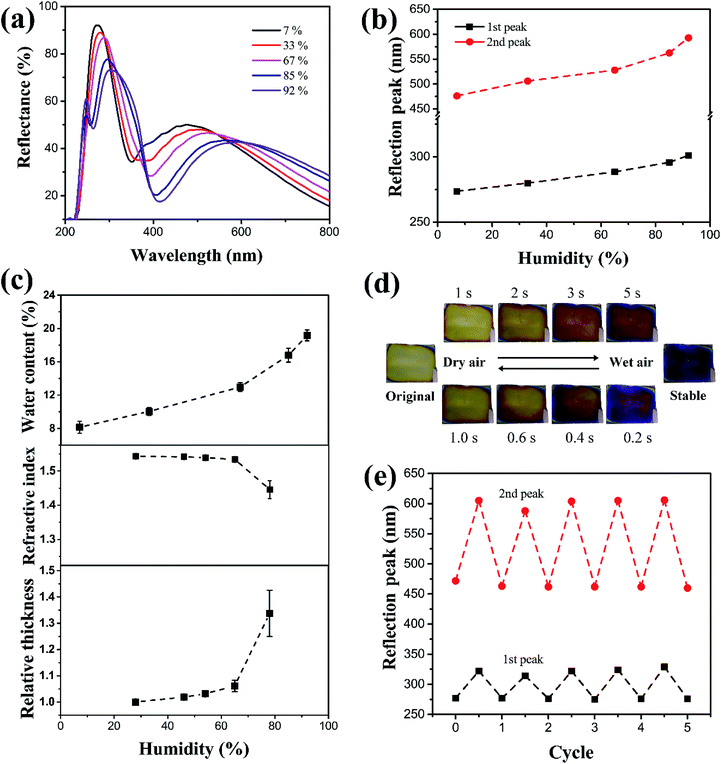
The SF films' sensitivity to humidity changes was characterized in a small closed chamber constructed using a Petri dish (90 mm in diameter and 15 mm in height). A 50 mL saturated salt (LiBr, MgCl2, NH4NO3, KCl, or Na2CO3) solution was placed inside the chamber, and the SF film was placed approximately 3–5 mm above the liquid surface. As the chamber was isolated from outer atmosphere, it was possible to control the humidity according to the fixed partial vapor pressure for a certain salt solution at constant temperature. The humidity was maintained between 7% and 92%. Due to the high hydrophilicity, it was possible to quickly characterize the film's sensitivity to humidity based on the shifting of the reflection peak when the decrease of peak intensity was small; larger shifts in peak wavelength in the visible light corresponded with clearer coloration changes and better identification of humidity. Fig. 2a shows a series of reflection spectra for SF films (coating rate 3000 rpm) kept under humidity of 7%, 33%, 67%, 85%, and 92% for 2 h. The spectrum shows two typical peaks at 273 nm and 476 nm respectively under 7% humidity. Increasing humidity resulted in clear red shifts for the two reflection peaks, accompanied with slight decreases in reflection intensity. The wavelength variations were plotted in Fig. 2b as a function of humidity.
At a relatively low humidity of <70%, the peaks move to 288 nm and 524 nm, and the shifts (Δλ) were no larger than 20 nm and 50 nm for the first and second peaks, respectively. When the humidity was higher than 80%, the peaks shifted to 50 (at 327 nm) and 130 nm (at 624 nm), representing clear color variation, which were even stronger than red shifting in some photonic crystals (the red shift is approximately 90 nm in ref. 36 and less than 50 nm in ref. 37).
The SF film physically absorbed on the Si wafer tightly, and it could not be peeled off from the substrate after drying and swelling for many times. Therefore, we believe that the size change of film in wet state mainly happened in thickness direction, while the expansion in plane direction was the minimal and negligible. The color change stems from two sources: film thickness and refractive index. When humidity increased, more water was absorbed into the SF film, consequently increasing film thickness. Below 70% humidity, the increase in thickness was small (∼5% as compared to the original thickness), because water uptake was limited (less than 13 wt%), as shown in Fig. 2c. When the humidity was higher than 80%, the thickness increased up to 30–35%, with a water uptake of nearly 20 wt%. The equation for Fabry–Perot interference of a dielectric thin film is the following:
2nd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = mλ, θ = mλ, |
In contrast, under high humidity and with water absorbed into the film, the refractive index measured at 632 nm decreased from 1.54 (for pure SF) to ∼1.44 (see Fig. 2c; note the refractive index for water is 1.33). According to thin-film interference, the decrease in refractive index n should theoretically cause a blue shift. However, since the magnitude of refractive index decrease was much smaller (approximately 6%) than the magnitude of thickness increase (30–35%), the overall response showed a remarkable red shift.
Furthermore, the humidity-responsive color change of SF film is very fast, in the range of several seconds. The humidity can be rapidly changed by pumping wet air or dry nitrogen gas into the Petri dish. Wet air caused the humidity to grow very quickly, increasing to nearly ∼90% in tens of seconds. The corresponding color change even took place in the first 5 s, as shown in Fig. 2d. When dry nitrogen was pumped to induce dryness, the film changed back to its original color even faster, at just 1 s. The difference in response time was due to the water-content-dependent thickness change, as shown in Fig. 2c. Only upon a certain water content (about 12–15%), the thickness change becomes much larger. Therefore, upon pumping the wet air, it takes time to reach such critical water content, while the drying makes it very rapid to decrease the water content to be below such content. Such rapid response of several seconds is much faster than many other humidity sensors, where typical response time is dozens of minutes or even longer. As all the films are only hundreds of nanometres in thickness, no difference in response time was observed for films with different thicknesses. Table 1 provides a comparison between SF films with many other multilayer or photonic humidity sensors. Considering the preparation process, this thin film has many advantages for applications in low-cost and fast humidity detections.
| Structures | Materials | Preparation process | Total response time | Peak-shifts | Ref. |
|---|---|---|---|---|---|
| Alternated thin films | PHEMA-co-PGMA, TiO2 | Layer by layer spin coating | ∼150 s | 150 nm | 38 |
| Multilayered Bragg stacks | TiO2, SiO2 | Layer by layer spin coating | — | 11 nm | 39 |
| Photonic crystal microdot | PNIPAm, poly(St-MMA-AA) particles | Inkjet printing, polymerization | 1.8 s | ∼100 nm | 36 |
| Photonic crystal film | Fe3O4@SiO2, PEG | Magnetic assembly of colloidal nanoparticles, photo-polymerization | 3–52 min | 160 nm | 40 |
| 3D photonic crystal | Fe3O4@C nanoparticles, polyacrylamide glycol gel | Magnetically induced self-assembly of colloidal nanoparticles, radical polymerization | 120 min | 153 nm | 41 |
| 3D-ordered microporous film | SiO2 nanoparticles, poly(ionic liquid) | Fabrication of opaline template, infiltration of ionic liquid monomer, photopolymerization, selective dissolution of template | 8 s | 148 nm | 42 |
| Inverse opal hydrogel | PS nanoparticles, polyacrylamide | Fabrication of opaline template, infiltration of monomer, photopolymerization | ∼30 s | 35 nm | 43 |
| One-layer thin film | Silk fibroin | Spin coating for only once | 5 s | 130 nm | Our work |
In addition to humidity sensitivity, the SF film also exhibited high reversibility and durability. To avoid the rapid variation of humidity and air pressure around the film, a larger chamber (a 20 cm × 20 cm × 20 cm glass box) was used to evaluate reversibility. The humidity inside the chamber was tuned from ∼10% to ∼90% by slowly pumping wet air or dry nitrogen, and the films were kept for a sufficiently long period of 1 min under each humidity condition, to allow equilibrium before each measurement. Fig. 2e presents the result of 5 cycles, showing that the reflectance peaks reversely changed with humidity. Thus, the films were concluded to have considerable reversibility and durability, and it is enough for some low-cost disposable humidity sensors.
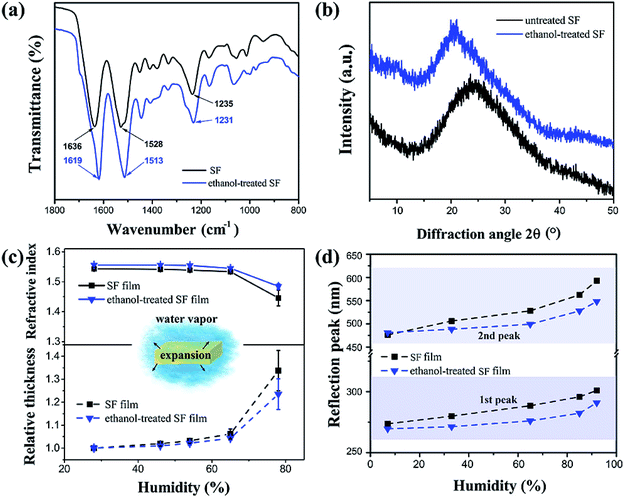
In order to reveal the mechanism for humidity sensitivity at the molecular level, a sample of SF film was treated with ethanol to modify its crystalline structure. After being soaked in ethanol/water (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 v/v), there were measurable changes in the SF film's Fourier transform infrared spectroscopy (FTIR) spectrum (Fig. 3a). The untreated film showed characteristic absorption bands at 1636, 1528, and 1235 cm−1 for the amide I, II, and III groups, representing the typical random coil protein conformation of the silk I structure.44 After the ethanol treatment, these bands shifted to 1619, 1513, and 1231 cm−1 respectively, indicating that the SF conformation had converted to the β-sheet formation of the silk II structure.45 In addition to the differences in FTIR, the X-ray diffraction (XRD) pattern showed a typical wide peak centred at 2θ = 24.7° for the untreated SF film (Fig. 3b), and a slightly narrowed peak centered at 20.7° for the ethanol-treated film. It was clear that the amorphous silk I structure had converted to the β-sheet silk II structure as a result of hydrogen bond rearrangement activated by low dielectric organic solvents.46–48 Typically, the silk II crystalline region contains densely arranged β-sheet macromolecular chains, which prevents water infiltration and thus hinders film expansion. Therefore, when the humidity increased, the thickness increase for the ethanol-treated film was less than 22% (Fig. 3c). The decrease in refractive index was also smaller after the ethanol treatment, decreasing by approximately 5% from 1.55 to 1.475. As shown in Fig. 3d, the wavelength changes (Δλ) were approximately 25 (from 270 nm to 292 nm) and 70 nm (from 479 nm to 548 nm) for the first and second peaks, respectively, at the 92% humidity. Therefore, the ethanol-treated SF films showed much smaller red shifts than the peak shifting for the untreated SF film (approximately 50 and 130 nm respectively).
25 v/v), there were measurable changes in the SF film's Fourier transform infrared spectroscopy (FTIR) spectrum (Fig. 3a). The untreated film showed characteristic absorption bands at 1636, 1528, and 1235 cm−1 for the amide I, II, and III groups, representing the typical random coil protein conformation of the silk I structure.44 After the ethanol treatment, these bands shifted to 1619, 1513, and 1231 cm−1 respectively, indicating that the SF conformation had converted to the β-sheet formation of the silk II structure.45 In addition to the differences in FTIR, the X-ray diffraction (XRD) pattern showed a typical wide peak centred at 2θ = 24.7° for the untreated SF film (Fig. 3b), and a slightly narrowed peak centered at 20.7° for the ethanol-treated film. It was clear that the amorphous silk I structure had converted to the β-sheet silk II structure as a result of hydrogen bond rearrangement activated by low dielectric organic solvents.46–48 Typically, the silk II crystalline region contains densely arranged β-sheet macromolecular chains, which prevents water infiltration and thus hinders film expansion. Therefore, when the humidity increased, the thickness increase for the ethanol-treated film was less than 22% (Fig. 3c). The decrease in refractive index was also smaller after the ethanol treatment, decreasing by approximately 5% from 1.55 to 1.475. As shown in Fig. 3d, the wavelength changes (Δλ) were approximately 25 (from 270 nm to 292 nm) and 70 nm (from 479 nm to 548 nm) for the first and second peaks, respectively, at the 92% humidity. Therefore, the ethanol-treated SF films showed much smaller red shifts than the peak shifting for the untreated SF film (approximately 50 and 130 nm respectively).
Fig. 4 presents a schematic illustration of the mechanism of humidity responses for SF films, proposed based on the above observations. SF is a hydrophilic–hydrophobic–hydrophilic polymer with alternating hydrophobic crystallite regions and hydrophilic amorphous blocks.49 The SF film contains amino acids with polar side groups, i.e. Ser, Tyr, Glu, and Asp, which have strong affinity to water, with the ability to absorb water molecules even in dry environments.50 In an untreated film, the fibroin polymers become entangled and form an amorphous structure; only few β-sheet crystalline domains are embedded randomly.51 According to our observations, the water absorption and film expansion are divided into two stages. In the first stage, when the humidity is lower than 70%, water molecules are physically, preferentially absorbed into the pores inside of the film without breakage of chemical bonds. This results in a small volume expansion. This behaviour is similar to the reaction of the ethanol-treated film, and thus, the overall red shifting is small (Fig. 3d).
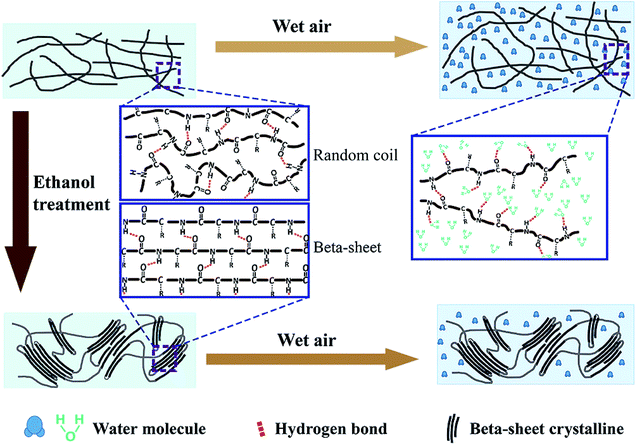 | ||
| Fig. 4 A schematic illustration showing the mechanism of humidity responses for the untreated and ethanol-treated SF films. | ||
When the humidity is higher than 80%, a second stage is activated, with many water molecules infiltrating into the internal pores of the cross-linked polymers. The water molecules de-bond the original hydrogen bonds between the fibroin polymers, and new hydrogen bonds form between the water molecule and the SF.52,53 The opening of cross-linked polymer chains results in volumetric expansion, therefore increasing the film thickness. However, due to the entanglement of long fibroin chains, the film cannot expand without limitation, but is restricted to a certain extent after water absorption equilibrium. Conversely, when the environment becomes drier, the infiltrated water molecules evaporate and the polymer chains cross-link again due to reformation of hydrogen bonds between the SF molecules. This process results in volume recovery and a decrease of thickness.
Ethanol treatment can modify the wetting process by increasing β-sheet crystalline domains. Following ethanol treatment, the hydrophobic blocks in the random coils of fibroin can assemble and organize to form micelles, eventually rearranging into regular and stable β-sheet crystallites.53 These insoluble crystallites can prevent the infiltration of water, subsequently hindering volumetric expansion.
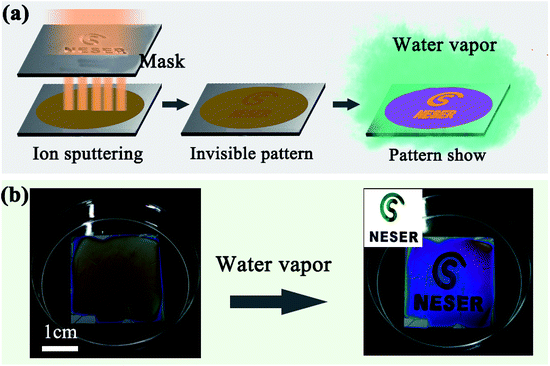
Due to their humidity responsivity and color variation characteristics, the simple SF film has a promising potential in a wide range of applications. Anti-counterfeit labelling applicability was demonstrated by locally suppressing the film expansion under high humidity. Part of the film surface was masked with a hydrophobic thin film to block water infiltration; under a wet environment, the masked region maintained its original color while color changes occurred in the unmasked part. Here, the masked region was prepared by ion sputtering a thin Au film using a patterned mask (Fig. 5a). As the Au film was only 2–3 nm in thickness, it was almost transparent and invisible on the silk film. When the film was placed under water vapor, the thin Au film prevented the infiltration of water molecules, while the unmasked region expanded and quickly changed color (Fig. 5b).
4 Conclusions
In conclusion, we demonstrated a facile procedure to fabricate sub-micron SF films with high humidity sensitivity indicated by quick color changing. The film color was tuned by adjusting the spin coating rate, and changed rapidly in just a few seconds in response to changes in environment humidity. At higher humidity above 80%, the red shift of the reflectance peak in the visible spectrum was even larger than 130 nm, corresponding to a significant color change as distinct as yellow to violet. As the volumetric expansion was induced by the de-bonding of hydrogen bonds between fibroin polymers during the water infiltration, subsequent water evaporation did not change the intrinsic molecular structure of the polymers. Consequently, the SF film exhibited superior reversibility and durability, and the response time and peak red-shifts are superior to many other conventional structural color based humidity sensors. An anti-counterfeit labelling application was demonstrated during experimentation, showing these coloration sensors to be highly applicable in various high performance optical devices.Acknowledgements
We gratefully thank the financial support from the National Science Foundation of China (51373110, 11404064, 51561145008, 51503137), Shanghai Pujiang Program (14PJ1401100), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, Qing Lan Project for Excellent Scientific and Technological Innovation Team of Jiangsu Province (2012), Project for Jiangsu Scientific and Technological Innovation Team (2013), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2015256).Notes and references
- P. Ball, Sci. Am., 2012, 306, 74–79 CrossRef PubMed.
- P. Vukusic and J. R. Sambles, Nature, 2003, 424, 852–855 CrossRef CAS PubMed.
- P. Vukusic, Phys. World, 2004, 17, 35–39 CrossRef.
- Y. Zhao, Z. Xie, H. Gu, C. Zhu and Z. Gu, Chem. Soc. Rev., 2012, 41, 3297–3317 RSC.
- H. Yin, B. Dong, X. Liu, T. Zhan, L. Shi, J. Zi and E. Yablonovitch, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 10798–10801 CrossRef CAS PubMed.
- Q. S. Li, Q. Zeng, L. Shi, X. H. Zhang and K. Q. Zhang, J. Mater. Chem. C, 2016, 4, 1752–1763 RSC.
- S. Kinoshita and S. Yoshioka, ChemPhysChem, 2005, 6, 1442–1459 CrossRef CAS PubMed.
- A. R. Parker, R. C. McPhedran, D. R. McKenzie, L. C. Botten and N. Nicorovici, Nature, 2001, 409, 36–37 CrossRef CAS PubMed.
- D. Gur, B. Leshem, M. Pierantoni, V. Farstey, D. Oron, S. Weiner and L. Addadi, J. Am. Chem. Soc., 2015, 137, 8408–8411 CrossRef CAS PubMed.
- F. Liu, B. Q. Dong, X. H. Liu, Y. M. Zheng and J. Zi, Opt. Express, 2009, 17, 16183–16191 CrossRef CAS PubMed.
- J. Teyssier, S. V. Saenko, D. van der Marel and M. C. Milinkovitch, Nat. Commun., 2015, 6, 6368 CrossRef CAS PubMed.
- H. M. Princen and S. G. Mason, J. Colloid Sci., 1965, 20, 453–463 CrossRef CAS.
- J. J. Richardson, M. Bjornmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- L. D. Bonifacio, B. V. Lotsch, D. P. Puzzo, F. Scotognella and G. A. Ozin, Adv. Mater., 2009, 21, 1641–1646 CrossRef CAS.
- M. Kolle, A. Lethbridge, M. Kreysing, J. J. Baumberg, J. Aizenberg and P. Vukusic, Adv. Mater., 2013, 25, 2239–2245 CrossRef CAS PubMed.
- B. V. Lotsch, C. B. Knobbe and G. A. Ozin, Small, 2009, 5, 1498–1503 CrossRef CAS PubMed.
- J. J. Walish, Y. Kang, R. A. Mickiewicz and E. L. Thomas, Adv. Mater., 2009, 21, 3078–3081 CrossRef CAS.
- A. T. Exner, I. Pavlichenko, B. V. Lotsch, G. Scarpa and P. Lugli, ACS Appl. Mater. Interfaces, 2013, 5, 1575–1582 CAS.
- L. Tong, W. Qi, M. Wang, R. Huang, R. Su and Z. He, Small, 2016, 12, 3433–3443 CrossRef CAS PubMed.
- Z. H. Wang, J. H. Zhang, J. X. Li, J. Xie, Y. F. Li, S. Liang, Z. C. Tian, C. A. Li, Z. Y. Wang, T. Q. Wang, H. Zhang and B. Yang, J. Mater. Chem., 2011, 21, 1264–1270 RSC.
- M. E. Calvo, S. Colodrero, N. Hidalgo, G. Lozano, C. Lopez-Lopez, O. Sanchez-Sobrado and H. Miguez, Energy Environ. Sci., 2011, 4, 4800–4812 Search PubMed.
- M. S. Park, Y. Lee and J. K. Kim, Chem. Mater., 2005, 17, 3944–3950 CrossRef CAS.
- J. A. Hiller, J. D. Mendelsohn and M. F. Rubner, Nat. Mater., 2002, 1, 59–63 CrossRef CAS PubMed.
- H. K. Raut, V. A. Ganesh, A. S. Nair and S. Ramakrishna, Energy Environ. Sci., 2011, 4, 3779–3804 CAS.
- C. Yao, J. Ren, C. Liu, T. Yin, Y. Zhu and L. Ge, ACS Appl. Mater. Interfaces, 2014, 6, 16727–16733 CAS.
- S. T. Parker, P. Domachuk, J. Amsden, J. Bressner, J. A. Lewis, D. L. Kaplan and F. G. Omenetto, Adv. Mater., 2009, 21, 2411–2415 CrossRef CAS.
- S. Ling, C. Li, K. Jin, D. L. Kaplan and M. J. Buehler, Adv. Mater., 2016, 28, 7783–7790 CrossRef CAS PubMed.
- F. G. Omenetto and D. L. KapLan, Nat. Photonics, 2008, 2, 641–643 CrossRef CAS.
- S. Kim, A. N. Mitropoulos, J. D. Spitzberg, H. Tao, D. L. Kaplan and F. G. Omenetto, Nat. Photonics, 2012, 6, 818–823 CrossRef CAS.
- H. Tao, J. M. Kainerstorfer, S. M. Siebert, E. M. Pritchard, A. Sassaroli, B. J. Panilaitis, M. A. Brenckle, J. J. Amsden, J. Levitt, S. Fantini, D. L. Kaplan and F. G. Omenetto, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 19584–19589 CrossRef CAS PubMed.
- M. Lee, H. Jeon and S. Kim, Nano Lett., 2015, 15, 3358–3363 CrossRef CAS PubMed.
- H. Kwon and S. Kim, ACS Photonics, 2015, 2, 1675–1680 CrossRef CAS.
- Y. Y. Diao, X. Y. Liu, G. W. Toh, L. Shi and J. Zi, Adv. Funct. Mater., 2013, 23, 5373–5380 CrossRef CAS.
- M. Morozova, P. Kluson, J. Krysa, P. Dzik, M. Vesely and O. Solcova, Sens. Actuators, B, 2011, 160, 371–378 CrossRef CAS.
- A. V. Yakovlev, V. A. Milichko, V. V. Vinogradov and A. V. Vinogradov, ACS Nano, 2016, 10, 3078–3086 CrossRef CAS PubMed.
- L. B. Wang, J. X. Wang, Y. Huang, M. J. Liu, M. X. Kuang, Y. F. Li, L. Jiang and Y. L. Song, J. Mater. Chem., 2012, 22, 21405–21411 RSC.
- L. Bai, Z. Xie, W. Wang, C. Yuan, Y. Zhao, Z. Mu, Q. Zhong and Z. Gu, ACS Nano, 2014, 8, 11094–11100 CrossRef CAS PubMed.
- Z. H. Wang, J. H. Zhang, J. Xie, C. A. Li, Y. F. Li, S. Liang, Z. C. Tian, T. Q. Wang, H. Zhang, H. B. Li, W. Q. Xu and B. Yang, Adv. Funct. Mater., 2010, 20, 3784–3790 CrossRef CAS.
- I. Pavlichenko, A. T. Exner, M. Guehl, P. Lugli, G. Scarpa and B. V. Lotsch, J. Phys. Chem. C, 2012, 116, 298–305 CAS.
- R. Y. Xuan, Q. S. Wu, Y. D. Yin and J. P. Ge, J. Mater. Chem., 2011, 21, 3672–3676 RSC.
- H. Hu, Q.-W. Chen, K. Cheng and J. Tang, J. Mater. Chem., 2012, 22, 1021–1027 RSC.
- J. Huang, C. A. Tao, Q. An, C. X. Lin, X. S. Li, D. Xu, Y. G. Wu, X. G. Li, D. Z. Shen and G. T. Li, Chem. Commun., 2010, 46, 4103–4105 RSC.
- R. A. Barry and P. Wiltzius, Langmuir, 2006, 22, 1369–1374 CrossRef CAS PubMed.
- H.-Y. Wang and Y.-Q. Zhang, Soft Matter, 2013, 9, 138–145 RSC.
- M. Sonoyama and T. Nakano, Appl. Spectrosc., 2000, 54, 968–973 CrossRef CAS.
- M. B. Dickerson, S. P. Fillery, H. Koerner, K. M. Singh, K. Martinick, L. F. Drummy, M. F. Durstock, R. A. Vaia, F. G. Omenetto, D. L. Kaplan and R. R. Naik, Biomacromolecules, 2013, 14, 3509–3514 CrossRef CAS PubMed.
- X. Chen, H. Cai, S. Ling, Z. Shao and Y. Huang, Appl. Spectrosc., 2012, 66, 696–699 CrossRef CAS PubMed.
- X. Hu, K. Shmelev, L. Sun, E.-S. Gil, S.-H. Park, P. Cebe and D. L. Kaplan, Biomacromolecules, 2011, 12, 1686–1696 CrossRef CAS PubMed.
- H.-J. Jin and D. L. Kaplan, Nature, 2003, 424, 1057–1061 CrossRef CAS PubMed.
- Z. B. Cao, X. Chen, J. R. Yao, L. Huang and Z. Z. Shao, Soft Matter, 2007, 3, 910–915 RSC.
- Y. Cheng, L.-D. Koh, D. Li, B. Ji, M.-Y. Han and Y.-W. Zhang, J. R. Soc., Interface, 2014, 11, 20140305 CrossRef PubMed.
- S. Y. Cho, Y. S. Yun, S. Lee, D. Jang, K. Y. Park, J. K. Kim, B. H. Kim, K. Kang, D. L. Kaplan and H. J. Jin, Nat. Commun., 2015, 6, 7145 CrossRef PubMed.
- S. Ryu, H. H. Kim, Y. H. Park, C. C. Lin, I. C. Um and C. S. Ki, J. Mater. Chem. B, 2016, 4, 4574–4584 RSC.
| This journal is © The Royal Society of Chemistry 2017 |