Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, characterization and photovoltaic studies of oligo(acriflavine) via chemical oxidative polymerization
Feyza Kolcu*ab and
İsmet Kaya *a
*a
aÇanakkale Onsekiz Mart University, Department of Chemistry, Polymer Synthesis and Analysis Laboratory, 17020, Çanakkale, Turkey. E-mail: feyzakolcu@comu.edu.tr; kayaismet@hotmail.com; Fax: +90 286 218 05 33
bÇanakkale Onsekiz Mart University, Lapseki Vocational School, Department of Chemistry and Chemical Processing Technologies, Çanakkale, Turkey
First published on 30th January 2017
Abstract
The present work reports a simple and inexpensive way for the synthesis of a novel water-soluble conjugated oligo(acriflavine) (OAC). Oligo(acriflavine) was synthesized via chemical oxidative polycondensation by H2O2 (30%) as the oxidant at an optimum reaction temperature of 110 °C without use of an additional external template. The chemical structure of the synthesized compound was confirmed by FT-IR, UV-Vis, 1H-NMR and 13C-NMR techniques. Further characterization was employed using, photophysical, electrochemical, photovoltaic thermogravimetric-differential thermal analysis (TG-DTA), fluorescence (PL), cyclic voltammetry (CV) and differential scanning calorimetry (DSC) measurements. The number average molecular weight of the oligomer was found to be 4950 Da with a polydispersity index of 1.18. Photoluminescence (PL) properties of the synthesized materials were determined in solution form. The solvent effect on the optical properties of OAC was studied in selected solvents. The spectral analysis displayed a bicolor emission behavior when OAC solution was irradiated at different wavelengths. The HOMO–LUMO energy levels and electrochemical (E′g) band gap values of OAC were determined by CV measurement. Optical and electrochemical band gaps of OAC with more conjugated structure were lower than for AC. XRD analysis signified that the oligomer was in a semi-crystalline form. To further study the morphologic properties of the OAC, Scanning Electron Microscopy (SEM) images were illustrated at different amplifications. It was demonstrated that the structure of OAC was essentially a ladder oligomer with phenazine rings. The best performing photovoltaic cell, based on OAC reached a Jsc of 0.14 mA cm−2, a Voc of 0.37 V, and a FF of 0.50, giving a power conversion efficiency (% η) of 0.027% under AM 1.5 irradiation (100 mW cm−2).
Introduction
Conducting polymers have been recently investigated for the production of technological applications.1–4 The area of applications encloses the production of biosensors,5 chemical gas sensors,6 electrochromic displays,7,8 photovoltaic and optical devices,9 light-emitting diodes and field effect transistors.10–13 Materials with a delocalized π-electron system absorb sunlight and transport charge carriers. This is now true of materials within the field of inherently conducting polymers like polyconjugated polymers using chemical oxidation polymerization. Interest in controlling electroactive organic materials, such as conducting polymers has recently grown. Solar cell applications triggered the search for the incorporation of semiconductors into dye-synthesized solar cells. To construct dye-sensitized solar cells, coumarine-,14,15 indoline-,16,17 cyanine18- and hemicyanine19,20- based dyes have recently been employed in photovoltaic applications.Water–alcohol soluble conjugated polymers have attracted considerable attention owing to their important applications in polymer solar cells as cathode interlayers and in PLEDs, organic photovoltaics, polymer cells as well as in drug delivery and detection of biomacromolecules.21–25 Oxidative polymerization (OP) commonly used is one of the suitable techniques to prepare conjugated conductive polymers. Using OP, the solubility problem of a conductive polymer can be overcome using amino-functionalized conjugated polymers that are soluble in alcohol in the presence of trace acetic acid due to weak interaction between the nitrogen atom of amino group and acetic acid.26
On the other hand, highly crystalline conjugated polymers are very important for efficient charge carrier transport, and a crystalline structure of the polymers will effectively enhance performance of their electronic or optical devices.27 Several crystalline conjugated polymers have been synthesized using chemical routes.28–30 Photovoltaic (PV) devices emerge as an attractive way of converting energy directly from the sunlight. Among all types of photovoltaic devices, polymer-based organic solar cells (OSCs) are particularly intriguing since they have the advantages of low production cost, processing at room temperature, mechanical flexibility, and suitability for mass production in addition to being relatively quiet, not producing toxic substances or greenhouse gases, and requiring little maintenance during operations.31
Encouraged by these explanations in this article we report the synthesis and characterization of π-conjugated structure of acriflavine (AC) having phenazine units in its backbone. AC is a dye used as antiseptic agent, bactericidal inhibitory, genetically active agent and reagent for drug analyses.32–34
A novel water-soluble conjugated polymer is presented by containing same side-chains bearing primary amino (–NH2) groups responsible for dissolving in aqueous solution. Chemical oxidative polymerization of aromatic diamine-AC where the nitrogen atoms of diamine group were the most susceptible site for the polymerization was simple and environmentally friendly. Polymer consists of purely head-to-tail or ortho-coupled coupled acriflavine monomers by employing the direct chemical oxidation of acriflavine.
Based on the foregoing information, we aimed to develop the potential applications of this new polymer in various disciplines, due to the fluorescent probe properties and high emission quantum yields. UV-Vis, CV, TGA, DSC, and PL techniques were carried out to characterize the photo physical, electrochemical and thermal properties of the new system. The electronic properties were analyzed using UV-Vis absorption spectrophotometer, fluorescence emission spectroscopy and cyclic voltammetry.
Experimental
Materials
Acriflavine (AC; 3,6-diamino-10-methylacridinium chloride) was purchased from Sigma-Aldrich Co. (USA). Methanol (MeOH), ethanol, acetic acid, chloroform (CHCl3), tetrahydrofurane (THF), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO), toluene, acetonitrile, 30% aqueous solution of hydrogen peroxide (H2O2) and all other solvents used were supplied by Merck Chemical Co. (Germany), and used as received without any purification.The synthesis of oligo-acriflavine (OAC)
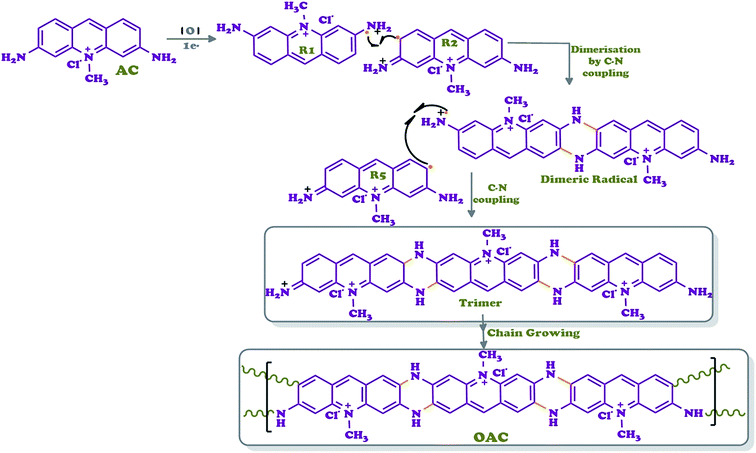
Acriflavine (AC) (0.01 mol, 2.59 g) dissolved in 40 mL of methanol was polymerized in 250 mL two-necked round bottom flask connected to a condenser by adding 3 drops of acetic acid and a solution of 1 mL of H2O2 dropwise within 30 min, respectively. The reaction was refluxed for 2 days at 105 °C. Then, the reaction mixture was cooled to room temperature to get the oxidative oligomer (OAC) of acriflavine. The black color precipitate was filtered and the unreacted monomer was removed by washing with ethanol (3 × 25 mL). The black product was then kept overnight in an oven at 60 °C for drying (yield 69%). Scheme 1 outlined the general synthetic route of OAC.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, aromatic ring), 1594 ν(C
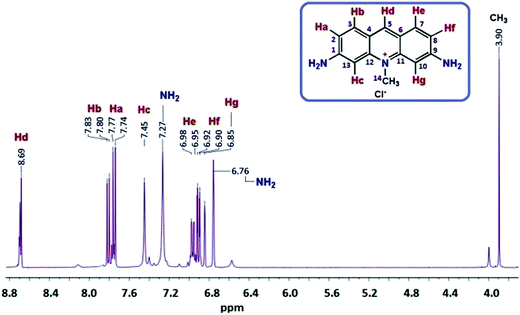
N, aromatic ring), 1594 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C phenyl), 1326 ν(C–N). 1H NMR (DMSO-d6): δH ppm, 8.69 (d, 1H, Hd), 7.81 (d, 1H, Hb), 7.76 (d, 1H, Ha), 7.45 (s, 1H, Hc), 6.97 (d, 1H, He), 6.91 (d, 1H, Hf), 6.85 (s, 1H, Hg), 7.27, 6.76 (s, 4H, 2NH2), 3.90 (s, 3H, CH3). 13C NMR (DMSO-d6): δ ppm, 157.88 (C1), 156.60 (C9), 144.09 (C11), 143.2 (C12), 142.93 (C5), 134.09 (C3), 132.18 (C7), 118.26 (C4), 117.2 (C6), 116.92 (C13), 116.89 (C10), 94.8 (C2), 94.03 (C8), 23.1 (C14).
C phenyl), 1326 ν(C–N). 1H NMR (DMSO-d6): δH ppm, 8.69 (d, 1H, Hd), 7.81 (d, 1H, Hb), 7.76 (d, 1H, Ha), 7.45 (s, 1H, Hc), 6.97 (d, 1H, He), 6.91 (d, 1H, Hf), 6.85 (s, 1H, Hg), 7.27, 6.76 (s, 4H, 2NH2), 3.90 (s, 3H, CH3). 13C NMR (DMSO-d6): δ ppm, 157.88 (C1), 156.60 (C9), 144.09 (C11), 143.2 (C12), 142.93 (C5), 134.09 (C3), 132.18 (C7), 118.26 (C4), 117.2 (C6), 116.92 (C13), 116.89 (C10), 94.8 (C2), 94.03 (C8), 23.1 (C14).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, aromatic ring), 1596 ν(C
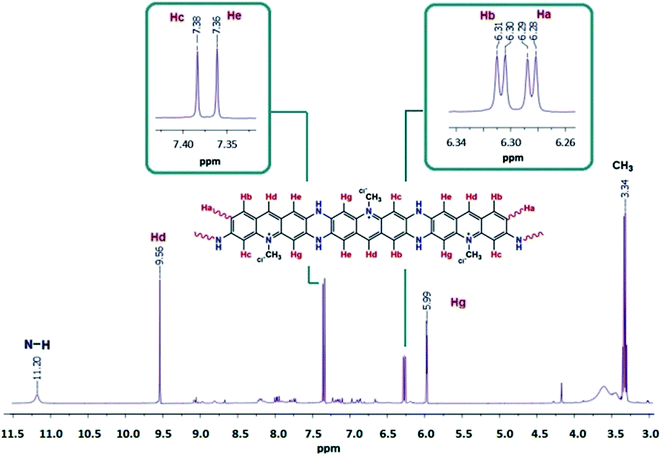
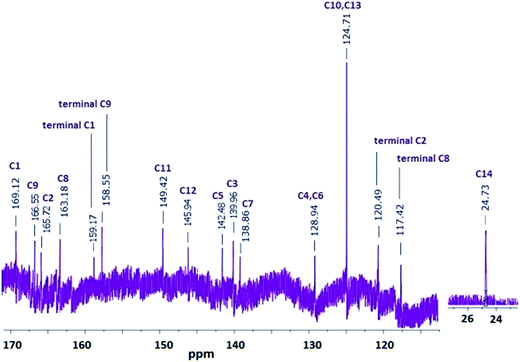
N, aromatic ring), 1596 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C phenyl). 1H NMR (DMSO-d6): δH ppm, 11.20 (s, 2H, aromatic N–H), 9.56 (s, 2H, Hd), 7.83 (d, 2H, Hb), 7.38 (s, 2H, Hc), 7.36 (s, 2H, He), 6.31 (d, 2H, Hb), 6.29 (d, 2H, Ha), 5.99 (s, 2H, Hg), 3.34 (s, 3H, CH3). 13C NMR (DMSO-d6): δ ppm, 169.12 (C1), 166.55 (C9), 165.72 (C2), 163.18 (C8), 159.17 (terminal C1), 158.55 (terminal C9), 149.42 (C11), 144.94 (C12), 142.48 (C5), 139.96 (C3), 138.86 (C7), 128.94 (C4, C6), 124.71 (C10, C13), 120.49 (terminal C2), 117.42 (terminal C8), 23.1 (C14).
C phenyl). 1H NMR (DMSO-d6): δH ppm, 11.20 (s, 2H, aromatic N–H), 9.56 (s, 2H, Hd), 7.83 (d, 2H, Hb), 7.38 (s, 2H, Hc), 7.36 (s, 2H, He), 6.31 (d, 2H, Hb), 6.29 (d, 2H, Ha), 5.99 (s, 2H, Hg), 3.34 (s, 3H, CH3). 13C NMR (DMSO-d6): δ ppm, 169.12 (C1), 166.55 (C9), 165.72 (C2), 163.18 (C8), 159.17 (terminal C1), 158.55 (terminal C9), 149.42 (C11), 144.94 (C12), 142.48 (C5), 139.96 (C3), 138.86 (C7), 128.94 (C4, C6), 124.71 (C10, C13), 120.49 (terminal C2), 117.42 (terminal C8), 23.1 (C14).Characterization techniques
The solubility tests were applied to OAC (1 mg) in different solvents (1 mL) at room temperature. The infrared (FT-IR) spectra were recorded using a Perkin Elmer FT-IR Spectrum One with the universal ATR sampling attachment (4000–650 cm−1) to study the chemical structures of AC and OAC. 1H and 13C-NMR spectra were recorded by using Bruker Avance DPX (USA) operating at the range of 400 and 100.6 MHz, respectively. The samples were dissolved in deuterated DMSO, and tetramethylsilane (TMS) was used as an internal standard at 25 °C. Thermogravimetric and Differential Thermal Analyses (TG-DTA) were carried out between 20–1000 °C using Perkin Elmer Diamond Thermal Analysis System. TG-DTA analyses were carried out at heating rate of 10 °C min−1 in a platinum crucible under nitrogen atmosphere (100 mL min−1). Perkin Elmer Sapphire Differential Scanning Calorimetry was placed in a covered aluminum pan with a 15 mg of the synthesized oligomer for DSC experiment between 25 and 420 °C (in N2, rate 10 °C min−1). The number average molecular weight (Mn), weight average molecular weight (Mw) and polydispersity index (PDI) of OAC were determined by Gel Permeation Chromatography-Light Scattering (GPC-LS) device by Malvern Viscotek GPC Dual 270 max. For GPC investigations a medium 300 × 8.00 mm Dual column addition 1 g L−1 of lithium bromide in DMF (1 mL min−1) was used as solvent. A Light Scattering Detector (LS) and a refractive index detector (RID) were used to analyze the products at 55 °C. X-ray diffraction (XRD) measurements were recorded by a PANalytical empyrean model X-ray diffractometer instrument (The Netherlands) with CuKα radiation at a wavelength of 1.54 Å over a range of 2θ from 5° to 90° with a scanning rate of 4° min−1.Optical properties
In order to investigate the optical properties of AC and OAC, the optical band gaps (Eg) recorded in Ultraviolet-visible (UV-vis) spectra (UK) were calculated from their absorption edges. Using Analytikjena Specord 210 Plus, the absorption spectra of the AC and OAC were recorded in selected solvents like DMF, DMSO, MeOH and water at 25 °C. The emission and excitation spectra were obtained by using a Shimadzu RF-5301PC spectrofluorophotometer (Japan) in different solvents including DMF, DMSO, methanol and water with 0.1 g L−1 as solution concentrations for all measurements. Photoluminescence (PL) spectra were obtained with a slit width of 3 nm. Fluorescence quantum yields (QYs) were determined using the comparative method as described in the literature.35,36 Fluorescein solution in 0.1 M of aqueous NaOH was used as a standard.Electrochemical properties
Cyclic voltammetry (CV) measurements were performed with a CHI 660C Electrochemical Analyzer (Texas, USA) at a potential scan rate of 25 mV s−1 to investigate electrochemical properties of AC and OAC. All the experiments were carried out in a dry box filled with argon atmosphere at room temperature. Silver wire as a reference electrode, glassy carbon electrode (GCE; d = 0.3 cm in diameter) as a working electrode and platinum wire as a counter electrode were used for all the measurements. The electrochemical potential of Ag was calibrated with respect to the ferrocene/ferrocenium (Fc/Fc+) couple. The half-wave potentials (E1/2) of (Fc/Fc+) in 0.1 M tetrabutylammoniumhexafluorophosphate (TBAPF6)/acetonitrile solution are 0.39 V and 0.38 V vs. Ag wire and supporting calomel electrolyte, respectively. The voltammetric measurements were carried out in acetonitrile for AC and DMSO/acetonitrile mixture, respectively. The electrochemical HOMO and LUMO energy levels and band gaps (E′g) of OAC were calculated from the oxidation and reduction onset values.37The photovoltaic characteristic of oligomer
The photovoltaic characteristics were studied by the illumination-dependent current–voltage measurements. Current–voltage characteristic of a solar cell gives information about maximum power point (MPP).38 The dye-sensitized solar cell was prepared using commercial Solaronix test cell kit (Solaronix 74992). TiO2 electrode was immersed into a 1 mM DMSO solution of OAC for 24 hours. Then, the dye layer was washed with ethanol and let to dry at room temperature. Subsequently, this electrode was clamped together with a Pt electrode (Solaronix) and applied Solaronix-AN50 electrolyte solution onto the interface between the two plates. The photocurrent–voltage characteristics of the prepared cell as a function of light intensity were monitored using MTI-BST8-STAT-EIS-LD, Single Channel Potentiostat/Galvanostat with Electrochemical Impedance Spectroscopy (USA) and under AM1.5 standard condition.Morphological and electrical properties
Scanning electron microscopy (SEM) photographs of oligomer were recorded using a JEOL JSM 7100F instrument (Japan) to observe morphology of oligomer particles. The oligomer solution was sprayed on an aluminum surface using a custom built electrospinning setup, which consisted of a 2.5 mL syringe containing the oligomer solution driven out of using an NE-300 single syringe pump (Pump Systems Inc., USA). A metal 25-gauge syringe needle tip and collecting plate were connected to a high voltage power supply (model 73030P, Genvolt Ltd., UK). The applied voltage was set at 7 kV and kept constant for all experiments. A flow rate of 1 mL h−1 was used in order to create a stable system.Solid state electrical conductivity of OAC in the form of a pellet was measured by four-point probe technique at room temperature and atmospheric pressure using Keithley 2400 Electrometer instrument. The oligomer was pressed in a hydraulic pressure developing up to 1687.2 kg cm−2 to prepare a pellet form.
Results and discussion
Synthesis and characterization of OAC
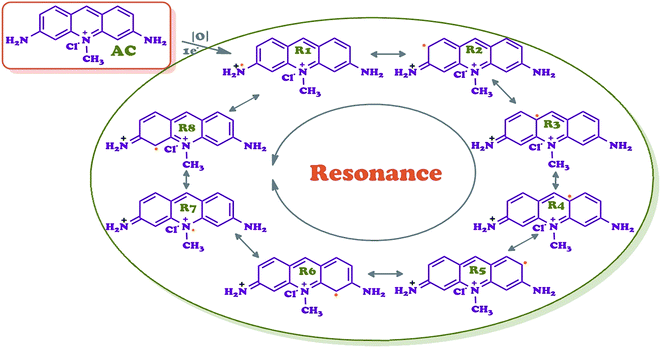
The oxidative polymerization of orange-colored acriflavine with H2O2 in methanol yielded an oligomer, OAC, as shown in Scheme 1. OAC was isolated as black-colored powder form with yield of 69%. This fact is an indication that the coupling of acriflavine molecules to chains took place. OAC was completely soluble in highly aprotic polar solvents, DMSO, DMF and THF in spite of partly solubility in some polar solvents, such as H2O and ethanol. While the polarity of the solvent was reduced, like dichloromethane, hexane and heptane, OAC became insoluble.Eight main coupling modes for the polymerization of acriflavine (AC) were identified and given in Scheme 2. The most probable coupling sites for the polymerization were through the radical formed as R1 and R2. AC was unit structure linked to each other via the C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C–NH–C bonds. In such a way that two acridine units could be regarded as forming one pyrazine ring as result of an intramolecular cyclization reaction, leading to the ladder-like structure.
C or C–NH–C bonds. In such a way that two acridine units could be regarded as forming one pyrazine ring as result of an intramolecular cyclization reaction, leading to the ladder-like structure.
Therefore, R1 and R2 radicals could favor the formation of pyrazine ring during the polymerization via C–N coupled dimer (Scheme 1). The formation of a cycle reduces the energy of the molecule owing to the generation of an aromatic structure.39 The ortho position was subsequently oxidized to form phenazine-like moieties along the backbone of the polymer. Phenazine cycles could further participate in the subsequent chain growth. In this way, a phenazine-containing oligomer is able to initiate the chain growth. The computational results suggest that intramolecular cyclization is thermodynamically favorable, leading to the formation of ladder segments during the course of oxidative polymerization of aromatic diamine polymer growth mechanism.40
FT-IR spectroscopy was used to confirm the chemical structure of AC and OAC. The peaks at 3300 and 3169 cm−1 attributed to the N–H stretching vibration of the –NH2 groups were replaced by one broader band at 3154 cm−1 for OAC which signified the transformation of the primary amine to a secondary amine through polymerization that took place on nitrogen atoms of primary amine groups and resulted in forming the C–N coupled structure. The absorption peak at 3028 cm−1 ascribable to the stretching modes of aromatic C–H bond was observed at 3034 cm−1 in the oligomer structure of OAC. The peak bands at 2844 cm−1 and 2905 cm−1 were corresponded to the aliphatic methylene (–CH3) of AC and OAC, respectively. FT-IR spectrum of AC indicated two peaks at 1480 and 1377 cm−1 for the C–H scissoring and methyl rock, respectively, became broader in that of OAC. Two sharp peaks at 1622 and 1594 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration modes for AC, became wider due to an increase in conjugation after polymerization and observed at 1660 and 1596 cm−1, respectively. The peaks at 1449 and 1510 cm−1 of OAC pointed to the ring stretching vibrations of newly formed pyrazine ring between two acridine units. The bending vibration of C–N bond for AC was observed at 1326 cm−1. In other words, phenazine ring could be considered as a part of the oligomer chain.41,42
C stretching vibration modes for AC, became wider due to an increase in conjugation after polymerization and observed at 1660 and 1596 cm−1, respectively. The peaks at 1449 and 1510 cm−1 of OAC pointed to the ring stretching vibrations of newly formed pyrazine ring between two acridine units. The bending vibration of C–N bond for AC was observed at 1326 cm−1. In other words, phenazine ring could be considered as a part of the oligomer chain.41,42
1H NMR of AC was also recorded for a comparison to OAC for further enlightment of the synthesized structure and shown in Fig. 1 and 2. In the spectrum, the peaks in the aromatic region observed at 7.81 (doublet), 7.76 (doublet), 7.45 (singlet), 6.97 (doublet), 6.91 (doublet) and 6.85 (singlet) ppm were attributed to the Hb, Ha, Hc, He, Hf and Hg protons of AC, respectively. Because of 1–4 interactions with Hb and He protons, the dublet–dublet peak seen more downfield at 8.69 ppm was related to the Hd proton. The peaks for Ha protons were observed as terminal protons in the spectrum of OAC. The peak for the methylene (–CH3) protons was observed as a singlet at 3.90 ppm. The signals of hydrogens in the phenylene unit shifted to lower fields after polymerization, which was significant for the π-conjugated polymers.43 The broad proton signals with respect to AC unit indicated that oligomer OAC consisted of repeating units with different chemical surroundings. The proton signals of NH2 group at 6.76 and 7.27 ppm almost disappeared and observed at 11.20 ppm as a secondary amine after polymerization due to C–N couplings of monomer units during the polycondensation reaction. The doublet signal for He proton of AC turned into a singlet peak at 7.36 ppm indicating that a ring closure between two radicals and ladder structure formation took place in the polymerization pathway of AC. The results elucidated that OAC was formed primarily through the formation of R1 and R2 radicals and the polymerization occurs via the formation of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–NH–C.44
C and C–NH–C.44
The polymerization proceeded by C–N coupling mechanism. The formation of C–N bond through cyclization resulted in shifting peaks for C1, C2, C8 and C9 downfield between 163.18 and 169.12 ppm. The peaks at 117.42, 120.49, 158.55 and 159.17 ppm indicated the presence of terminal C1, C9, C2 and C8, respectively. C10 and C13 peaks were observed at 116.92 and 116.89 ppm for AC appeared at 124.71 ppm in the spectrum of OAC the proposed structure of oligomer is confirmed according to the 1H NMR and 13C NMR spectral results in Fig. 2 and 3. The proposed structure for the oligomer was supported by UV-Vis and FT-IR analyses. Our claim for the polymerization of AC was that polymerization primarily took place via C1 and C2 phenylene units form both sides of acriflavine.
The molecular weight of OAC was determined using GPC chromatogram indicating that Mn and Mw weight were calculated as 4200 and 4950 Da, respectively, with a mono modal particle size distribution by RID detector, resulting in that OAC was oligomeric structured. OAC had a polydispersity index (PDI) value of 1.18. According to the average molecular weight value, the average constitutional units in OAC were nearly 19–22.
To research the crystal structures of AC and OAC, the powder XRD analysis was conducted. The crystallite sizes of the monomer and the oligomer are given in Table 1. The calculated (D) values gave information about the types of crystal structure and the interlayer spacing (d). The analyzed samples of AC and OAC were finely grounded and homogenized. Using the diffraction data, the mean crystallite sizes of the monomer and the oligomer, D, were determined according to the Debye–Scherer equation D = (0.9λ)/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), where λ was X-ray wavelength (1.5406 Å), θ was Bragg diffraction angle, and β was the full width at half maximum of the diffraction peak.45,46 In view of the importance of knowing crystal sizes and interlayer spacings (d) obtained from the angle at which the corresponding peak is diffracted in AC and OAC, it can be proposed the percent of the crystallinity of AC and OAC. The peak at 2θ = 26.8° for AC disappeared, substituted by a new weakened broad peak at around 2θ = 27° for OAC. A decrease in the percentage crystalline was observed from 33 to 19% for AC and OAC, respectively, due to the loss of peaks at 2θ value of 18.4° and the decrease in peak intensities. Highly intense four diffraction peaks were observed at 2θ = 32.8°, 44.7°, 65.1° and 78.25° and four less intense peaks observed at 2θ = 18.4°, 26.8° and 47.6° for the monomer AC. The four intense peaks were observed at 2θ = 32.8°, 44.7°, 65.03° and 78.24° for the oligomer OAC. The peak corresponding to AC seen at 2θ = 26.17° became lower and broader in OAC, which revealed that the sample was partially crystallized.47 The low crystallinity could result from the long range ordering of the oligomer chains.48 Less intense peaks of OAC were attributed to the periodicity parallel to oligomer chains. A decrease in the crystallite size caused an increase in the width of the diffraction as peak broadening. On either side of the Bragg angle, the diffracted beam destructively interfered and resulted in a sharp peak.
θ), where λ was X-ray wavelength (1.5406 Å), θ was Bragg diffraction angle, and β was the full width at half maximum of the diffraction peak.45,46 In view of the importance of knowing crystal sizes and interlayer spacings (d) obtained from the angle at which the corresponding peak is diffracted in AC and OAC, it can be proposed the percent of the crystallinity of AC and OAC. The peak at 2θ = 26.8° for AC disappeared, substituted by a new weakened broad peak at around 2θ = 27° for OAC. A decrease in the percentage crystalline was observed from 33 to 19% for AC and OAC, respectively, due to the loss of peaks at 2θ value of 18.4° and the decrease in peak intensities. Highly intense four diffraction peaks were observed at 2θ = 32.8°, 44.7°, 65.1° and 78.25° and four less intense peaks observed at 2θ = 18.4°, 26.8° and 47.6° for the monomer AC. The four intense peaks were observed at 2θ = 32.8°, 44.7°, 65.03° and 78.24° for the oligomer OAC. The peak corresponding to AC seen at 2θ = 26.17° became lower and broader in OAC, which revealed that the sample was partially crystallized.47 The low crystallinity could result from the long range ordering of the oligomer chains.48 Less intense peaks of OAC were attributed to the periodicity parallel to oligomer chains. A decrease in the crystallite size caused an increase in the width of the diffraction as peak broadening. On either side of the Bragg angle, the diffracted beam destructively interfered and resulted in a sharp peak.
| Compounds | Peak pos. [°2θ] | β (B obs. [°2θ]) | cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
Crystallite size [nm] | d-Spacing [Å] |
|---|---|---|---|---|---|
| Major peaks of AC | 32.84 | 0.307 | 0.95 | 31.08 | 0.44 |
| 44.71 | 0.307 | 0.92 | 32.24 | 0.31 | |
| 65.11 | 0.307 | 0.92 | 35.48 | 0.20 | |
| 78.25 | 0.409 | 0.78 | 28.63 | 0.21 | |
| Major peaks of OAC | 32.84 | 0.205 | 0.96 | 47.98 | 0.28 |
| 44.72 | 0.307 | 0.92 | 32.24 | 0.31 | |
| 65.03 | 0.205 | 0.96 | 54.87 | 0.13 | |
| 78.24 | 0.409 | 0.78 | 28.32 | 0.21 |
Optical and electrochemical properties
The UV-vis spectra of AC and OAC are recorded using DMSO at room temperature and shown in Fig. 4. The peaks concerning π → π* electronic transitions for the phenylene ring were observed in the range of below 300 nm. n → π* transition of imine bond for AC and OAC appeared at 468 and 473 nm, respectively, due to the fact that as π-conjugated systems get larger, the energy gap for a n → π* transition becomes narrow and the corresponding wavelength of light absorbed becomes longer. In comparison to the starting material (AC), it was possible to verify that the maximum peak at 475 nm for OAC is red-shifted with a broadened absorption. The observable band broadening for OAC in Fig. 4a was common for conductive polymers of a polyconjugated structure, indicating that polymerization was accomplished. It is well known that phenazine cycles have an optical absorption at a wavelength of 450–600 nm.49 Phenazine cycles participated in the subsequent chain growth.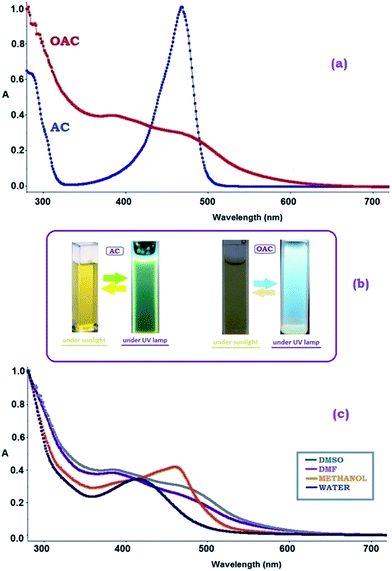 | ||
| Fig. 4 Absorption spectra of (a) (AC) and (OAC) in DMSO, (b) the colors of AC and OAC under room light and UV light, (c) absorption spectra of OAC in different solvents. | ||
From an analysis of the absorption spectra, it is possible to evaluate the optical band gap energy. The optical band gap values (Eg) are obtained by using the following equation as in the literature and were given in Table 2.
| Eg = 1242/λonset | (1) |
The effect of solvent polarity on UV-vis spectra was also examined and shown in Fig. 4c. The effect of solvent polarity was clearly seen on the positions, intensities and shapes of the absorption bands of OAC. The spectral shifts in DMSO, DMF, methanol and water with different polarities mainly depends on the strength of the intermolecular hydrogen bonds between the –NH group of OAC and the –OH, carbonyl (CO) or sulfonyl (SO) functional groups of the solvent molecules.51,52 The maxima of the absorption curves appear to undergo blue shifts with increasing solvent polarity. As seen in Fig. 4c, λmax values were found to be 490, 459, 458 and 414 in DMF, DMSO, methanol and water, respectively. The free pair of electrons on nitrogen atom of OAC were lost via H-bonding leading to the hyposochromically (blue shift) shift of π–π* bands due to a decrease in conjugation and delocalization of the π-electrons when the solvent polarity increased. The shift to shorter wavelength was more enhanced in polar protic solvents like methanol and water than that in apolar protic solvents. The clarification could be based on that a blue shift induced by increasing the polarity of the solvent signified a decrease in the polar quinoid resonance forms. The benzene substituents in OAC are electron donors with unshared electron pairs that may partake in hydrogen bonds with the solvent. Blue shifts would be accounted for that the interaction with polar solvents contributes to the stability of the ground state more than it does to the excited state.53
Therefore, OAC was expected to show a semiconductor behavior as result of oxidative polymerization. Additionally, the lowest optical band gap was expected to observe in DMF or DMSO due to their aprotic polar solvent property to prevent the formation of a hydrogen bond between OAC and the solvent, concurrent with an increase in conjugation and delocalization of the π-electrons.
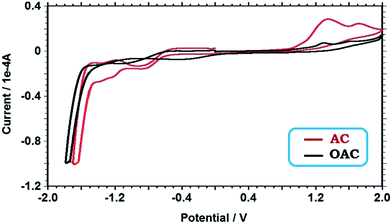
The electrochemical properties and redox behavior of AC and its oligomer were studied using cyclic voltammograms (CVs) carried out in acetonitrile as the supporting electrolyte at room temperature under argon atmosphere are given in Fig. 5. Based on the correlations between the HOMO and LUMO energy levels and redox potentials, the value of the energy levels can be calculated using the onset values of the oxidation and reduction peaks. Making the correlation between redox process and the energy levels, it was possible to determine the electrochemical band gap energy (E′g) of the oligomer. The HOMO–LUMO energy levels and electrochemical band gaps (E′g) were calculated using the oxidation (Eox) and reduction peak potentials (Ered) as shown in Table 2. The determination of HOMO–LUMO energy levels were possible by using the empirical formulas:54
| EHOMO = −(4.39 + Eox) | (2) |
| ELUMO = −(4.39 + Ered) | (3) |
| E′g = ELUMO − EHOMO | (4) |
As displayed in Fig. 5, the oxidation of acriflavine initially produced the radical cation that reacted with another monomer molecule to produce oligo(acriflavine). Two oxidation peaks observed at 1.34 and 1.30 V can be attributed to the oxidation of free NH2 group of AC to form polaron structure (˙+NH), and the oxidation of –NH2 group to form cation radical for OAC in the anodic region, respectively, which are not stable and undergo chemical reactions with parent molecule or dimerization reactions. The acriflavine unit is first oxidized by the formation of a radical cation which then, combines to form dimer and higher oligomers.55 The reduction peak potentials were concerned with the neutralization of the oxidized states of the oligomer.
The diagrams revealed the tendency of the HOMO elevation and on the contrary, the LUMO reduction through the oligomer formation (Table 2). Since the electrochemical band gap of OAC was lower than that of AC, the electrochemical band gap (E′g) values confirmed the optical band gap values (Eg).
Fluorescence characteristics
Since acriflavine has been used as color development reagent for spectrophotometric and fluorimetric determination,56,57 it was necessary to study the fluorescence properties of its polymerization product (OAC). The photoluminescence (PL) spectra of AC and OAC in different solvents were recorded as shown in Fig. 6.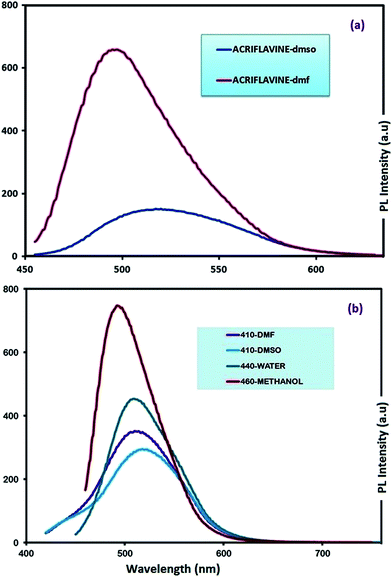 | ||
| Fig. 6 Emission spectra of AC (a) and OAC (b) solutions in selected solvents. Slit width: 3 nm and conc.: 10 mg L−1 in all measurements. | ||
Photoluminescence (PL) spectra of monomer (AC) could be recorded in only DMF and DMSO with a slit width of 3 nm, because the emission exceeded 1000 a.u of PL intensity in water and methanol. The monomer was a green light emitter with an emission maximum at 517 and 497 nm in DMF and DMSO, respectively. The intensities in DMF and DMSO were observed as 659 and 151 a.u, respectively (Fig. 6a). On the other hand, the oligomer had a different property from the monomer with an extra emission of blue color when excited by UV radiation. Fig. 6b gives information about the emission spectra of OAC in various solvents. As seen in Fig. 5b, the fluorescence intensity of OAC decreases with increasing the polarity of solvent. Maximum emission wavelength (λmax) of OAC in DMSO, DMF, H2O and MeOH appeared at 437, 440, 441 and 441 nm with the appearance of a blue color emission and 521, 518, 510 and 521 nm with the emission of yellow color when excited by UV and visible light, respectively. The obtained results revealed that the spectrum was affected by polarity depending on protic–aprotic nature of solvent.
In MeOH and H2O protic solvents, OAC emitted bicolored fluorescent light with blue and green colors when excited at 380 and 460 nm, respectively as seen in Fig. 7. In DMF and DMSO aprotic solvents, the excitation of OAC with 380 and 460 nm resulted in the blue and green fluorescence light at around 440 and 520 nm, respectively, as seen in Fig. 8. The intensities of bicolored fluorescent light emission were 300 and 800 with the excitation wavelengths at 380 and 460 nm, respectively. The behavior of bicolor emission when excited at different wavelengths can be explained in a way that polymers have a broad chain length distribution, and the longer conjugated chains have lower energy gaps with an emission light at longer wavelengths. Therefore, the wavelength of fluorescent emission increased as the wavelength of the excitation source increased. Quantum yield (QY) is another important contribution to PL characteristics. The oligomer OAC has the emission QY of 6% in DMSO and 3% in DMF for green PL emission.
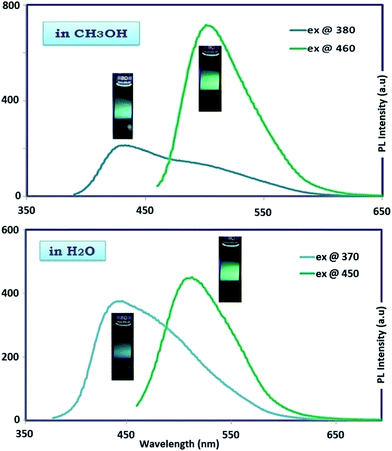 | ||
| Fig. 7 Photographs and the corresponding of maximum emission spectra of OAC solution in CH3OH and H2O. Slit width: 3 nm and conc.: 10 mg L−1 in all measurements. | ||
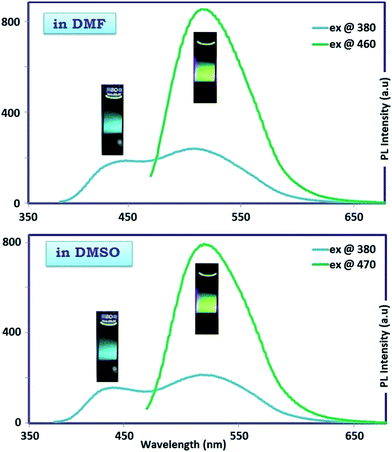 | ||
| Fig. 8 Photographs and the corresponding of maximum emission spectra of OAC solution in DMF and DMSO. Slit width: 3 nm and conc.: 10 mg L−1 in all measurements. | ||
To observe the multicolor emission with change of excitation wavelength, confocal microscope analysis was also performed in Fig. 9. A certain amount of OAC solution was dropped on a glass sheet and dried. The emission colors of blue and green colors were separately obtained by using confocal microscope with the excitation wavelengths of 360 and 470 nm agreed with the PL ones very well. OAC displayed bicolor emission behavior in all selected polar solvents. OAC showed nearly similar emission characteristics under the same excitation wavelength in selected polar solvents such as DMF, DMSO, MeOH and water.
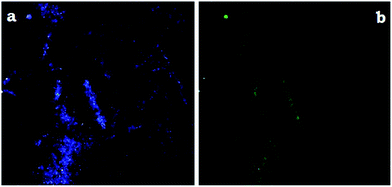 | ||
| Fig. 9 Confocal microscope images of OAC. The excitation wavelengths were 380 (a), and 470 (b), respectively. | ||
Based on foregoing results, OAC can be a very promising candidate for materials in light-emitting diodes due to their bicolor (blue and green) light-emitting property as fluorescent water soluble conjugated oligomer.
Thermal properties
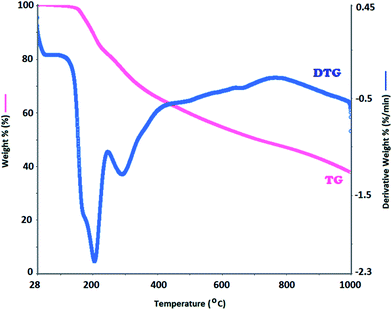
The thermal degradation curves (TGA-DTG) for the synthesized oligomer AC and OAC at heating rates of 10 °C from ambient temperature to 1000 °C under nitrogen atmosphere are depicted in Fig. 10. TG-DTG analyses reveal that thermal degradation of AC and OAC happened in two stages. It was clearly seen in Fig. 10 that 5–7% weight loss assigned to the removal of the adsorption water for AC and OAC was observed around 50–120 °C.58,59 As stressed in Table 3, despite the high thermal stability of the monomer (AC) the oligomer (OAC) showed low thermal resistance, because initial degradation temperatures (Ton) of AC and OAC were obtained to be 290 and 160 °C, respectively. The temperatures corresponding to 20% (T20) weight losses were calculated as 373 and 266 °C, and the temperatures corresponding to 50% (T50) weight losses were figured out as 628 and 719 °C for AC and OAC, respectively. The maximum rate of weight loss (Tmax) values obtained from the derivative thermogravimetry (DTG) curves were found to be 333 and 430 °C for AC, and 206 and 292 °C for OAC, indicating that the thermal decomposition took place in two stages. The glass transition temperature (Tg) of OAC studied by differential scanning calorimeter (DSC) technique was found to be 139 °C. In addition, the char% amounts of AC and OAC at 1000 °C were obtained as 49.7% and 37.7%, respectively. Based on foregoing thermal analysis data, it can be said that the synthesized oligomer is not thermally stable.| Compounds/thermal data | AC | OAC |
|---|---|---|
| a The onset temperature.b Maximum weight loss temperature.c Glass transition temperature.d Change of specific heat during glass transition. | ||
| Tona | 290 | 160 |
| Tmaxb | 333, 430 | 206 |
| 20% weight loss | 373 | 266 |
| 50% weight loss | 628 | 719 |
| % char at 1000 °C | 49.7 | 37.7 |
| DTA (exo/endo) | —/— | —/— |
| DSC [Tgc (°C)/ΔCpd (J g−1 K−1)] | —/— | 139/0.821 |
Photovoltaic properties
The photovoltaic power conversion efficiency of a solar cell (η) is described as the ratio of generated electricity from the solar cell to incoming light energy. The efficiency of a solar cell is determined as the fraction of incident power which is converted to electricity and is defined as:
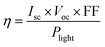 | (5) |
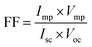 | (6) |
The characteristic intersections with abscissa and ordinate are the open circuit voltage (Voc) and the short circuit current (Isc), respectively, as seen in Fig. 11. The largest power output (Pmax) is determined using the point at which the product of voltage and current has maximum value. Division of Pmax by the product of Isc and Voc yields the fill factor FF. We have achieved 0.027 as % η under AM 1.5 irradiation (100 mW cm−2) with a dye-sensitized solar cells based on OAC, exhibiting a short circuit photocurrent density Jsc of 0.14 mA cm−2, open circuit voltage Voc of 0.37 V, and the fill factor FF of 0.50. It can be concluded that OAC is moderately effective as an organic-dye photosensitizer for use in dye-sensitized solar cells. The reason regarding to performance of the organic photovoltaics depends on the following factors. (1) Broad and strong absorption in visible and near IR region to match with solar spectrum (2) suitable LUMO energy level of acceptor and HOMO energy level of donor to maintain Voc (3) optimum morphology donor–acceptor with nanoscale phase separation within active layer which influences Jsc, Voc and FF.60
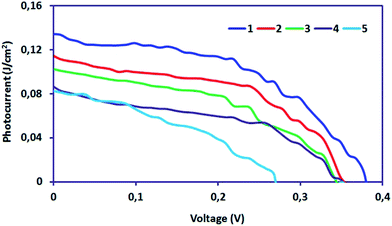 | ||
Fig. 11 J–V curves under different light intensities (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 mW cm−2; 2 100 mW cm−2; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 mW cm−2; 3 80 mW cm−2; 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 mW cm−2; 4 60 mW cm−2; 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mW cm−2; 5 40 mW cm−2; 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mW cm−2). 20 mW cm−2). | ||
Morphological and electrical properties
The morphology of OAC has been characterized by scanning electron microscopy (SEM). SEM views at different amplifications are shown in Fig. 12. According to the SEM images, the polymer surface morphology was smooth without any noticeable pinholes or cracks within the conventional SEM resolution seen in Fig. 12. The size of the OAC particles can be as large as several micrometers. The molecular structure of OAC was relatively flat and regular which meant that the polymerization took place over forming one pyrazine ring as a result of an intramolecular cyclization, leading to the ladder segments and phenazine structures along oligomer chain of OAC.By the exposure of the pellet to iodine vapor in a dessicator at room temperature, the conductivity of iodine doped oligomer depending on time could be followed.61 The virgin electrical conductivity of OAC was measured to be 2.35 × 10−9 S cm−1. Upon doping with iodine at room temperature in a vacuum desiccator, its conductivity increased to 5.90 × 10−9 S cm−1 in 6 h and increased by about two orders of magnitude reached to 1.86 × 10−7 S cm−1 in 24 hours. The values were 3.1 × 10−7, 3.96 × 10−7, 7 × 10−7, 9.94 × 10−7 and 1.08 × 10−6 S cm−1 by doping in 48, 72, 96 and 120 hours, respectively. Since nitrogen is an electronegative element, iodine doped oligomer generally occurs by coordination of iodine with nitrogen atoms in the oligomer structure. The results revealed that the electrical conductivity of iodine-doped pellet of OAC increased about three orders of magnitude and reached to about 10−6 S cm−1 value in 120 hours. The conductivity first increased with doping time, then tend to level-off. As emphasized, there is an opposite correlation between the values of HOMO–LUMO band gap and the conductivity. A lower band gap makes the electronic transition easier with obtaining higher conductivity values. Consequently, it can be said that OAC is semi-conducting structured.
Conclusion
The oxidation of acriflavine with hydrogen peroxide resulted in the formation of an oxidation product with the molar mass of several thousands, leading to having an oligomeric nature rather than an oligomeric structure. By a combined application of spectroscopic and spectroelectrochemical techniques, the structural details of the formation of phenazine-like units has been studied in detail. From the application point of view, the combined effects of inclusion of acriflavine and polymerization azomethine-based compounds improve the photophysical, thermal and morphological properties. Fluorescent spectral studies indicated that OAC was a good candidate for blue- and green-light emitter. Furthermore, OAC manifested thermal stability and smooth homogeneous surfaces. With these attractive properties, the synthesized oligomer has many potential applications in various fields. Thus, it can be inferred that the introduction of acriflavine to the procedure in oligomer formation would significantly improve the photophysical and thermal properties. These results provide important structure–activity relationship that can be used to predict the morphological and electro-optical properties of conjugated oligomer.Conjugated organic dyes, which have OAC moiety as the electron donor showed moderate performance as a photosensitizer in dye-sensitized solar cells. A maximum solar-energy-to-electricity conversion efficiency (η) of 0.027% under AM 1.5 irradiation (100 mW cm−2) with a dye-sensitized solar cell. The crystalline nature and bicolored emission property of oligo(acriflavine) provide potential applications in bicolored fluorescent labeling in bioscience and the fabrication of nano-devices. The study may be carried forward by designing a better structure of OAC with light absorbing low band gap donor–acceptor morphology in nanoscale for better photon harvesting and thereby may increase the photocurrent in solar cell.
Acknowledgements
The authors thank Çanakkale Onsekiz Mart University scientific research project commission for support with the project number (Project Nu.: FBA-2016-710).References
- S. P. Economopoulos, A. K. Andreopoulou, V. G. Gregoriou and J. K. Kallitsis, Chem. Mater., 2005, 17, 1063–1071 CrossRef CAS.
- W. Y. Huang, H. Yun, H. S. Lin, T. K. Kwei and Y. Okamoto, Macromolecules, 1999, 32, 8089–8093 CrossRef CAS.
- A. Kimyonok, X. Y. Wang and M. Weck, J. Macromol. Sci., Polym. Rev., 2006, 46, 47–77 CrossRef CAS.
- A. S. Anselmo, L. J. Lindgren, J. Rysz, A. Bernasik, A. Budkowski, M. R. Andersson, K. Svensson, J. van Stam and E. Moons, Chem. Mater., 2011, 23, 2295–2302 CrossRef CAS.
- R. Wilson and A. P. F. Turner, Biosens. Bioelectron., 1992, 7, 165–185 CrossRef CAS.
- S. Hellstrom, L. J. Lindgren, Y. Zhou, F. L. Zhang, O. Inganas and M. R. Andersson, Polym. Chem., 2010, 1, 1272–1280 RSC.
- M. K. Ram, N. S. Sundaresan and B. D. Malhotra, J. Mater. Sci. Lett., 1994, 13, 1490–1493 CrossRef CAS.
- W. H. Meyer, H. Kiess, B. Binggeli, E. Meier and G. Harbeke, Synth. Met., 1985, 10, 255–259 CrossRef CAS.
- Handbook of organic conductive materials and polymers, ed. H. S. Nalwa, Wiley, New York, 1997 Search PubMed.
- V. Saxena and B. D. Malhotra, Curr. Appl. Phys., 2003, 3, 293–305 CrossRef.
- A. Rizzo, N. Solin, L. J. Lindgren, M. R. Andersson and O. Inganas, Nano Lett., 2010, 10, 2225–2230 CrossRef CAS PubMed.
- S. Kobayashi and A. Makino, Chem. Rev., 2009, 109, 5288–5353 CrossRef CAS PubMed.
- W. Liu, S. Bian, L. Li, L. Samuelson, J. Kumar and S. Tripathy, Chem. Mater., 2000, 12, 1577–1584 CrossRef CAS.
- K. Hara, K. Sayama, Y. Ohga, A. Shinpo, S. Suga and H. Arakawa, Chem. Commun., 2001, 569–570 RSC.
- K. Hara, M. Kurashige, Y. Dan-oh, C. Kasada, A. Shinpo, S. Suga, K. Sayama and H. Arakawa, New J. Chem., 2003, 27, 783–785 RSC.
- T. Horiuchi, H. Miura and S. Uchida, Chem. Commun., 2003, 21, 3036–3037 RSC.
- T. Horiuchi, H. Miura and S. Uchida, J. Photochem. Photobiol., A, 2004, 164, 29–32 CrossRef CAS.
- A. Ehret, L. Stuhl and M. T. Spitler, J. Phys. Chem. B, 2001, 105, 9960–9965 CrossRef CAS.
- Q. H. Yao, F. S. Meng, F. Y. Li, H. Tian and C. H. Huang, J. Mater. Chem., 2003, 13, 1048–1053 RSC.
- Z. S. Wang, F. Y. Li and C. H. Huang, Chem. Commun., 2000, 20, 2063–2064 RSC.
- H. H. Lu, Y. S. Ma, N. J. Yang, G. H. Lin, Y. C. Wu and S. A. Chen, J. Am. Chem. Soc., 2011, 133, 9634–9637 CrossRef CAS PubMed.
- C. V. Hoven, A. Garcia, G. C. Bazan and N. Thuc-Quyen, Adv. Mater., 2008, 20, 3793–3810 CrossRef CAS.
- X. Lu, Q. Fan, G. Zhang, K. Pu and W. Huang, Sci. China: Chem., 2010, 53, 1122–1127 CrossRef CAS.
- K. Zhang, X. Guan, F. Huang and Y. Cao, Acta Chim. Sin., 2012, 70, 2489–2495 CrossRef CAS.
- F. Huang, H. B. Wu and Y. Cao, Chem. Soc. Rev., 2010, 39, 2500–2521 RSC.
- B. Liu, W. L. Yu, Y. H. Lai and W. Huang, Macromolecules, 2002, 35, 4975–4982 CrossRef CAS.
- M. Onoda, K. Tada, M. Ozaki and K. Yoshino, Thin Solid Films, 2000, 363, 9–12 CrossRef CAS.
- R. E. Bauer, V. Enkelmann, U. M. Wiesler, A. J. Berresheim and K. Müllen, Chem.–Eur. J., 2002, 8, 3858–3864 CrossRef CAS.
- M. J. Winokur and B. R. Mattes, Macromolecules, 1998, 31, 8183–8191 CrossRef CAS.
- Z. Mo, K. B. Lee, Y. B. Moon, M. Kobayashi, A. J. Heeger and F. Wudl, Macromolecules, 1985, 18, 1972–1977 CrossRef CAS.
- Y. F. Zhou, M. Eck and M. Kruger, Energy Environ. Sci., 2010, 3, 1851–1864 CAS.
- Y. Li, H. Kuwabara, Y. K. Gong, Y. Takaki and K. Nakashima, J. Photochem. Photobiol., B, 2003, 70, 171–176 CrossRef CAS.
- S. H. Choi, J. Y. Cho, Y. S. Chung, E. K. Hong, Y. B. Han and S. G. Kim, Int. J. Immunopharmacol., 2000, 22, 775–787 CrossRef CAS PubMed.
- T. I. Grishaeva and A. I. Zakharov, Zh. Anal. Khim., 1990, 45, 1333–1337 CAS.
- A. T. R. Williams, S. A. Winfield and J. N. Miller, Analyst, 1983, 108, 1067–1071 RSC.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Kluwer Academic/Plenum Publishers, New York, 2nd edn, 1999 Search PubMed.
- R. Cervini, X. C. Li, G. W. C. Spencer, A. B. Holmes, S. C. Moratti and R. H. Friend, Synth. Met., 1997, 84, 359–360 CrossRef CAS.
- H. Hoppe and N. S. Sariciftci, J. Mater. Chem., 2004, 19, 1924–1945 CAS.
- G. Ćirić-Marjanović, M. Trchová and J. Stejskal, Int. J. Quantum Chem., 2008, 108, 318–333 CrossRef.
- G. Ćirić-Marjanović, N. V. Blinova, M. Trchová and J. Stejskal, J. Phys. Chem. B, 2007, 111, 2188–2199 CrossRef PubMed.
- M. B. Mitchell, G. R. Smith and W. A. Guillory, J. Chem. Phys., 1981, 75, 44–48 CrossRef CAS.
- K. Chiba, T. Ohsaka, Y. Ohnuki and N. Oyama, J. Electroanal. Chem., 1987, 219, 117–124 CrossRef CAS.
- A. Bilici, F. Dogan, M. Yıldırım and İ. Kaya, React. Funct. Polym., 2011, 71, 675–683 CrossRef CAS.
- A. Hathoot, M. Abdel-Kader and M. Abdel-Azzem, Int. J. Electrochem. Sci., 2009, 4, 208–222 CAS.
- B. P. Baranwal, T. Fatma and A. Varna, J. Mol. Struct., 2009, 920, 472–477 CrossRef CAS.
- M. A. Short and P. L. Walker, Carbon, 1963, 1, 3–9 CrossRef CAS.
- J. Han, G. P. Song and R. Guo, Eur. Polym. J., 2007, 43, 4229–4235 CrossRef CAS.
- X. Lu, H. Mao, D. Chao, X. Zhao, W. Zhang and Y. Wei, Mater. Lett., 2007, 61, 1400–1403 CrossRef CAS.
- D. He, Y. Wu and B. Q. Xu, Eur. Polym. J., 2007, 43, 3703–3709 CrossRef CAS.
- K. Colladet, M. Nicolas, I. Goris, I. Lutsen and D. Vanderzande, Thin Solid Films, 2004, 451, 7–11 CrossRef.
- A. V. Kulinich, N. A. Derevyanko and A. A. Ishchenko, Russ. J. Gen. Chem., 2006, 76, 1441–1457 CrossRef CAS.
- B. W. Domagalska, K. A. Wilk and R. Zielinski, J. Photochem. Photobiol., A, 2006, 184, 193–203 CrossRef CAS.
- H. E. Ungnada, J. Am. Chem. Soc., 1953, 75, 432–434 CrossRef.
- W. Liu, S. Lee, S. Yang, S. Bian, L. Lia, L. A. Samuelson, J. Kumar and S. K. Tripathy, J. Macromol. Sci., Part A: Pure Appl. Chem., 2001, 38, 1355–1370 CrossRef.
- E. T. Seo, R. F. Nelson, J. M. Fritsh, L. S. Marcoux, D. W. Leedy and N. Adams, J. Am. Chem. Soc., 1966, 88, 3498–3503 CrossRef CAS.
- R. Segarra Guerrero, C. Gomez Benito and J. Martinez Calatayud, Talanta, 1996, 43, 239–246 CrossRef CAS PubMed.
- J. M. Calatayud, J. V. G. Mateo and V. David, Analyst, 1998, 123, 429–434 RSC.
- M. Yıldırım and İ. Kaya, Synth. Met., 2012, 162, 436–443 CrossRef.
- R. Solmaz and G. Kardas, Prog. Org. Coat., 2009, 64, 81–88 CrossRef CAS.
- N. B. Kolhe, S. Shinde, B. Saibal and S. K. Asha, Organic Photonics and Photovoltaics, 2015, 3, 71–100 CrossRef.
- F. R. Diaz, J. Moreno, L. H. Tagle, G. A. East and D. Radic, Synth. Met., 1999, 100, 187–193 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |