Open Access Article
Open Access ArticleA novel modified MIL-101-NH2 ligand for CuI-catalyzed and air promoted oxidation of secondary alcohols†
Hui Liua,
Daniele Ramellab,
Peng Yu *a and
Yi Luan
*a and
Yi Luan *c
*c
aScience College of Hunan Agricultural University, Hunan 410128, China. E-mail: pengy7505@hunau.net; Tel: +86-731-84617022
bTemple University-Beury Hall, 1901, N. 13th Street, Philadelphia, PA 19122, USA
cSchool of Materials Science and Engineering, University of Science and Technology Beijing, 30 Xueyuan Road, Haidian District, Beijing 100083, P. R. China. E-mail: yiluan@ustb.edu.cn
First published on 24th April 2017
Abstract
An efficient Cu(I)-catalyzed aerobic alcohol oxidation system was developed utilizing a novel metal–organic framework (MOF) ligand at room temperature. Several relatively inert secondary alcohols were converted to their corresponding ketones in high yields and selectivities in the presence of a Cu(I) catalyst and air as the oxidant. This newly developed Cu(I)/MOF ligand system can also be easily extended to the aerobic oxidation of primary alcohols including aliphatic ones. Furthermore, the MIL-101-N-2-pyc ligand can be recycled several times without compromising reaction activity.
Introduction
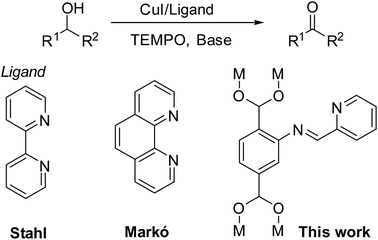
Efficient and selective oxidation of alcohols to aldehydes or ketones is a fundamental step in the synthesis of several natural products and other useful compounds.1 In the past, there have been several well-known selective oxidation processes realized in an industrial setting, such as the preparation of cumene peroxide, acetaldehyde, and propylene oxide. Among the several oxygen sources available for the selective alcohol oxidation reaction, molecular oxygen would be the most environmentally and economically advantageous.2 During the past few decades, copper complexes have overcome other and noble metals such as Pd,3 Au,4 Ru5 and Rh6 as inexpensive, environmentally benign and efficient transition metal catalysts for the aerobic alcohol oxidation.7 Stahl and co-workers reported systems formed by [Cu(MeCN)4]X (X = TfO, BF4, or PF6) and 2,2′-bipyridine (bpy) ligand for primary alcohol oxidation in the presence of air8 (Fig. 1). Markó also developed a catalytic system, based on 1,10-phenanthroline as the ligand, CuCl as the catalyst and DBADH2/O2 as the oxidizer, which smoothly transformed secondary alcohols into the desired ketones in toluene (Fig. 1).9 Several other organic ligands were also studied in combination with copper salts for similar processes.10 Although many catalytic systems did display satisfactory efficiency for alcohol oxidation, only a very limited number of successful examples of aerobic oxidations of secondary alcohols has been reported to date.11 Furthermore, oxidation of secondary alcohols using oxygen or air remains a major challenge.Metal–organic frameworks (MOFs) are attractive microporous materials for a wide range of applications in gas absorption, chemical sensing, molecular separation, drug delivery and catalysis.12 Thanks to the ability of the organic linkers to be post-synthetically modified, MOFs are versatile platforms for a variety of transition metal-based catalytic systems.13 Stanley and Huang has developed a bipyridine MOF for the Pd(II) coordination and utilized the complex for an efficient Suzuki–Miyaura cross-coupling reaction.14 Given its very versatile nature, the N,N′-chelating ligand was employed in the oxidation of several secondary alcohols.15 Due to the toxicity and carcinogenicity of the commonly used N,N′-bidentate ligands, recyclable MOF/N,N′-ligands are desirable from a green chemistry point of view. Furthermore, their use lowers the cost of the oxidation process.16 However, the synthesis of an ideal solid ligand for copper salt immobilization featuring N,N′-chelating ligand for efficient aerobic secondary alcohol oxidation is still a challenge.17
In this study, we wish to report a novel MOF/CuI combination for the effective oxidation of secondary alcohols using air as the stoichiometric oxidant. A newly designed iminopyridine derived MIL-101-N-2-pyc MOF was utilized as the solid and recyclable ligand; it complexes with the copper ion from CuI to form the CuI/MIL-101-N-2-pyc catalyst in situ. To our knowledge, this is the first example of utilization of an MOF solid ligand in the oxidation of alcohols using air as a free and stoichiometric oxidant. Air is a more challenging oxidant than others because it is a low concentration form of molecular oxygen. The CuI/MIL-101-N-2-pyc MOF developed as a highly efficient aerobic oxidation catalyst for secondary alcohols could also be easily utilized for the oxidation of primary alcohols. In addition, the MIL-101-N-2-pyc solid ligand offers the great advantage of being recyclable.
Results and discussion
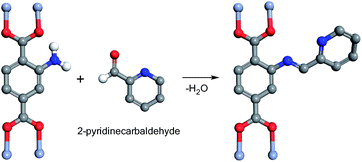
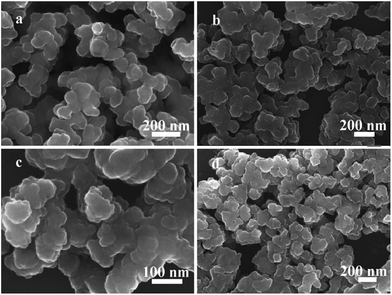
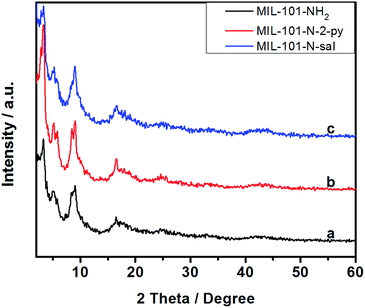
The MIL-101-NH2 used for post-synthetic modification in this project was obtained through a one-step synthetic procedure. This procedure furnished large quantity of MIL-101-NH2 directly from a chromium salt and 2-aminoterephthalic acid (NH2-H2BDC). Then, the facile synthesis of MIL-101-N-2-pyc was accomplished by reacting MIL-101-NH2 with 2-pyridinecarbaldehyde; a similar strategy was recently developed by Yaghi and co-workers (Fig. 2).18 As shown in Fig. 1 and S1,† the iminopyridine moiety on MIL-101-N-2-pyc bears no electron-withdrawing group, providing an ideal coordination environment for a copper salt.Scanning electron microscopy (SEM) of MIL-101-NH2 and MIL-101-N-2-pyc are shown in Fig. 3. The crystals of MIL-101-N-2-pyc MOF appear to have a spherical morphology with diameters of around 150 nm, which was retained after the post-synthetic modification with 2-pyridinecarbaldehyde. The powder X-ray diffraction (pXRD) pattern of MIL-101-NH2 was in agreement with the literature data.19 As expected, the pXRD patterns observed for the MIL-101-N-2-pyc material possessed the same crystalline reflections as its precursor MIL-101-NH2. The high similarity in pXRD patterns indicated the structural topology of MIL-101-N-2-pyc throughout the post-synthetic modification was maintained (Fig. 4). Not surprisingly, salicylaldehyde modified MIL-101-NH2 also showed the same XRD pattern as expected (Fig. 4c).
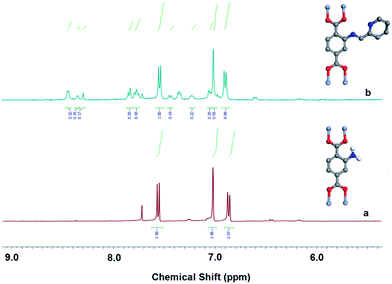
The post-synthetically modified MIL-101-NH2 was characterized by proton nuclear magnetic resonance (1H NMR) to determine its modification ratio (Fig. 5 and S2†). Nuclear magnetic resonance studies of the digested sample indicated that 24% of the amino groups are functionalized as aromatic iminopyridine. The amount of ligand usage is calculated according to the amount of functionalized amino groups based on the 1H-NMR results. However, FT-IR spectra failed to provide any evidence for internal structural alternation of MIL-101-NH2; this is probably due to the weakness of the peak corresponding to the iminopyridine group and to its low modification ratio (Fig. S3†).
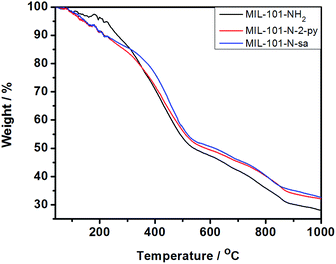
The MIL-101-NH2 and MIL-101-N-2-pyc were then examined by thermal gravimetric analysis (TGA) to confirm the thermal and structural stability of iminopyridine derived MOF ligand. Both MIL-101-NH2 and MIL-101-N-2-pyc showed good thermal stability with a weight loss at ∼372 °C as observed on the TGA curve. This observation indicates that the post-synthetic modification with 2-pyridinecarbaldehyde does not result in structural instability (Fig. 6).
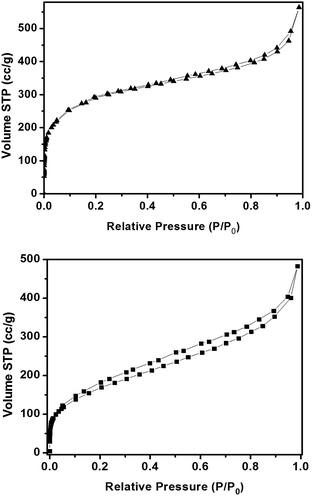
The high porosity of the parent MIL-101-NH2 was confirmed by nitrogen adsorption isotherms. Also, the porosity is retained after post-synthetic modification, even though the surface area decreased due to occupation by the imine functional groups of the pores. As a result, MIL-101-N-2-pyc has a calculated surface area of 1352.14 m2 g−1 as determined by the Brunauer–Emmett–Teller (BET) method. Although the BET surface area is reduced from the measured value of 1998.58 m2 g−1, for the parent chromium derived MIL-101-NH2 (Fig. 7), the surface area reduction of MIL-101-N-2-pyc has no negative impact on the excellent catalytic activity of the MIL-101-N-2-pyc ligand.
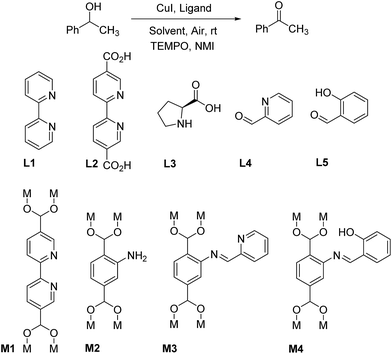
A solvent screening was performed in order to determine the optimal reaction conditions for the oxidation of 1-phenylethan-1-ol at room temperature. It was not surprising to observe the low yield of the reaction when run in toluene or ethanol since such solvents do not usually function well under aerobic oxidation reaction conditions (Table 1, entries 1 and 2).22 It was noticed that acetonitrile is the most suitable solvent for this transformation, even though DMF offered better catalytic results in terms of conversion and yield (Table 1, entries 3 and 4). Although not surprising, it was highly interesting to observe the superior catalytic result obtained with the use of DMF as solvent, since excellent results of DMF as a solvent for different catalytic systems has been reported in literature,20 although the strong basic and ligand effects of DMF were not thoroughly studied. In addition, the disadvantage of the use of DMF as solvent is significant since the removal of high boiling point DMF during the catalytic reaction is tedious, and because of DMF's known carcinogenicity. With the optimal reaction conditions in hand, a variety of organic ligands was further evaluated, either in homogeneous or heterogeneous form. 2,2′-Bipyridine L1 functioned as an excellent homogeneous organic ligand in the reaction and its role and mechanism were studied in detail by Stahl (Table 1, entry 5).21 The reaction is sluggish in the absence of the 2,2′-bipyridine ligand, indicating the crucial chelating effect the ligand has on CuI during the catalytic process (Table 1, entry 6). Unfortunately, the electron-withdraw functional group on the 2,2′-bipyridine ligand diminishes the yield of the desired product (L2, Table 1, entry 7). Since the carboxylate moiety is crucial for the formation of the MOF structure, we turned our attention towards looking for other possible ligands. N,O ligands, such as proline L3 and 2-pyridinecarbaldehyde L4 showed not to be suitable ligands for Cu(I) promoted aerobic oxidation reaction (Table 1, entries 8 and 9). Salicylaldehyde L5 also functioned poorly in the oxidation of secondary alcohols (Table 1, entry 10). As a result, only N,N′-compounds were determined to be efficient ligands for CuI catalysis and we focused our efforts on synthesizing a solid MOF ligand bearing a N,N′ bidentate ligand.
| Entry | Copper | Ligand | Solvent | Yieldb |
|---|---|---|---|---|
| a 5 mol% Cu catalyst, 5 mol% ligand, 5 mol% TEMPO, 10 mol% NMI in the presence of air at room temperature for 6 h.b Yield was calculated based on the GC-MS conversion and selectivity. | ||||
| 1 | CuI | L1 | PhCH3 | 27% |
| 2 | CuI | L1 | EtOH | 34% |
| 3 | CuI | L1 | DMF | 99% |
| 4 | CuI | — | DMF | 99% |
| 5 | CuI | L1 | CH3CN | 97% |
| 6 | CuI | — | CH3CN | 23% |
| 7 | CuI | L2 | CH3CN | <5% |
| 8 | CuI | L3 | CH3CN | 3% |
| 9 | CuI | L4 | CH3CN | 19% |
| 10 | CuI | L5 | CH3CN | 10% |
| 11 | CuI | M1 (Zr) | CH3CN | 25% |
| 12 | CuI | M2 (Cr) | CH3CN | 34% |
| 13 | CuI | M3 (Cr) | CH3CN | 95% |
| 14 | CuI | M4 (Cr) | CH3CN | 72% |
| 15 | CuBr | M3 (Cr) | CH3CN | 28% |
| 16 | CuCl | M3 (Cr) | CH3CN | 17% |
MOF M1 was synthesized according to the literature procedure.23 It was not surprising to observe an extremely poor reaction yield provided by M1 ligand (Table 1, entry 11), since the electron-withdrawing effect was investigated in entry 7 of Table 1. This observation excludes the further utilization of M1 ligand for aerobic oxidation studies of secondary alcohols. MIL-101-NH2 M2 only provided a moderate yield since the aromatic amino group is not a good coordinating functional group for Cu(I) (Table 1, entry 12). With all this information in hand, we intended to synthesize a MOF structure as the heterogeneous Cu(I) ligand, in which the pyridine moiety was not affected by carboxylate electron-withdraw groups. As a result, the M3 (Cr) ligand, which utilized 2-pyridinecarbaldehyde for the PSM of MIL-101-NH2, gave the best yield (95%) in presence of air under room temperature (Table 1, entry 13). This observation strongly supported our catalyst design strategy, which furnishes the successful and efficient solid ligand M3 (Cr). In the end, salicylaldehyde functionalized MIL-101-NH2 was not able to achieve a similarly high yield, presumably because of the poor coordination ability originated from the phenol group (Table 1, entry 14). CuBr and CuCl were much less efficient than CuI and they were excluded from further catalytic studies (Table 1, entries 15 and 16). In summary, MIL-101-N-2-pyc (M3 (Cr)) was chosen as the most optimal solid ligand, in combination with CuI for further catalytic studies. Our catalytic system showed great efficiency under extremely mild reaction conditions and using air as the oxidant. The reaction time and catalyst loading can be further lowered under elevated reaction temperatures; the observed catalytic activity is superior to that of several systems reported in literature for processes run under similar reaction condition (Table S1†).
As shown in Table 2, various substituted secondary alcohols were evaluated using the MIL-101-N-2-pyc/CuI system in combination with TEMPO and N-methylimidazole (NMI). Electron-rich phenylethanols were oxidized to their corresponding ketones in excellent yields and selectivities (Table 2, entries 1–3). Electron-deficient phenylethanols instead afforded lower yields even under extended reaction times; this is likely due to the lower electron-density of the benzylic carbon (Table 2, entries 4–6). Furthermore, para-phenylphenylethanol was evaluated as an electron-rich bulky alcohol; excellent yield and selectivity were observed (Table 2, entry 7). This suggests that our catalytic system is compatible with relatively larger alcohols. However, biphenylmethanol gave a lower yield due to its bulkiness (Table 2, entry 8). The decrease in yield for bulky substrates was also observed in other MOF catalytic studies; it was explained as due to the insufficient pore size of the 3D framework structure.
| Entry | R1 | R2 | Time (h) | Yield (%) |
|---|---|---|---|---|
| a Reaction condition: 5 mol% CuI, 5 mol% ligand, 5 mol% TEMPO, 10 mol% NMI in the presence of air at room temperature for 6 h. | ||||
| 1 | 4-CH3C6H4 | CH3 | 6 | 94 |
| 2 | 4-CH3OC6H4 | CH3 | 6 | 99 |
| 3 | 3-CH3OC6H4 | CH3 | 6 | 99 |
| 4 | 4-FC6H4 | CH3 | 12 | 90 |
| 5 | 4-ClC6H4 | CH3 | 12 | 85 |
| 6 | 3-CF3C6H4 | CH3 | 12 | 71 |
| 7 | 4-PhC6H4 | CH3 | 6 | 93 |
| 8 | Ph | Ph | 12 | 67 |
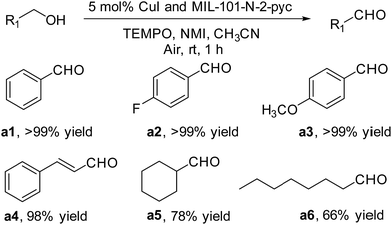
Under similar reaction conditions, the MIL-101-N-2-pyc/CuI system was further utilized in the aerobic oxidation of several primary alcohols over a shorter reaction time (Fig. 8). The aromatic alcohols, either bearing electron-donating or electron-withdrawing groups, reacted smoothly using the MIL-101-N-2-pyc/CuI system in presence of air for 1 h (Fig. 8, aldehydes a1–a3). In addition, (E)-3-phenylprop-2-en-1-ol was evaluated as an enol and a yield of 98% was achieved (Fig. 8, aldehyde a4). Cyclohexylmethanol and octan-1-ol were tested as aliphatic primary alcohols but only moderate yields were observed (Fig. 8, aldehydes a4 and a5). It can be concluded that the MIL-101-N-2-pyc/CuI system is highly efficient for the oxidation of primary alcohols under extremely mild reaction conditions.
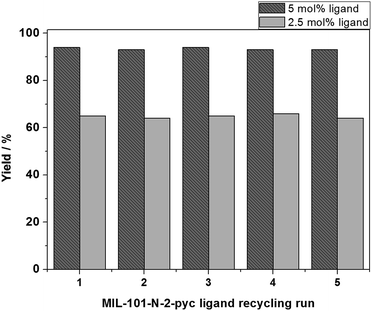
The MIL-101-N-2-pyc solid ligand was recycled for five times in order to examine its recyclability and chemical stability. The ligand was centrifuged from the reaction mixture and then washed with CH3CN. In this study, the high efficiency of the MIL-101-N-2-pyc ligand was maintained beyond the fifth reaction cycle (Fig. 9). Furthermore, the recycling experiment was also conducted at lower catalyst loading (2.5 mol% of MIL-101-N-2-pyc ligand and CuI). Compromised yield and similar selectivity was observed for the 1-phenylethan-1-ol oxidation reaction. As we expected, the yield of acetophenone product remained to be 65% after five reaction cycle, which is almost identical as the yield after the first reaction cycle. The strong covalent bond between the iminopyridine moiety and the aromatic group ensures stability to the ligand during the reaction. A hot filtration test was performed and the oxidation reaction stopped after the solid ligand was isolated from the reaction solution (Fig. S6†). This observation indicated that the MIL-101-N-2-pyc ligand is crucial for the reaction and that there was no leaching of the iminopyridine moiety. Also, the X-ray powder diffraction pattern of the MIL-101-N-2-pyc ligand remains unvaried even after five catalytic cycles (Fig. S4†). These facts clearly demonstrate that there was no alternation in internal MOF structure or decomposition over the aerobic oxidation process (Fig. S5†).
Conclusions
In conclusion, a novel 2-pyridinecarbaldehyde functionalized amino MOF was synthesized and utilized as a solid and recyclable ligand for Cu(I) catalysis. The newly synthesized MIL-101-N-2-pyc showed comparable efficiency with regard to synthetic ease and for the convenience of using the 2,2′-bipyridine ligand. An efficient aerobic alcohol oxidation system was designed for secondary alcohols based on the combination of CuI and of the solid MIL-101-N-2-pyc ligand under mild reaction conditions. A variety of primary and secondary alcohols was successfully oxidized to ketones under the optimized reaction conditions in high yields and selectivities. To our knowledge, it is the first example of secondary alcohol oxidation using a copper salt, a solid ligand and molecular oxygen in air as the oxidant. This study also highlights the use of air as a green and inexpensive oxidant and the recyclability of the MOF ligand.Experimental section
All the chemicals were used without further purification.Preparation of MIL-101-N-2-pyc ligand
The synthesis of amino-functionalized MIL-101 was done according to the literature procedure.17 1.39 g of MIL-101-NH2 (1.0 mmol based on MW of 1392 g mol−1, with 7 amino groups) were suspended in 15 mL of CH3CN; 21.0 mmol of 2-pyridinecarbaldehyde (2.25 g, 21.0 mmol) were then added. The mixture was stirred slowly at 40 °C for 12 h, after which the solvent was decanted. Fresh CH3CN (10 mL) was used to rinse the crystals once a day for three days. The crystals were dried under vacuum at 80 °C before use.Catalytic selective oxidation of alcohols
In general, the catalytic properties of the catalysts were examined for the alcohol oxidation with air in a 25 mL round-bottom flask. In a typical process, a mixture of acetonitrile (5.0 mL) and alcohol (1.0 mmol) was added into a 25 mL round-bottom flask, together with 5 mol% of the chosen copper species, 5 mol% of ligand, 5 mol% of TEMPO, and 10 mol% of NMI. After a certain reaction time, n-dodecane (0.2 mmol) was added as an internal standard for the determination of yield and selectivity. The filtered liquid samples were analyzed by GC-MS and 1H-NMR.MIL-101-N-2-pyc ligand recycling
At the end of each oxidation reaction cycle, the MIL-101-N-2-pyc ligand was recovered by centrifugation of the solution mixture followed by washing with 5–10 mL of CH3CN. After being immersed in the solvent for 12 h and dried at 40 °C under vacuum for 12 h, the MIL-101-N-2-pyc solid ligand was reused.Material characterization
Scanning electron microscope (SEM) images of samples were obtained with a ZEISS SUPRA 55 (the samples for the SEM measurements were first dispersed in ethanol, sonicated for a few minutes, and then supported onto the silicon slice and the holey carbon film on a Cu grid, respectively). The BET surface area, pore volume and pore diameter were measured by an AUTOSORB-1C analyzer. The phase composition of the products was characterized by X-ray powder diffraction (XRD, Cu Kα radiation, λ = 0.1542 nm) via a M21X diffractometer. Fourier transform infrared spectra (FT-IR) were collected by a Nicolet 6700 spectrometer. Thermogravimetric analysis (TGA) was conducted by using a Netzsch STA449F at a heating rate of 10 °C min−1 under the N2 flow. 1H-NMR spectra were recorded on a Varian Unity Plus 400 instrument using CDCl3 as the solvent. The results were analyzed by gas chromatography-mass spectrometry using n-dodecane as the internal standard (GC-MS, Agilent 7890/5975C-GC/MSD, HP5-MS column, Ar carrier gas, 200 °C).Acknowledgements
This work is supported by Beijing Natural Science Foundation (2172037) and National Natural Science Foundation of China (No. 51503016). P. Y. thanks the State Key Laboratory of Chemical Resource Engineering and Y. L. thanks the Fundamental Research Funds for the Central Universities (Grant No. FRF-TP-16-004A3) for funding support.Notes and references
- (a) J. C. J. Bart and S. Cavallaro, Ind. Eng. Chem. Res., 2015, 54, 567–576 CrossRef CAS; (b) M. G. Clerici and O. A. Kholdeeva, Liquid Phase Oxidation Via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications, John Wiley & Sons, Hoboken, 2013 Search PubMed.
- T. Mallat and A. Baiker, Chem. Rev., 2004, 104, 3037–3058 CrossRef CAS PubMed.
- (a) M. S. Sigman and D. R. Jensen, Acc. Chem. Res., 2006, 39, 221–229 CrossRef CAS PubMed; (b) V. Pascanu, A. B. Gomez, C. Ayats, A. E. Platero-Prats, F. Carson, J. Su, Q. Yao, M. À. Pericas, X. Zou and B. M. ín-Matute, ACS Catal., 2015, 5, 472–479 CrossRef CAS; (c) C. Qi, Y. Xiong, V. Eschenbrenner-Lux, C. Cong and J. A. Porco Jr, J. Am. Chem. Soc., 2016, 138, 798 CrossRef CAS PubMed.
- (a) Y. Luan, Y. Qi, H. Gao, N. Zheng and G. Wang, J. Mater. Chem. A, 2014, 2, 20588–20596 RSC; (b) L. Wang, J. Li, W. Dai, Y. Lv, Y. Zhang and S. Gao, Green Chem., 2014, 16, 2164–2173 RSC; (c) C. Qi, T. Qin, D. Suzuki and J. A. Porco Jr, J. Am. Chem. Soc., 2014, 136, 3374 CrossRef CAS PubMed; (d) Y. Luan, Y. Qi, H. Gao, R. S. Andriamitantsoa, N. Zheng and G. Wang, J. Mater. Chem. A, 2015, 3, 17320–17331 RSC.
- (a) K. Yamaguchi, K. Mori, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2000, 122, 7144–7145 CrossRef CAS; (b) H. Shimizu, S. Onitsuka, H. Egami and T. Katsuki, J. Am. Chem. Soc., 2005, 127, 5396–5413 CrossRef CAS PubMed.
- L. Liu, M. Yu, B. B. Wayland and X. Fu, Chem. Commun., 2010, 46, 6353–6355 RSC.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234–6458 CrossRef CAS PubMed.
- (a) J. M. Hoover and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 16901–16910 CrossRef CAS PubMed; (b) J. M. Hoover and S. S. Stahl, Org. Synth., 2013, 90, 240–250 CrossRef CAS.
- I. E. Markó, P. R. Giles, M. Tsukazaki, I. Chellé-Regnaut, A. Gautier, S. M. Brown and C. J. Urch, J. Org. Chem., 1999, 64, 2433–2439 CrossRef.
- (a) M. F. Semmelhack, C. R. Schmid, D. A. Cortes and C. S. Chou, J. Am. Chem. Soc., 1984, 106, 3374–3376 CrossRef CAS; (b) P. Gamez, I. W. C. E. Arends, J. Reedijk and R. A. Sheldon, Chem. Commun., 2003, 2414–2415 RSC; (c) N. Jiang and A. J. Ragauskas, J. Org. Chem., 2006, 71, 7087–7090 CrossRef CAS PubMed; (d) P. J. Figiel, M. Leskela and T. Repo, Adv. Synth. Catal., 2007, 349, 1173–1179 CrossRef CAS; (e) P. J. Figiel, A. Sibaouih, J. U. Ahmad, M. Nieger, M. T. Raisanen, M. Leskela and T. Repo, Adv. Synth. Catal., 2009, 351, 2625–2632 CrossRef CAS; (f) S. Mannam, S. K. Alamsetti and G. Sekar, Adv. Synth. Catal., 2007, 349, 2253–2258 CrossRef CAS.
- (a) I. E. Markó, P. R. Giles, M. Tsukazaki, S. M. Brown and C. J. Urch, Science, 1996, 274, 2044–2046 CrossRef; (b) V. Pascanu, A. Bermejo Gómez, C. Ayats, A. E. Platero-Prats, F. Carson, J. Su, Q. Yao, M. A. Pericàs, X. Zou and B. Martín-Matute, ACS Catal., 2015, 5, 472–479 CrossRef CAS; (c) T. Mitsudome, Y. Mikami, K. Ebata, T. Mizugaki, K. Jitsukawa and K. Kaneda, Chem. Commun., 2008, 4804–4806 RSC; (d) P. J. Figiel, M. N. Kopylovich, J. Lasri, M. F. C. Guedes da Silva, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, Chem. Commun., 2010, 46, 2766–2768 RSC.
- S. M. Cohen, Chem. Sci., 2010, 1, 32–36 RSC.
- (a) S. J. Garibay, Z. Wang and S. M. Cohen, Inorg. Chem., 2010, 49, 8086–8091 CrossRef CAS PubMed; (b) W. Zhu, C. He, P. Wu, X. Wu and C. Duan, Dalton Trans., 2012, 41, 3072–3077 RSC.
- X. Li, R. V. Zeeland, R. V. Maligal-Ganesh, Y. Pei, G. Power, L. Stanley and W. Huang, ACS Catal., 2016, 6, 6324–6328 CrossRef CAS.
- (a) L. Rogan, N. Louise Hughes, Q. Cao, L. M. Dornana and M. J. Muldoon, Catal. Sci. Technol., 2014, 4, 1720–1725 RSC; (b) M. M. Hossaina and S.-G. Shyu, Adv. Synth. Catal., 2010, 352, 3061–3068 CrossRef.
- (a) J. Canivet, S. Aguado, Y. Schuurman and D. Farrusseng, J. Am. Chem. Soc., 2013, 135, 4195–4198 CrossRef CAS PubMed; (b) C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492–9493 CrossRef CAS PubMed; (c) A. Sasmal, E. Garribba, C. Rizzoli and S. Mitra, Inorg. Chem., 2014, 53, 6665–6674 CrossRef CAS PubMed.
- (a) J. Yang, P. Li and L. Wang, Catal. Commun., 2012, 27, 58–62 CrossRef; (b) J. Liu, X. Zhang, J. Yang and L. Wang, Appl. Organomet. Chem., 2014, 28, 198–203 CrossRef CAS.
- C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492–9493 CrossRef CAS PubMed.
- Y. Lin, C. Kong and L. Chen, RSC Adv., 2012, 2, 6417–6419 RSC.
- (a) G. Zhang, X. Han, Y. Luan, Y. Wang, X. Wen, L. Xu and C. Ding, Chem. Commun., 2013, 49, 7908–7910 RSC; (b) D. Damodara, R. Arundhathi and P. R. Likhar, Adv. Synth. Catal., 2014, 356, 189–198 CrossRef CAS; (c) H.-Y. Sun, Q. Hua, F.-F. Guo, Z.-Y. Wang and W.-X. Huang, Adv. Synth. Catal., 2012, 354, 569–573 CrossRef CAS.
- (a) J. M. Hoover, B. L. Ryland and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 2357–2367 CrossRef CAS PubMed; (b) J. M. Hoover, J. E. Steves and S. S. Stahl, Nat. Protoc., 2012, 7, 1161–1166 CrossRef CAS PubMed.
- Y. Qi, Y. Luan, J. Yu, X. Peng and G. Wang, Chem.–Eur. J., 2015, 21, 1589–1597 CrossRef CAS PubMed.
- S. Øien, G. Agostini, S. Svelle, E. Borfecchia, K. A. Lomachenko, L. Mino, E. Gallo, S. Bordiga, U. Olsbye, K. P. Lillerud and C. Lamberti, Chem. Mater., 2015, 27, 1042–1056 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00296c |
| This journal is © The Royal Society of Chemistry 2017 |