Open Access Article
Open Access ArticlePreparation of [18F]-NHC-BF3 conjugates and their applications in PET imaging†
Kantapat Chansaenpak‡
ac,
Mengzhe Wang‡a,
Hui Wanga,
Benjamin C. Giglioa,
François P. Gabbaï b,
Zhanhong Wua and
Zibo Li
b,
Zhanhong Wua and
Zibo Li *a
*a
aBiomedical Research Imaging Center, Department of Radiology, University of North Carolina, Chapel Hill, North Carolina, USA 27514. E-mail: ziboli@med.unc.edu
bDepartment of Chemistry, Texas A&M University, College Station, Texas, USA 77843
cNational Nanotechnology Center (NANOTEC), National Science and Technology Development Agency (NSTDA), 111 Thailand Science Park, Pathum Thani, Thailand 12120
First published on 22nd March 2017
Abstract
N-Heterocyclic carbene boron trifluoride (NHC-BF3) conjugates were successfully radiofluorinated by SnCl4-promoted 18F–19F isotopic exchange reaction in one labelling step. This method afforded in vivo stable PET agents that efficiently bound to targeted tumours yielding PET images with good tumour-to-background contrast.
Fluorine-18 is typically incorporated into molecular tracers at the last step of the overall synthesis process due to its short half-life (110 minutes). One of the recent methods for late-stage radiofluorination is a pre-functionalization approach in which a 18F-captor such as a BF3 or a BF2 unit is preinstalled into bioactive molecules.1 Several examples, including the radiofluorinations of alkylammoniomethyltrifluoroborate2 (AMBF3) (Scheme 1A), BODIPY3 (Scheme 1B) and phosphonium aryltrifluoroborate4 (PArBF3) (Scheme 1C) conjugates, were demonstrated.
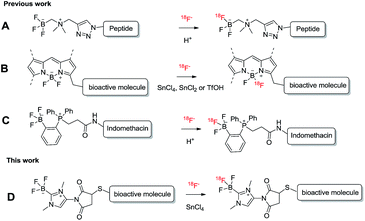 | ||
| Scheme 1 Scheme showing the late-stage radiofluorinations of boron-based radiotracers shown in previous reports (A–C) and this report (D). | ||
In the case of AmBF3 and PArBF3 conjugates, the 18F–19F isotopic exchange of the BF3 moiety could be simply processed in acidic aqueous media.2d,4,5 However, the 18F–19F isotopic exchange of the BF2 unit in BODIPY conjugates could only be efficiently done in a dried organic solvent with strong Lewis/BrØnsted acid promotors (SnCl4, SnCl2, or TfOH).3,6
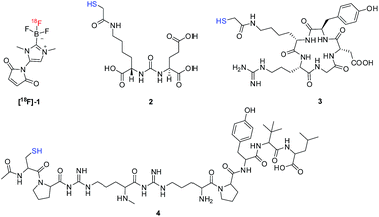
Recently, we demonstrated that the [18F]-maleimide functionalized NHC-BF3 ([18F]1) (Fig. 1) could be prepared via SnCl4-promoted 18F–19F isotopic exchange and it could be easily conjugated to Cys-Phe dipeptide.7 However, the 18F direct labelling of the NHC-BF3 pre-functionalized bioactive molecule (Scheme 1D) has not been reported. With this in mind, we decided to validate the late-stage radiofluorination in NHC-BF3 conjugated bioactive molecules, including glutamate–urea–lysine (2), cyclic RGD-containing peptide (3), and neurotensin (NT) peptide (4) (Fig. 1). The results on radiosynthesis and PET imaging of these [18F]-NHC-BF3 conjugates are presented in this report.
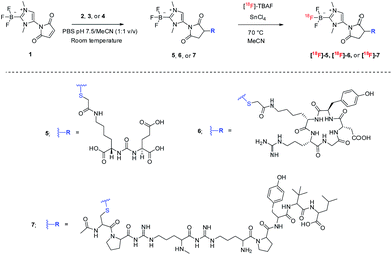
As a starting point, NHC-BF3 conjugated glutamate–urea–lysine (5), cyclic RGD-containing peptide (6), and neurotensin peptide (7) were prepared by thiol-Michael addition click reaction of 1 with 2, 3, and 4, respectively (Scheme 2). This reaction was performed by mixing the solution of 1 in MeCN with the solution of 2, 3, or 4 in phosphate buffer solution (PBS) pH 7.5. The reaction mixture was then incubated at room temperature for 2 hours before purifying with high-performance liquid chromatography (HPLC). The detection of the molecular ions by positive-mode electrospray mass spectrometry at m/z 653.1999 for 5, 953.3689 for 6 and 1332.6725 for 7 confirmed the identity of the conjugates.
Next, we labelled our new NHC-BF3 conjugates by SnCl4-promoted 18F–19F isotopic exchange reaction. The solution of NHC-BF3 conjugates and tin(IV)chloride (excess) in anhydrous MeCN was mixed with azeotropically dried tetrabutylammonium [18F]-fluoride (TBAF) (Scheme 2). The mixture was heated at 70 °C for 10 min, then quenched with 1 mL of water and passed through a Sep-Pak light alumina N cartridge to remove an unreacted 18F-fluoride.
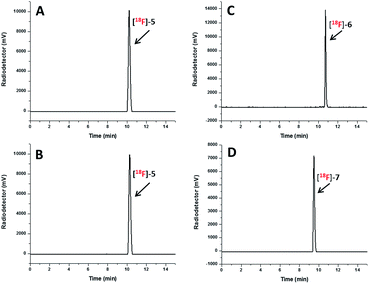
The radio-HPLC profiles of [18F]5, [18F]6 and [18F]7 obtained after passing through Sep-Pak alumina N cartridge are shown in Fig. 2A, C and D, respectively. The identity of [18F]-NHC-BF3 conjugates was confirmed by the comparison of their retention times with those of reference compounds as shown in Fig. 2A and B in the case of [18F]5. The UV HPLC profiles of 6 and 7 are displayed in the ESI.† The radio-HPLC profiles revealed that the [18F]5, [18F]6 and [18F]7 were obtained as a major product. The small radio-HPLC peaks of the impurities were also detected, which could be the results from decomposition due to exposure to SnCl4. Although the HPLC purification is required, the radiolabelling time can be reasonably shortened compared with our previous report7 as the radio-bioconjugation step between [18F]-1 and the bioactive molecule is removed.
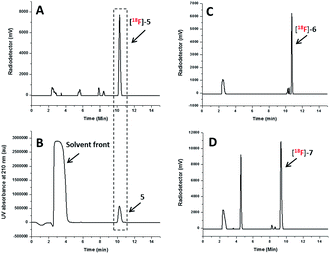 | ||
| Fig. 2 Radio HPLC profile of the [18F]5 (A), [18F]6 (C) and [18F]7 (D) obtained after passing through Sep-Pak alumina N cartridge and UV HPLC profile of 5 (B) as the reference compound. | ||
The decay-corrected radiochemical yield, and specific activity for [18F]5, [18F]6 and [18F]7 are shown in Table 1. The radiochemical yields ranging from 21.4 to 29.6% are comparable with those obtained from SnCl4-promoted radiolabelling of BODIPY-RGD conjugate3a and slightly lower than SnCl2 or TfOH-promoted radiolabelling of BODIPY conjugates reported in previous publications.3 The specific activities of all [18F]-NHC-BF3 conjugates are considerably higher than that of [18F]-NHC-BF3-Cys-Phe prepared by two-step radiosynthesis.7
| Entry | Cpd | Amount | SnCl4 (μL) | Temp. (°C) | RCYa (%) | SAb (GBq μmol−1) | |
|---|---|---|---|---|---|---|---|
| μg | μmol | ||||||
| a Radiochemical yields (RCY) were calculated by dividing the 18F-activity of the isolated product by the starting 18F activity.b Specific activities (SA) were determined by dividing the isolated product activity by the amount of the product (based on the integration of UV-HPLC profile and compare it with the standard calibration curve). The radiochemical yields were decay corrected. The specific activities were measured at the end of synthesis (EOS). | |||||||
| 1 | [18F]5 | 200 | 0.31 | 1 | 70 | 29.6 | 6.0 |
| 2 | [18F]6 | 300 | 0.32 | 1 | 70 | 25.6 | 3.5 |
| 3 | [18F]7 | 400 | 0.30 | 1 | 70 | 21.4 | 3.4 |
In term of the in vitro stability, [18F]5, [18F]6 and [18F]7 demonstrated negligible decomposition at both 60 and 120 min after incubation in phosphate buffer solution pH 7.5 under ambient temperature (Fig. 3 and ESI†). These results are consistent with the extremely long hydrolysis half-life of 1 observed by 19F NMR spectroscopy and the in vitro stability studies of [18F]-NHC-BF3-Cys-Phe shown in the previous report.7 Next, we chose [18F]5, the prostate-specific membrane antigen (PSMA)-based radiotracer, to perform the in vivo stability study in mice.
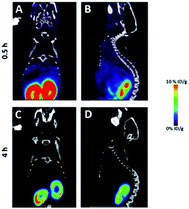
The microPET/CT images obtained at 0.5 h and 4 h post injection revealed that [18F]5 mainly localized in kidneys due to its hydrophilic nature (Fig. 4), as seen with other PSMA-based radiotracers.8 The high accumulation of [18F]5 in kidneys is partially due to high expression of PSMA within proximal renal tubules.9 Based on PET quantification, the kidney uptake of [18F]5 was determined to be 12.1 and 2.3% ID per g at 0.5 h and 4 h post injection, respectively. This finding indicated that [18F]5 was slowly cleared from kidneys. Additionally, we did not observe any bone uptake signal due to tracer decomposition even at 4 h post injection which is similar to the in vivo stability of the [18F]-NHC-BF3-Cys-Phe.7
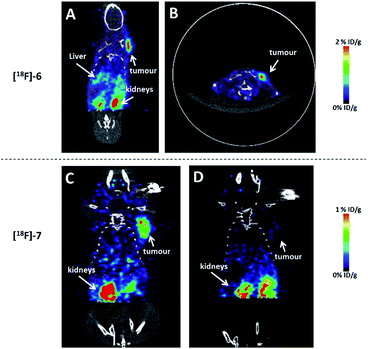
In order to validate whether these agents could be used for cancer imaging, the glioblastoma (U87MG) and pancreatic (PANC1) tumour bearing mice were injected with the radiolabelled RGD and NT conjugates, respectively. As shown in Fig. 5A–C, [18F]6 and [18F]7 nicely visualized tumour in a good contrast and showed negligible uptake in bone. Additionally, both tracers barely localized in liver due to the hydrophilic nature of the NHC-BF3 conjugates which is different from the bio-distributions of the [18F]-BODIPY-peptide conjugates3a,6a and the [18F]-ortho-(Ph2MeP)C6H4(BF3)-indomethacin conjugate.4 The target specificity of [18F]7 was confirmed by blocking experiment in which the radiotracer was co-injected with an excess amount (200 μg) of NT peptide (4). In this blocking experiment, the tumour uptake of [18F]7 was remarkably inhibited by nonradioactive NT peptide, confirming the receptor specificity of this imaging agent. These results are significant as they are the first example showing that the 18F-radiolabelled NHC-BF3 conjugates can image the targeted tumour in vivo. Clearly, the specific activity is high enough for tumour imaging application. However, we do like to point out that the specific activity may need to be improved further for neuroimaging applications where the receptor number is limited.
The quantification of the activity accumulation in the major organs (Fig. 6A and B) was calculated by measuring the regions of interest (ROIs) encompassing the entire organ. Although the degrees of tumour uptake of [18F]6 and [18F]7 are different (1.50 ± 0.013% ID per g for [18F]6 and 0.54 ± 0.031% ID per g for [18F]7), the tumour-to-muscle ratios of both conjugates are comparable (9.85 ± 0.45 for [18F]6 and 9.33 ± 0.42 for [18F]7) yielding tumour images with good contrast (Fig. 5A and B for [18F]6, Fig. 5C for [18F]7). The low liver uptake (0.086 ± 0.0064% ID per g) and the comparatively high kidney uptake (0.94 ± 0.10) of [18F]7 at 1 h post injection indicate that [18F]7 rapidly cleared from liver which is similar to our previous reports on the PET imaging of 18F-vinyl sulfone conjugated NT-peptide.10
In summary, we have successfully synthesized [18F]-NHC-BF3 conjugated bioactive molecules via SnCl4 promoted 18F–19F isotopic exchange reaction. This approach allows the fluorine-18 to be introduced at late stage of the synthesis. These agents showed excellent in vitro and in vivo stability due to the zwitterionic nature of the NHC-BF3 moiety. As shown in PET imaging, both RGD and NT conjugates ([18F]6 and [18F]7) demonstrated prominent tumour uptake with good tumour-to-background contrast. This approach provides broad applications for the radiolabelling of thiol-containing bioactive molecules.
Acknowledgements
This work was supported by the National Cancer Institute (P30-CA016086-35-37), and the Biomedical Research Imaging Center, University of North Carolina at Chapel Hill. All animal-related experiments were performed in compliance with the guidelines of Public Health Service (PHS) policy on Human Care and Use of Laboratory Animals approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (IACUC).Notes and references
- (a) K. Chansaenpak, B. Vabre and F. P. Gabbai, Chem. Soc. Rev., 2016, 45, 954–971 RSC; (b) D. M. Perrin, Acc. Chem. Res., 2016, 49, 1333–1343 CrossRef CAS PubMed; (c) P. W. Miller, N. J. Long, R. Vilar and A. D. Gee, Angew. Chem., Int. Ed., 2008, 47, 8998–9033 CrossRef CAS PubMed; (d) A. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford and P. J. H. Scott, Chem. Sci., 2014, 5, 4545–4553 RSC; (e) V. Bernard-Gauthier, J. J. Bailey, Z. Liu, B. Wängler, C. Wängler, K. Jurkschat, D. M. Perrin and R. Schirrmacher, Bioconjugate Chem., 2016, 27, 267–279 CrossRef CAS PubMed.
- (a) Z. Liu, M. Pourghiasian, F. Bénard, J. Pan, K.-S. Lin and D. M. Perrin, J. Nucl. Med., 2014, 55, 1499–1505 CrossRef CAS PubMed; (b) Z. B. Liu, M. A. Radtke, M. Q. Wong, K. S. Lin, D. T. Yapp and D. M. Perrin, Bioconjugate Chem., 2014, 25, 1951–1962 CrossRef CAS PubMed; (c) Z. Liu, G. Amouroux, Z. Zhang, J. Pan, N. Hundal-Jabal, N. Colpo, J. Lau, D. M. Perrin, F. Bénard and K.-S. Lin, Mol. Pharm., 2015, 12, 974–982 CrossRef CAS PubMed; (d) Z. Liu, M. Pourghiasian, M. A. Radtke, J. Lau, J. Pan, G. M. Dias, D. Yapp, K.-S. Lin, F. Bénard and D. M. Perrin, Angew. Chem., Int. Ed., 2014, 53, 11876–11880 CrossRef CAS PubMed; (e) M. Pourghiasian, Z. Liu, J. Pan, Z. Zhang, N. Colpo, K.-S. Lin, D. M. Perrin and F. Bénard, Bioorg. Med. Chem., 2015, 23, 1500–1506 CrossRef CAS PubMed; (f) Z. Zhang, S. Jenni, C. Zhang, H. Merkens, J. Lau, Z. Liu, D. M. Perrin, F. Bénard and K.-S. Lin, Bioorg. Med. Chem. Lett., 2016, 26, 1675–1679 CrossRef CAS PubMed; (g) Z. Liu, H. Chen, K. Chen, Y. Shao, D. O. Kiesewetter, G. Niu and X. Chen, Sci. Adv., 2015, 1(8), e1500694 Search PubMed; (h) Z. Liu, K.-S. Lin, F. Benard, M. Pourghiasian, D. O. Kiesewetter, D. M. Perrin and X. Chen, Nat. Protoc., 2015, 10, 1423–1432 CrossRef CAS PubMed.
- (a) S. Liu, D. Li, Z. Zhang, G. K. Surya Prakash, P. S. Conti and Z. Li, Chem. Commun., 2014, 50, 7371–7373 RSC; (b) E. J. Keliher, J. A. Klubnick, T. Reiner, R. Mazitschek and R. Weissleder, ChemMedChem, 2014, 9, 1368–1373 CrossRef CAS PubMed.
- K. Chansaenpak, M. Wang, S. Liu, Z. Wu, H. Yuan, P. S. Conti, Z. Li and F. P. Gabbai, RSC Adv., 2016, 6, 23126–23133 RSC.
- Z. Li, K. Chansaenpak, S. Liu, C. R. Wade, H. Zhao, P. S. Conti and F. P. Gabbaï, MedChemComm, 2012, 3, 1305–1308 RSC.
- (a) S. Liu, T.-P. Lin, D. Li, L. Leamer, H. Shan, Z. Li, F. P. Gabbaï and P. S. Conti, Theranostics, 2013, 3, 181–189 CrossRef CAS PubMed; (b) K. Chansaenpak, H. Wang, M. Wang, B. Giglio, X. Ma, H. Yuan, S. Hu, Z. Wu and Z. Li, Chem.–Eur. J., 2016, 22, 12122–12129 CrossRef CAS PubMed.
- K. Chansaenpak, M. Wang, Z. Wu, R. Zaman, Z. Li and F. P. Gabbai, Chem. Commun., 2015, 51, 12439–12442 RSC.
- (a) S. M. Hillier, K. P. Maresca, F. J. Femia, J. C. Marquis, C. A. Foss, N. Nguyen, C. N. Zimmerman, J. A. Barrett, W. C. Eckelman, M. G. Pomper, J. L. Joyal and J. W. Babich, Cancer Res., 2009, 69, 6932–6940 CrossRef CAS PubMed; (b) S. M. Hillier, K. P. Maresca, G. Lu, R. D. Merkin, J. C. Marquis, C. N. Zimmerman, W. C. Eckelman, J. L. Joyal and J. W. Babich, J. Nucl. Med., 2013, 54, 1369–1376 CrossRef CAS PubMed; (c) Y. Chen, C. A. Foss, Y. Byun, S. Nimmagadda, M. Pullambhatla, J. J. Fox, M. Castanares, S. E. Lupold, J. W. Babich, R. C. Mease and M. G. Pomper, J. Med. Chem., 2008, 51, 7933–7943 CrossRef CAS PubMed.
- (a) D. A. Silver, I. Pellicer, W. R. Fair, W. D. Heston and C. Cordon-Cardo, Clin. Cancer Res., 1997, 3, 81–85 CAS; (b) B. S. Slusher, G. Tsai, G. Yoo and J. T. Coyle, J. Comp. Neurol., 1992, 315, 217–229 CrossRef CAS PubMed.
- Z. Wu, L. Li, S. Liu, F. Yakushijin, K. Yakushijin, D. Horne, P. S. Conti, Z. Li, F. Kandeel and J. E. Shively, J. Nucl. Med., 2014, 55(7), 1178–1184 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28806e |
| ‡ These two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |