Open Access Article
Open Access ArticleSynthesis, characterization and optical properties of novel dendronized azo-dyes containing a fullerene C60 unit and well-defined oligo(ethylene glycol) segments†
Jesús Ortíz-Palaciosa,
Gerardo Zaragoza-Galánb,
Edgar Aguilar-Ortíza,
Efraín Rodríguez-Albaa and
Ernesto Rivera *a
*a
aInstituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior Ciudad Universitaria, C. P. 04510, D.F, Mexico. E-mail: riverage@unam.mx
bFacultad de Ciencias Químicas, Universidad Autónoma de Chihuahua, Campus Universitario, #2, Apartado Postal 669, Chihuahua, Mexico
First published on 16th March 2017
Abstract
Herein, we report the preparation and characterization of a novel series of dendronized azo-dyes containing a fullerene C60 unit and well-defined oligo(ethylene glycol) spacers. The azobenzene units present in these dyes were substituted in the 4′-position with different functional groups (–H, –OCH3, –C4H9, –CN and –NO2). The optical properties of these compounds were studied by absorption spectroscopy as a function of the dipole moment of the azobenzene moieties. All fullerene C60-azobenzene derivatives exhibited trans–cis photoisomerization and photoprotonation under irradiation with UV-light. The results were compared to those obtained with their precursor azo-dyes without fullerene C60. It was found that the presence of the fullerene unit significantly quenches the photoisomerization yield, whereas the photoprotonation was not affected by the presence of this chromophore.
1 Introduction
Fullerene C60 has been widely studied and employed in the elaboration of many opto-electronic and photovoltaic devices because of its remarkable electronic properties. This chromophore is considered one of the best molecular electron-acceptor groups able to participate in Förster Resonance Energy Transfer (FRET) and Charge Transfer (CT) phenomena either in solution or in the solid state.1–3 However, the applications of fullerene C60 itself are very limited because of its poor solubility in organic solvents. The incorporation of alkyl chains and other functional groups into fullerene C60 has led to the development of novel molecular structures with enormous potential optical applications.4–8 Very recently, some hybrid systems based on azo-dyes containing fullerene C60 units have been investigated due to their synergistic effect. Wang et al. reported the functionalization of fullerene C60 via the addition of a substituted azobenzene as a carbene active intermediate.9 Kay et al. reported the synthesis and optical properties of a novel azo-dendrimer bearing a fullerene C60 unit as core.10 Other related structures combining azobenzene-fullerene, exhibiting interesting optical properties, have been reported in the literature.11–13 Some of them led to the development of new materials for molecular photoswitching,9,10,14 smart optoelectronic devices15 and organic solar cells.16,17Rau classified azobenzenes into three main categories based on their photochemical behaviour.18 Unsubstituted photochromic azobenzene belong to the first category, known as “azobenzenes”. The thermally stable trans isomer exhibits an intense absorption band at 350 nm due to the π–π* transition, as well as a weak intensity band at 440 nm related to the n–π* transition, whereas the cis isomer undergoes similar transitions but with a more intense n–π* band. Moreover, “azobenzenes” have a relatively poor overlap of the π–π* and n–π* bands. The second category, known as “aminoazobenzenes” usually includes azobenzenes that are substituted by an electron-donor group and are characterized by the significant overlap of the π–π* and n–π* bands. Finally, azobenzenes bearing an electron-donor and an electron-acceptor group belong to the third category, named “pseudostilbenes”, where the π–π* and n–π* bands are practically superimposed and inverted on the energy scale.18
When donor–acceptor substituted azobenzenes are incorporated into a polymer backbone, they generate very versatile photoactive materials. In particular, the irradiation of these polymers with linear polarized light produces rapid trans–cis cis–trans photoisomerization of “pseudostilbene” azobenzenes. In consequence, polarized light allows the selective activation of “pseudostilbenes” bearing a polarization axis parallel to the absorbing radiation, which causes the photoalignment of the azobenzene moieties until they become perpendicular to the light polarization axis.16–22
Our research group has developed different series of azo-dyes23–26 and azo-polymers27,28 with different structures. Very recently, we reported the preparation and optical properties of some photoactive azo-dendrons and azo-dendrimers bearing a liquid crystalline behaviour, which exhibited a photoprotonation phenomenon.29 In this work, we describe the synthesis and characterization of a novel series of dendronized azo-dyes containing fullerene C60 units, flexible alkyl and oligo(ethylene glycol) spacers. The amino-azobenzenes reported in this work were substituted in the 4′-position with different functional groups (–H, –OCH3, –C4H9, –CN and –NO2). The Bingel reaction was used in order to obtain novel fullerene C60-azobenzene branched systems (Fig. 1 vide infra), which exhibit trans–cis photoisomerization and photoprotonation under irradiation with UV-light. The optical properties of these compounds were studied in detail by absorption spectroscopy. The aim of this work is to demonstrate the ability of these compounds to modify their optical properties by photoprotonation and their susceptibility to favor the energy transfer phenomenon instead of the trans–cis photoisomerization due to the strong acceptor character of the fullerene unit.
2 Experimental
2.1 General conditions
All reagents used in the synthesis of the fullerene C60-azobenzene derivatives were purchased from Aldrich and used as received without further purification. Dichloromethane was dried by distillation over calcium hydride and anhydrous toluene was employed. Precursor azo-dyes were synthesized according to the method previously reported by us,23,24,30 whereas N-ethyl-N-(2-hydroxyethyl)-4-(4-nitrophenylazo) aniline (Disperse Red-1, DR1) was purchased from Aldrich. The fullerene C60 was incorporated into azobenzene derivatives as described in the literature.31,321H and 13C NMR spectra of the intermediates and final compounds involved in the synthesis were recorded in CDCl3 solution at room temperature on a Bruker Avance 400 MHz spectrometer, operating at 400 MHz and 100 MHz for 1H and 13C, respectively. All compounds were dissolved in spectral quality solvents purchased from Aldrich, and their absorption spectra were recorded on a Varian Cary 1 Bio UV-vis (model 8452A) spectrophotometer at room temperature, using 1 cm width quartz cuvettes. MALDI-TOF mass spectra were obtained on a Bruker Daltonic Felx Analysis, using dithranol as matrix.
Photoisomerization experiments were carried out in solution and solid state (cast film). For the disubstituted malonic derivatives (9, 10, 11) and the fullerene C60-azobenzene derivatives (15, 16, 17) the photoisomerization experiments were conducted in DMF solution (2.5 × 10−4 M) at room temperature. Cast films were prepared by depositing a saturated solution of the azo-dye on quartz substrates. Solutions and films were irradiated using a Compact UV lamp model UVGL-25, 254/365 nm (6 W). The samples were irradiated with intervals of 10 s and the spectral changes were monitored by absorption spectroscopy. Meanwhile, photoprotonation experiments with the fullerene C60-azobenzene derivatives were carried out in CHCl3 solution. Compounds 15 and 19 were irradiated at 254 nm and monitored by absorption spectroscopy with intervals of 10 and 30 s, respectively.
2.2 Synthesis
1H NMR (400 MHz, CDCl3): δ = 6.49 (t, J = 2 Hz, 2H, H1), 6.37 (t, J = 2 Hz, 1H, H2), 4.61 (s, 2H, PhCH2OH), 3.93 (t, J = 7 Hz, 4H, PhOCH2), 1.79–1.72 (m, 4H, PhOCH2CH2), 1.47–1.26 (m, 36H, all CH2 of the aliphatic chain), 0.88 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 160.52 (2C, Cc), 143.16 (1C, Ca), 105.02 (2C, Cb), 100.52 (1C, Cd), 68.03 (2C, PhOCH2), 65.44 (1C, PhCH2OH), 31.90, 29.61–29.18, 26.02, 25.70, 22.87 (20C, all CH2 of the aliphatic chain), 14.09 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3) δ = 6.47 (d, J = 2 Hz), 6.42 (t, J = 2 Hz), 5.14, (s, 2H), 3.93 (t, J = 6 Hz, 4H), 3.50 (s, 2H), 1.77 (m, 4H), 1.26 (m, 36H), 0.88 (t, J = 6 Hz, 6H) ppm.
13C NMR (100 MHz, CDCl3): δ = 171.38, 166.37, 160.39, 136.88, 106.38, 101.22, 68.01, 67.46, 40.78, 31.87, 29.55–29.16, 25.97, 22.62, 14.05 ppm.
2.3 Synthesis of the precursor azo-dyes
Precursor azo-dyes (4), (5) and RED-PEG-4 (7) were prepared according to the method previously reported by us.30,33,35For the synthesis of azobenzenes (E)-2-(4-(phenyldiazenyl)phenyl)-5,8,11-trioxa-2-azatridecan-13-ol (3) and (E)-4-((4-((2-(2-(2-(2-hydroxyethoxy)ethoxy)ethoxy)ethyl)(methyl)amino)phenyl)diazenyl)benzonitrile (6) (see ESI†).
2.4 Synthesis of the disubstituted malonic ester
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluent to yield compound 9 (Scheme 1a). Yield: 55%.
2) as eluent to yield compound 9 (Scheme 1a). Yield: 55%.
1H NMR (400 MHz, CDCl3): δ = 7.87 (d, J = 9 Hz, 2H, H5), 7.84 (d, J = 9 Hz, 2H, H4), 7.47–7.37 (m, 3H, H6–H), 6.75 (d, J = 9 Hz, 2H, H3), 6.47 (d, J = 2 Hz, 2H, H2), 6.41 (t, J = 2 Hz, 1H, H1), 5.09 (s, 2H, PhCH2OOC), 4.29 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.92 (t, J = 6 Hz, 4H, PhOCH2 of the aliphatic chain), 3.68–3.59 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.46 (s, 2H, OOCCH2COO), 3.07 (s, 3H, CH3N), 1.80–1.71 (m, 4H, PhOCH2CH2), 1.46–1.39 (m, 4H, PhO(CH2)2CH2), 1.36–1.27 (m, 32H, all CH2 of the aliphatic chain), 0.90 (t, J = 6 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.17 (1C, Cg), 166.01 (1C, Ce), 160.28 (2C, Cb), 153.00 (1C, Ch), 151.22 (1C, Cl), 143.45 (1C, Ck), 137.08 (1C, Cd), 129.14 (1C, Co), 128.70 (2C, Cn), 124.85 (2C, Cj), 122.02 (2C, Cm), 111.21 (2C, Ci), 106.20 (2C, Cc), 100.94 (1C, Ca), 70.58–70.41, 68.37 (6C, OCH2 of the tetra(ethylene glycol) chain), 68.59 (2C, PhOCH2 of the aliphatic chain), 67.85 (1C, PhCH2O), 66.96 (1C, NH2(CH2)2), 64.35 (1C, COOCH2 of the tetra(ethylene glycol) chain), 51.97 (1C, NCH2), 41.18 (1C, Cf), 39.00 (1C, NCH3), 31.75, 29.50–29.07, 25.88, 22.52 (20C, all CH2 of the aliphatic chain), 13.97 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.83 (d, J = 9 Hz, 2H, H5), 7.74 (d, J = 8 Hz, 2H, H4), 7.26 (d, J = 8 Hz, 2H, H6), 6.75 (d, J = 9 Hz, 2H, H3), 6.46 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.09 (s, 2H, PhCH2OOC), 4.29 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.92 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.69–3.60 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.45 (s, 2H, OOCCH2COO), 3.09 (s, 3H, CH3N), 2.67 (t, J =8 Hz, 2H, PhCH2), 1.79–1.70 (m, 4H, PhOCH2CH2), 1.68–1.57 (m, 2H, Ph(CH2)2), 1.45–1.26 (m, 38H, Ph(CH2)3 and all CH2 of the aliphatic chain), 0.95 (t, J = 7 Hz, 6H, CH3), 0.89 (t, J = 6 Hz, 3H, Ph(CH2)CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.37 (1C, Cg), 166.21 (1C, Ce), 160.43 (2C, Cb), 151.38 (1C, Ch), 151.14 (1C, Cl), 144.63 (1C, Co), 143.68 (1C, Ck), 137.18 (1C, Cd), 128.90 (2C, Cn), 124.77 (2C, Cm), 122.09 (2C, Cj), 111.38 (2C, Ci), 106.39 (2C, Cc), 101.10 (1C, Ca), 70.74–70.57, 68.76 (6C, OCH2 of the tetra(ethylene glycol) chain), 67.17 (2C, PhOCH2 of the aliphatic chain), 68.52 (1C, PhCH2O), 68.05 (1C, N(CH2)2) 64.53 (1C, COOCH2 of the tetra(ethylene glycol) chain), 52.15 (1C, NCH2), 41.34 (1C, Cf) 39.18 (1C, NCH3), 35.46 (1C, PhCH2), 33.48 (1C, PhCH2CH2), 31.88, 29.63–29.21, 26.01, 22.65 (20C, all CH2 of the aliphatic chain), 22.30 (1C, Ph(CH2)2CH2), 14.08 (2C, CH3), 13.91 (1C, Ph(CH2)3CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.83 (d, J = 9 Hz, 2H, H5), 7.81 (d, J = 9 Hz, 2H, H4), 6.97 (d, J = 9 Hz, 2H, H6), 6.76 (d, J = 9 Hz, 2H, H3), 6.46 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.09 (s, 2H, PhCH2OOC), 4.29 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.92 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.86 (s, 3H, PhOCH3), 3.69–3.61 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.46 (s, 2H, OOCCH2COO), 3.08 (s, 3H, CH3N), 1.78–1.75 (m, 4H, PhOCH2CH2), 1.44–1.26 (m, 36H, all CH2 of the aliphatic chain), 0.88 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.35 (1C, Cg), 166.19 (1C, Ce), 160.78 (1C, Co), 160.42 (2C, Cb), 150.92 (1C, Ch), 147.43 (1C, Cl), 143.64 (1C, Ck), 137.18 (1C, Cd), 124.53 (2C, Cj), 123.76 (2C, Cm), 114.02 (2C, Cn), 111.41 (2C, Ci), 106.38 (2C, Cc), 101.09 (1C, Ca), 70.73–70.56, 68.75 (6C, OCH2 of the tetra(ethylene glycol) chain), 68.52 (2C, PhOCH2 of the aliphatic chain), 68.04 (1C, PhCH2O), 67.15 (1C, N(CH2)2), 64.52 (1C, COOCH2 of the tetra(ethylene glycol) chain), 55.43 (1C, NCH2), 52.16 (1C, OCH3), 41.33 (1C, Cf), 39.15 (1C, NCH3), 31.87, 29.62–29.20, 26.00, 22.64 (20C, all CH2 of the aliphatic chain), 14.07 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.86 (d, J = 9 Hz, 2H, H5), 7.85 (d, J = 10 Hz, 2H, H4), 7.70 (d, J = 9 Hz, 2H, H6), 6.75 (d, J = 9 Hz, 2H, H3), 6.45 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.08 (s, 2H, PhCH2OOC), 4.29 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.91 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.68–3.60 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.45 (s, 2H, OOCCH2COO), 3.11 (s, 3H, CH3N), 1.79–1.74 (m, 4H, PhOCH2CH2), 1.45–1.25 (m, 36H, all CH2 of the aliphatic chain), 0.88 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.26 (1C, Cg), 166.09 (1C, Ce), 160.36 (2C, Cb), 155.31 (1C, Cl), 152.24 (1C, Ch), 143.48 (1C, Ck), 137.11 (1C, Cd), 132.90 (2C, Cn), 125.77 (2C, Cm), 122.57 (2C, Cj), 118.84 (1C, Co), 111.70 (1C, PhCN), 111.34 (2C, Ci), 106.31 (2C, Cc), 100.99 (1C, Ca), 70.70–70.50, 68.68 (6C, OCH2 of the tetra(ethylene glycol) chain), 67.07 (PhOCH2 of the aliphatic chain), 68.44 (1C, PhCH2O), 67.97 (1C, N(CH2)2), 64.42 (1C, COOCH2 of the tetra(ethylene glycol) chain), 52.07 (1C, NCH2), 41.27 (1C, Cf), 39.18 (1C, NCH3), 31.80, 29.55–29.13, 25.93, 22.56 (20C, all CH2 of the aliphatic chain), 14.01 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 8.27 (d, J = 9 Hz, 2H, H6), 7.88 (d, J = 9 Hz, 2H, H5), 7.86 (d, J = 9 Hz, 2H, H4), 6.75 (d, J = 9 Hz, 2H, H3), 6.45 (d, J = 2 Hz, 2H, H2), 6.38 (t, J = 2 Hz, 1H, H1), 5.08 (s, 2H, PhCH2OCO), 4.29 (t, J = 5 Hz, 2H, PhOCH2 of the tetra(ethylene glycol) chain), 3.90 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.69–3.60 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.45 (s, 2H, OOCCH2COO), 3.11 (s, 3H, CH3N), 1.75–1.70 (m, 4H, PhOCH2CH2), 1.45–1.38 (m, 4H, PhO(CH2)2CH2), 1.33–1.24 (m, 32H, all CH2 of the aliphatic chain), 0.87 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.21 (1C, Ce), 166.06 (1C, Cg), 160.45 (2C, Cb), 156.74 (1C, Cl), 152.54 (1C, Ch), 147.34 (1C, Co), 143.77 (1C, Ck), 137.21 (1C, Cd), 125.99 (2C, Cj), 124.50 (2C, Cn), 122.49 (2C, Cm), 111.46 (2C, Ci), 106.42 (2C, Cc), 101.18 (1C, Ca), 70.77–70.57, 68.74 (6C, OCH2 of the tetra(ethylene glycol) chain), 67.08 (2C, PhOCH2 of aliphatic chain), 68.55 (1C, PhCH2O), 68.06 (1C, N(CH2)2), 64.44 (1C, COOCH2 of the tetra(ethylene glycol) chain), 52.17 (1C, NCH2), 41.31 (1C, Cf), 39.17 (1C, NCH3), 31.81, 29.56–29.19, 25.97, 22.57 (20C, all CH2 of the aliphatic chain), 13.98 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 8.31 (d, J = 9 Hz, 2H, H6), 7.91 (d, J = 9 Hz, 2H, H5), 7.89 (d, J = 10 Hz, 2H, H4), 6.76 (d, J = 9 Hz, 2H, H3), 6.46 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.09 (s, 2H, PhCH2OCO), 4.36 (t, J = 6 Hz, 2H, NCH2CH2OCO), 3.91 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.66 (t, J = 6 Hz, 2H, CH3CH2NCH2), 3.51–3.46 (m, 2H, OCOCH2CH2NCH2) 3.45 (s, 2H, OOCCH2COO), 1.77–1.70 (m, 4H, PhOCH2CH2), 1.45–1.38 (m, 4H, PhO(CH2)2CH2), 1.31–1.20 (m, 35H, all CH2 of the aliphatic chain and NCH2CH3), 0.88 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 166.19 (1C, Ce), 165.91 (1C, Cg), 160.54 (2C, Cb), 156.74 (1C, Cl), 151.10 (1C, Ch), 147.54 (1C, Co), 144.01 (1C, Ck), 137.16 (1C, Cd), 126.21 (2C, Cn), 124.58 (2C, Cj), 122.63 (2C, Cm), 111.48 (2C, Ci), 106.61 (2C, Cc), 101.21 (1C, Ca), 68.15 (2C, PhOCH2 of the aliphatic chain), 67.26 (1C, PhCH2O), 62.23 (1C, CH3CH2NCH2CH2O), 48.57 (1C, CH3CH2NCH2), 45.67 (1C, CH3CH2N), 41.43 (1C, Cf), 31.88 (2C, CH3(CH2)2), 29.63–29.25 (14C, all CH2 of the aliphatic chain), 26.03 (2C, PhO(CH2)3), 22.64 (2C, CH3CH2), 14.04 (1C, CH3), 12.27 (1C, NCH2CH3) ppm.
2.5 Synthesis of the fullerene C60-azobenzene derivatives
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used as eluent in order to remove unreacted fullerene C60. In the second column, a mixture of hexane/ethyl acetate (7
1) was used as eluent in order to remove unreacted fullerene C60. In the second column, a mixture of hexane/ethyl acetate (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 6
3 and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was employed to give the pure desired compound 15 (Scheme 2a). Yield: 31%. MALDI-TOF: C115H83N3O9 calcd: [M + H]+ 1650.90 found (m/z): [M + H]+ 1651.93.
4) was employed to give the pure desired compound 15 (Scheme 2a). Yield: 31%. MALDI-TOF: C115H83N3O9 calcd: [M + H]+ 1650.90 found (m/z): [M + H]+ 1651.93.
1H NMR (400 MHz, CDCl3) (Scheme 2a): δ = 7.85 (d, J = 9 Hz, 2H, H5), 7.83 (d, J = 8 Hz, 2H, H4), 7.48–7.38 (m, 3H, H6–H), 6.75 (d, J = 9 Hz, 2H, H3), 6.58 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.43 (s, 2H, PhCH2OCO), 4.63 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.89 (t, J = 6.50 Hz, 4H, PhOCH2 of the aliphatic chain), 3.83 (t, J = 5, 2H, OCH2), 3.68–3.62 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.09 (s, 3H, CH3N), 1.77–1.73 (m, 4H, PhOCH2CH2), 1.43–1.25 (m, 36H, all CH2 of the aliphatic chain), 0.89 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3) (Scheme 2a): 163.46 (1C, Cg), 163.29 (1C, Ce), 160.47 (2C, Cb), 153.17 (1C, Ch), 151.34 (1C, Cl), 145.20, 145.16, 145.11, 144.97, 144.83, 144.63, 144.60, 144.55, 144.48, 144.41, 143.80, 143.78, 143.64, 143.01, 142.96, 142.89, 142.13, 141.85, 141.77, 140.84, 140.82, 139.34, 138.66 (fullerene carbons), 136.55 (1C, Cd), 129.31 (1C, Co), 128.87 (2C, Cn), 125.02 (2C, Cj), 122.17 (2C, Cm), 111.38 (2C, Ci), 107.15 (2C, Cc), 101.64 (1C, Ca), 71.40 (fullerene carbon), 70.75–70.64, 68.91, 68.66 (7C, OCH2 of the chain tetra(ethylene glycol) chain), 68.53 (2C, PhOCH2 of the aliphatic chain), 68.11 (1C, PhCH2O), 66.18 (1C, NCH2), 52.15 (1C, Cf), 39.24 (1C, NCH3), 31.90, 29.67–29.26, 26.10, 22.67 (20C, CH2 of the aliphatic chain), 14.12 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.83 (d, J = 9 Hz, 2H, H5), 7.75 (d, J = 8 Hz, 2H, H4), 7.27 (d, J = 7 Hz, 2H, H6), 6.75 (d, J = 9 Hz, 2H, H3), 6.58 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.43 (s, 2H, PhCH2OOC), 4.62 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.89 (t, J = 6 Hz, 4H, PhOCH2 of the aliphatic chain), 3.83 (t, J = 5 Hz, OCH2), 3.69–3.62 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.09 (s, 3H, CH3N), 2.67 (t, J = 8 Hz, 2H, PhCH2), 1.77–1.70 (m, 4H, PhOCH2CH2), 1.67–1.60 (m, 2H, Ph(CH2)2), 1.42–1.25 (m, 38H, Ph(CH2)3 and all CH2 of the aliphatic chain), 0.95 (t, J = 7 Hz, 6H, CH3), 0.89 (t, J = 6 Hz, 3H, Ph(CH2)CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 163.46 (1C, Cg), 163.31 (1C, Ce), 160.54 (2C, Cb), 151.46 (1C, Ch), 151.18 (1C, Cl), 144.67 (1C, Co), 143.84 (1C, Ck), 145.24, 145.20, 145.15, 145.05, 145.02, 144.87, 143.05, 143.00, 142.17, 141.89, 141.82, 140.88, 139.37, 138.71 (fullerene carbons), 136.60 (1C, Cd), 128.91 (2C, Cn), 124.82 (2C, Cm), 122.16 (2C, Cj), 111.46 (2C, Ci), 107.24 (2C, Cc), 101.77 (1C, Ca), 71.49 (fullerene carbon), 70.76–70.69, 68.94 (6C, OCH2 of the tetra(ethylene glycol) chain) 68.52 (PhOCH2 of the aliphatic chain), 68.61 (1C, PhOCH2), 68.18 (1C, PhCH2O), 66.22 (1C, NCH2), 52.23 (1C, Cf), 39.22 (1C, NCH3), 35.49 (1C, PhCH2), 33.49 (1C, PhCH2CH2), 31.92, 29.69–29.30, 26.13, 22.68 (20C, all CH2 of the aliphatic chain), 22.33 (1C, Ph(CH2)2CH2), 14.11 (2C, CH3), 13.93 (1C, Ph(CH2)3CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.82 (d, J = 9 Hz, 2H, H5), 7.81 (d, J = 9 Hz, 2H, H4), 6.98 (d, J = 8.98 Hz, 2H, H6), 6.76 (d, J = 9 Hz, 2H, H3), 6.58 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.43 (s, 2H, PhCH2OOC), 4.62 (t, J = 5 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.89 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.82 (t, J = 5 Hz, OCH2), 3.87 (s, 3H, PhOCH3), 3.69–3.62 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.08 (s, 3H, CH3N), 1.77–1.70 (m, 4H, PhOCH2CH2), 1.45–1.25 (m, 36H, all CH2 of the aliphatic chain), 0.89 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 163.47 (1C, Cg), 163.31 (1C, Ce), 160.85 (1C, Co), 160.54 (2C, Cb), 150.98 (1C, Ch), 147.54 (1C, Cl), 145.24, 145.21, 145.16, 145.06, 144.88, 144.68, 144.60, 144.54, 144.46, 143.85, 143.06, 143.00, 142.18, 141.90, 141.83, 140.89, 139.38, 138.71 (fullerene carbons), 136.61 (1C, Cd), 124.60 (2C, Cj), 123.83 (2C, Cm), 114.10 (2C, Cn), 111.50 (2C, Ci), 107.26 (2C, Cc), 101.78 (1C, Ca), 71.50 (fullerene carbon), 70.77–70.70, 68.71 (6C, OCH2 of the tetra(ethylene glycol) chain), 68.96 (PhOCH2 of the aliphatic chain), 68.63 (1C, PhCH2O), 68.20 (1C, OOCCH2 of the tetra(ethylene glycol) chain), 66.23 (1C, NCH2), 55.50 (1C, OCH3), 52.26 (1C, Cf), 39.21 (1C, NCH3), 31.93, 29.70–29.31, 26.13, 22.69 (20C, all CH2 of the aliphatic chain), 14.11 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 7.88 (d, J = 9 Hz, 2H, H5), 7.86 (d, J = 9 Hz, 2H, H4), 7.73 (d, J = 8 Hz, 2H, H6), 6.76 (d, J = 9 Hz, 2H, H3), 6.58 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.43 (s, 2H, PhCH2OOC), 4.62 (t, J = 6 Hz, 2H, OOCCH2 of the tetra(ethylene glycol) chain), 3.90 (t, J = 6 Hz, 4H, PhOCH2 of the aliphatic chain), 3.83 (t, J = 5 Hz, OCH2), 3.68–3.62 (m, 14H, CH2N y OCH2 of the tetra(ethylene glycol) chain), 3.12 (s, 3H, CH3N), 1.78–1.68 (m, 4H, PhOCH2CH2), 1.45–1.25 (m, 36H, all CH2 of the aliphatic chain), 0.89 (t, J = 6 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 163.47 (1C, Cg), 163.33 (1C, Ce), 160.48 (2C, Cb), 149.98 (1C, Cl), 147.83 (1C, Ch), 145.21, 145.17, 145.13, 144.99, 144.84, 144.65, 144.58, 144.43, 143.80, 142.97, 142.91, 142.15, 141.86, 141.78, 140.86, 140.83, 139.36, 136.56 (fullerene carbons), 129.33 (1C, Cd), 128.90 (2C, Cn), 125.03 (2C, Cm), 122.18 (2C, Cj), 118.01 (1C, Co), 111.39 (2C, PhCN), 111.34 (1C, Ci), 107.18 (2C, Cc), 101.66 (1C, Ca), 72.87 (fullerene carbon), 70.76–70.66, 68.67 (6C, OCH2 of the tetra(ethylene glycol) chain) 68.44 (2C, PhOCH2 of the aliphatic chain), 68.93 (1C, PhCH2O), 68.54 (1C, PhCH2O), 68.12 (1C, OOCCH2 of the tetra(ethylene glycol) chain), 66.19 (1C, NCH2), 52.16 (1C, Cf), 39.25 (1C, NCH3), 31.91, 29.68–29.27, 26.11, 22.68 (20C, all CH2 of the aliphatic chain), 14.13 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 8.32 (d, J = 9 Hz, 2H, H6), 7.91 (d, J = 9 Hz, 2H, H5), 7.88 (d, J = 9 Hz, 2H, H4), 6.77 (d, J = 9 Hz, 2H, H3), 6.58 (d, J = 2 Hz, 2H, H2), 6.40 (t, J = 2 Hz, 1H, H1), 5.43 (s, 2H, PhCH2OCO), 4.63 (t, J = 5 Hz, 2H, PhOCH2 of the tetra(ethylene glycol) chain), 3.89 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.83 (t, J = 5 Hz, OCH2), 3.69–3.62 (m, 14H, CH2N and OCH2 of the tetra(ethylene glycol) chain), 3.13 (s, 3H, CH3N), 1.76–1.70 (m, 4H, PhOCH2CH2), 1.45–1.37 (m, 4H, PhO(CH2)2CH2), 1.35–1.25 (m, 32H, all CH2 of the aliphatic chain), 0.89 (t, J = 7 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 163.45 (1C, Ce), 163.30 (1C, Cg), 160.56 (2C, Cb), 156.83 (1C, Cl), 152.55 (1C, Ch), 147.44 (1C, Co), 145.24, 145.23, 145.17, 145.04, 145.01, 144.88, 144.69, 144.64, 144.59, 144.46, 143.84, 143.06, 143.01, 142.95, 142.18, 141.90, 141.81, 140.88, 139.36, 138.71 (fullerene carbons), 136.59 (1C, Cd), 126.12 (2C, Cj), 124.65 (2C, Cn), 122.60 (2C, Cm), 111.54 (2C, Ci), 107.27 (2C, Cc), 101.75 (1C, Ca), 71.49 (fullerene carbon), 70.85–70.71, 68.72 (6C, OCH2 of the tetra(ethylene glycol) chain), 68.96 (2C, PhOCH2 of the aliphatic chain), 68.62 (1C, PhCH2O), 68.20 (1C, COOCH2), 66.20 (1C, NCH2), 52.25 (1C, Cf), 39.35 (1C, NCH3), 31.92, 29.69–29.30, 26.13, 22.68 (20C, all CH2 of the aliphatic chain), 14.10 (2C, CH3) ppm.
1H NMR (400 MHz, CDCl3): δ = 8.30 (d, J = 9 Hz, 2H, H6), 7.88 (d, J = 7 Hz, 2H, H5), 7.87 (d, J = 7 Hz, 2H, H4), 6.83 (d, J = 9 Hz, 2H, H3), 6.60 (d, J = 2 Hz, 2H, H2), 6.41 (t, J = 2 Hz, 1H, H1), 5.41 (s, 2H, PhCH2OCO), 4.68 (t, J = 6 Hz, 2H, NCH2CH2OCO), 3.89 (t, J = 7 Hz, 4H, PhOCH2 of the aliphatic chain), 3.79 (t, J = 6 Hz, 2H, CH3CH2NCH2), 3.57–3.51 (m, 2H, OCOCH2CH2NCH2), 1.76–1.68 (m, 4H, PhOCH2CH2), 1.43–1.36 (m, 4H, PhO(CH2)2CH2), 1.34–1.24 (m, 35H, all CH2 of the aliphatic chain and NCH2CH3), 0.89 (t, J = 6 Hz, 6H, CH3) ppm.
13C NMR (100 MHz, CDCl3): δ = 163.35 (1C, Ce), 163.18 (1C, Cg), 160.53 (2C, Cb), 156.63 (1C, Cl), 151.20 (1C, Ch), 147.40 (1C, Co), 145.21, 145.18, 145.11, 144.89, 144.83, 144.59, 144.56, 144.43, 143.99, 143.79, 143.76, 142.98, 142.86, 142.80, 142.11, 142.08, 141.81, 141.69, 140.78, 140.75, 139.21, 138.60 (fullerene carbons), 136.47 (1C, Cd), 126.47 (2C, Cn), 124.61 (2C, Cj), 122.63 (2C, Cm), 111.56 (2C, Ci), 107.33 (2C, Cc), 101.52 (1C, Ca), 71.18 (fullerene carbon), 68.95 (1C, PhCH2O), 68.15 (2C, PhOCH2), 63.96 (1C, NCH2CH2O), 51.59 (1C, Cf), 48.23 (1C, CH3CH2NCH2), 45.34 (1C, NCH2), 31.91 (2C, CH3(CH2)2), 29.68–29.25 (14C, all CH2 of the aliphatic chain), 26.10 (2C, PhO(CH2)3), 22.68 (2C, CH3CH2), 14.13 (2C, CH3), 12.29 (1C, NCH2CH3) ppm.
3 Results and discussion
3.1 Synthesis of the fullerene C60-azobenzene derivatives
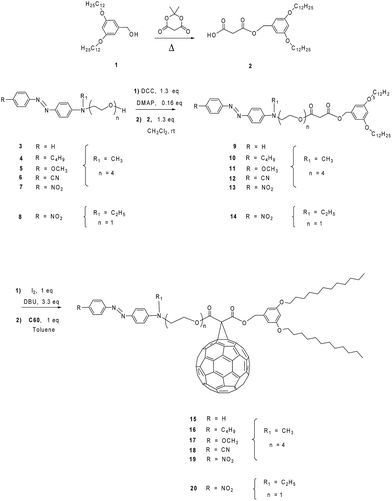
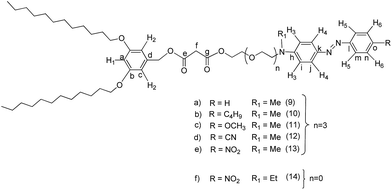
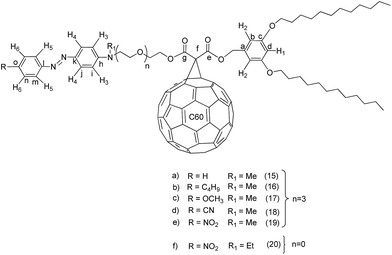
Generally, the synthesis of the fullerene C60-azobenzene derivatives was carried out according to the synthetic sequence illustrated in Fig. 1. Six different substituted azobenzenes (amino, amino-butyl, amino-methoxy, amino-cyano, amino-nitro, DR1) (3, 4, 5, 6, 7 and 8) bearing different dipole moment values were prepared and incorporated into a substituted malonate containing a phenyl group with aliphatic chains, to give the corresponding precursor compounds (9, 10, 11, 12, 13 and 14). The last step consisted on the incorporation of fullerene C60 into the precursor malonates by means of a Bingel reaction to give six different fullerene C60-azobenzene derivatives (15, 16, 17, 18, 19 and 20).Precursor substituted azobenzenes 4, 5, and 7 have been prepared according to the method previously reported by us,30,33 whereas substituted azobenzenes 3 and 6 were synthesized using a similar procedure.23 Intermediate 2-(2-{2-[2-(methyl-phenyl-amino)-ethoxy]-ethoxy}-ethoxy)-ethanol was prepared according to our method previously reported in the literature and the diazonium salts were prepared in situ. Aniline (1 eq.) was reacted with NaNO2 (1 eq.) in an HCl solution (30%) at 0 °C. Afterwards, 2-(2-{2-[2-(methyl-phenyl-amino)-ethoxy]-ethoxy}-ethoxy)-ethanol (1 eq.) was added dropwise to the reaction mixture in order to obtain the amino substituted azobenzene (3) with 68% yield.30 Similarly, the diazonium salt of 4-cyanoaniline was used to give the corresponding amino-cyano substituted azobenzene (ESI†).
Dendron GIOH (1) (1 eq.) was treated in the presence of Meldrum's acid (1 eq.) to afford the monosubstituted malonic ester (2). This intermediate was further reacted with intermediate (3) in the presence of N,N-dimethyl-4-aminopyridine (DCC) and N,N-dimethyl-4-aminopyridine (DMAP) in dry dichloromethane to give the azobenzene containing disubstituted malonic ester (9). Finally, this compound was reacted with fullerene C60, iodine and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) in anhydrous toluene to give the corresponding fullerene C60-azobenzene derivative (15). The other fullerene C60-azobenzene systems were obtained according to the same synthetic method, under the same reaction conditions described above. In the same manner, compounds (10), (11), (12), (13) and (14) were reacted under the Bingel reaction conditions to give the corresponding fullerenes C60-azobenzene derivatives (16), (17), (18), (19) and (20), respectively (Fig. 1).
3.2 Characterization of the fullerene C60-azobenzenes derivatives
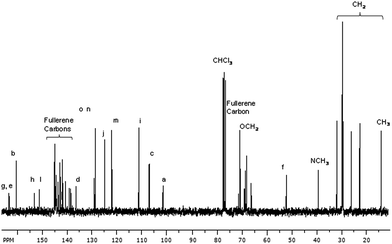
The precursor disubstituted malonic azo-dyes and their corresponding fullerene C60-azobenzene derivatives were fully characterized by 1H and 13C NMR spectroscopies. The molecular weights and purity of the final products were confirmed by MALDI-TOF mass spectrometry using dithranol as matrix.For instance, in the 1H NMR spectrum of compound 9 (Fig. 2a), we observe six signals in the aromatic region at 7.87, 7.84, 7.47–7.37, 6.75, 6.47 and 6.41 ppm, due to the aromatic protons present in the azobenzene unit and the phenyl group, corresponding to H5, H4, H6–H, H3, H2 and H1, respectively. In the aliphatic zone, we can see a singlet at 5.09 (PhCH2OCO), and two triplets at 4.29 (OOCCH2) and 3.92 ppm (PhOCH2). In addition, we perceive a multiplet at 3.68–3.59 ppm related to the protons OCH2 of the oligo(ethylene glycol) segments, as well as two singlets at 3.46 and 3.07 ppm due to the α protons of the malonate (OOCCH2COO) and the methyl group (NCH3), respectively. Finally, the protons corresponding to methylenes (CH2) present in the aliphatic chain appear at 1.80–1.71, 1.46–1.39, 1.36–1.27 and 0.90 ppm.
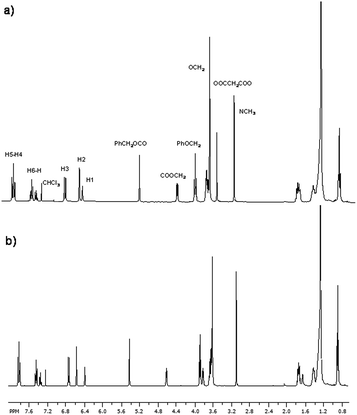 | ||
| Fig. 2 1H NMR spectra in CDCl3: (a) precursor malonic azo-dye 9, (b) fullerene C60-azobenzene derivative 15. | ||
In the 1H NMR spectrum of fullerene C60-azobenzene derivative 15, the chemical shifts of the protons are very similar (Fig. 2b). As we can notice, the signal due to the α protons of the malonate (OOCCH2COO), previously observed at 3.46 ppm, totally disappeared. This is an indication that the Bingel coupling reaction between the fullerene C60 and the precursor malonic azo-dye successfully occurred.
On the other hand, in the aromatic region of the 13C NMR spectrum of the malonic azo-dye 9, there are 14 signals at 163.46, 163.29, 160.47, 153.17, 151.34, 143.78, 136.55, 129.31, 128.87, 125.02, 122.17, 111.38, 107.15, 101.64 ppm, assigned to the aromatic carbons of the phenyl groups present in the structure. Moreover, in the aliphatic region, we observe various signals at 70.75–70.64, 68.91, 68.66 ppm due to the methylenes present in the tetra(ethylene glycol) spacers. In addition, the carbons PhOCH2 of the aliphatic chain appear at 68.53. Four more signals at 68.11, 66.18, 52.15 and 32.24 ppm, corresponding to carbons (PhCH2O), (NCH2), (Cf) and (NCH3), respectively, were also observed. Finally, the signals due to all the CH2 and CH3 present in the aliphatic chains appeared at 31.90, 29.67–29.26, 26.10, 22.67, and 14.12 ppm.
In the 13C NMR spectrum of fullerene C60-azobenzene derivative 15, the signals of the aromatic rings did not show any significant chemical shift with respect to its precursor compound 9 (Fig. 3). However, we observe the appearance of the signals arising from the fullerene C60 sp2 carbons at 145.20, 145.16, 145.11, 144.97, 144.83, 144.63, 144.60, 144.55, 144.48, 144.41, 143.80, 143.78, 143.64, 143.01, 142.96, 142.89, 142.13, 141.85, 141.77, 140.84, 140.82, 139.34, and 138.66 ppm. Meanwhile, in the aliphatic region, we can notice an additional signal of the fullerene C60 sp3 carbons and the carbon α to the carbonyl group (Cf) at 71.40 and 52.15 ppm, respectively.
The structure and purity of these compounds were confirmed by MALDI-TOF mass spectrometry. All fullerene C60-azobenzene derivatives showed the expected molecular ion peaks, which are in agreement with the calculated molecular weights. The 13C NMR the fullerene-azobenzene derivatives reported here can be found in the ESI.†
3.3 Optical properties of the fullerene C60-azobenzene derivatives
The optical properties of the fullerene C60-azobenzene derivatives were studied by absorption spectroscopy in the UV-vis region; the results are summarized in Tables 1 and 2. The absorption spectra of the precursor malonic azo-dyes without fullerene were recorded in CHCl3, MeOH and DMF solution, whereas those of the fullerene C60-azobenzene derivatives were carried out in CHCl3 and DMF.| Compound | CHCl3 | DMF | MeOH | |||
|---|---|---|---|---|---|---|
| λmax (nm) | Cut off (nm) | λmax (nm) | Cut off (nm) | λmax (nm) | Cut off (nm) | |
| 9 | 410 | 528 | 418 | 530 | 410 | 428 |
| 10 | 410 | 528 | 418 | 530 | 410 | 428 |
| 11 | 410 | 528 | 418 | 530 | 410 | 428 |
| 12 | 448 | 562 | 462 | 574 | 450 | 568 |
| 13 | 478 | 596 | 496 | 628 | 478 | 610 |
| 14 | 468 | 584 | 490 | 632 | 478 | 610 |
| Compound | CHCl3 λmax (nm) | DMF λmax (nm) | % photoisomerization yield cis isomer Δabsb/Δabsa | Film λmax (nm) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Absorbance of the precursor malonic azo-dyes.b Absorbance of the fullerene C60-azobenzene system. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 15 | 408 | 416 | 6.4 | 408 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 16 | 408 | 416 | 3 | 408 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 17 | 408 | 416 | 15.5 | 408 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 18 | 446–450 | 462 | — | 446 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 19 | 480 | 492–494 | — | 480 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 20 | 470–472 | 488 | — | 476 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The absorption spectra of the precursor malonic azo-dyes without fullerene were normalized for a better comparison. As we can notice, the absorption spectra of compounds 9, 10 and 11, in CHCl3 solution, showed a maximum absorption band at λmax = 410 nm followed by red-shifted shoulder at 421 nm due to the π–π* and n–π* transitions, respectively. These compounds have a low dipole moment value and belong to aminoazobenzenes category, according to Rau's classification.18,19 By contrast, compounds 12, 13 and 14 exhibited well defined absorption bands at 448, 478 and 468 nm, respectively (Fig. 4a). These compounds possess high dipole moment values and belong to the pseudostilbenes category, so that they exhibit a total overlap of the π–π* and n–π* bands in other words, only one absorption band can be observed in the UV-vis spectra.18,19 However, the absorption bands of these compounds in MeOH solution did not show any significant red shift. On the contrary, compound 14 showed a red-shift of 10 nm in its absorption band with respect to that observed in CHCl3 (Table 1), and in DMF a significant solvatochromic effect was observed. Compounds having low dipole moments (9, 10 and 11) showed a red-shift of 8 nm in their adsorption bands, whereas compounds bearing high dipole moments (12, 13 and 14) exhibited red-shifts of 14, 18 and 22 nm, respectively (Fig. 4b, Table 1). On the other hand, malonic azo-dyes bearing NO2 as acceptor group (13, 14) exhibited a maximum absorption band at λmax = 478 nm in MeOH solution, showing slight shifts of 10 and 6 nm in CHCl3 and DMF solution, respectively (Table 1).
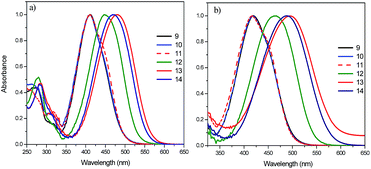 | ||
| Fig. 4 Normalized absorption spectra of the precursor azo-dyes: (a) in CHCl3, and (b) in DMF solution. | ||
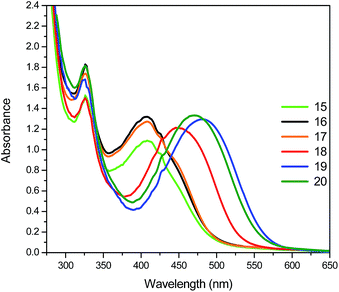
Regarding the optical properties of the fullerene C60-azobenzene derivatives, we noticed that in CHCl3 solution the absorption band of compounds 15, 16 and 17 (low dipole moment) are blue-shifted by 2 nm with respect to their precursor malonic azo-dyes without fullerene (Table 2). These compounds exhibited a maximum absorption band at λmax = 408 nm with a red-shifted shoulder at 422 nm arising from the π–π* and n–π* transitions, respectively. In addition, we note the presence of a blue-shifted shoulder at 394 nm, which reveals the presence of H-aggregates (Fig. 5). Alternatively, azo-compounds bearing high dipole moment values (18, 19, 20) show adsorption bands at 448, 480 and 472 nm, respectively. These compounds exhibited also a blue-shifted shoulder at 432 nm, which indicates the presence of H-aggregation (Fig. 5, Table 2).36
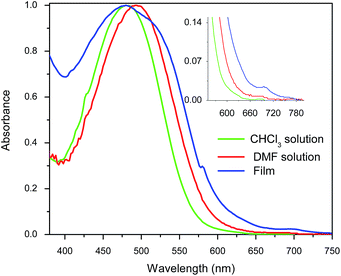
As well, the optical properties of fullerene C60-azobenzene derivatives were studied in DMF solution (ESI, Fig. S6†). These hybrid systems having low dipole moments (15, 16, 17) exhibited a maximum absorption band which is 8 nm red-shifted with respect to the absorption value in CHCl3 solution. However, fullerene C60-azobenzene derivatives bearing high dipole moment values (18, 19 and 20) showed maximum absorption bands at λmax = 462, 492–494, 488, respectively. They also exhibited a blue-shifted shoulder at 428 nm, which can be due to intramolecular azobenzene–fullerene interactions.36
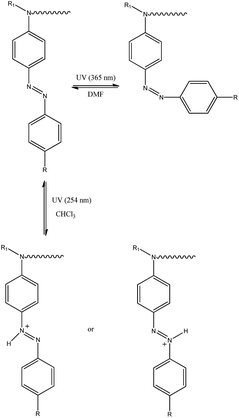
3.4 Protonation effect upon irradiation
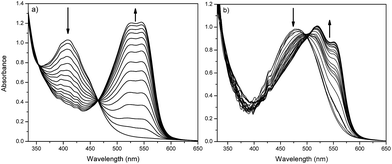
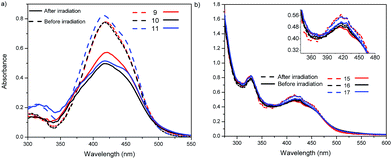
We decided to study the photoprotonation effect on the series of azo-dyes containing first generation Fréchet type dendrons. In these compounds, the formation of an azo-hydrazone via tautomerism can be induced by irradiating with UV light at 254 nm.29,37 Particularly, we investigated the photoprotonation effect of the fullerene C60-azobenzene derivatives 15 and 19, having the lowest and the highest dipole moment value, respectively. After irradiation with UV light for 140 s, compound 15 reached a photostationary state (PPS). However, over time we noticed that absorption band at 410 nm decreases drastically in intensity and two new absorption bands appear in the visible region at 524 nm and 546 nm, respectively; the isobestic point was located at 430 nm (Fig. 6a). Similarly, fullerene C60-azobenzene derivative 19, bearing a high dipole moment value, exhibited the same photochromic effect as its homologue with low dipole moment 15. Upon irradiation the absorption band at 480 nm decreased drastically in intensity and the appearance of two additional bands at 520 and 552 nm was observed; in this case the isosbestic point was situated at 500 nm (Fig. 6b). This fullerene C60-azobenzene system reached a photostationary state (PPS) after irradiation for 600 s. From these results, we can remark that the photochromic behavior of this azo-dye was not perturbed at all by the incorporation of the fullerene unit (Fig. 8).3.5 trans–cis photoisomerization
Photoisomerization experiments were performed with the samples in DMF solution, which were irradiated with UV light at 365 nm for 10 min. After this time, we noticed that the intensity of the absorption band as well as the extinction molar coefficient of compounds 9, 10 and 11 decreased, due to the photoisomerization from the trans (E) to the cis (Z) isomer. Moreover, in the UV region of the absorption spectrum of these compounds there is a shoulder at 450 nm, related to the n–π* transition of the cis isomer (Fig. 7a). Meanwhile, in the absorption spectra of the fullerene C60-azobenzenes derivatives 15, 16 and 17 the intensity of the bands did not vary significantly (Fig. 7b). The photoisomerization yield in these compounds depends in large measure on the donor/acceptor character of the substituents present in the azobenzene units (Table 2). Fullerene C60-azobenzene derivative 17 exhibited a photoisomerization yield of 13%, which can attributed to the electron-donor effect of the (OCH3) group. Finally, compounds 15 and 17 bearing low dipole moment values exhibited photoisomerization yields of 6.4 and 3.0%, respectively (Table 2). These low yields are due to the fact that the fullerene C60 strongly absorb in the UV region, which causes a partial quenching the photoisomerization process (Fig. 8).3.6 Optical properties and trans–cis photoisomerization in cast films
The optical properties of the fullerene C60-azobenzenes derivatives were also studied in cast films, and the results are summarized in Table 2. The normalized absorption spectra of the fullerene C60-azobenzene derivative 19 in CHCl3, DMF and cast film are shown in Fig. 9. As we can see, the absorption band in a cast film did not show any significant shift with respect to the absorption band observed in CHCl3 solution. However, in DMF this absorption band was red-shifted by 8 nm, and we note a shoulder at 524 nm due to the presence of J-aggregates,28 followed by two discrete shoulders at 580 and 702 nm, respectively (Table 2).38On the other hand, photoisomerization experiments were carried out in cast film samples. The precursor malonic azo-dyes without fullerene bearing low dipole moments (9, 10 and 11) exhibited trans–cis photoisomerization when they were irradiated with UV light at 365 nm for 10 minutes. After this time, we noticed a decrease in intensity of the absorption band at 410 nm, jointly with the appearance of a shoulder at 450 nm due to the n–π* transition of the cis isomer (Fig. 10a). On the contrary, when the fullerene C60-azobenzene derivatives (15, 16, 17) were irradiated with UV light for 10 minutes, the absorption bands did not change at all (Fig. 10b). This is certainly due to a quenching of the photoisomerization process by the presence of the fullerene unit in these derivatives.
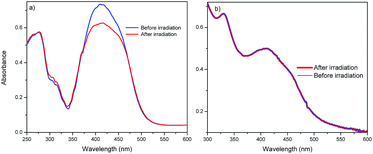 | ||
| Fig. 10 Absorption spectrum in casted film of: (a) precursor azo-dye 9, (b) fullerene C60-azobenzene derivative 15, after irradiation for 10 minutes. | ||
4 Conclusions
Six different fullerene C60-azobenzene derivatives (15, 16, 17, 18, 19 and 20) were successfully synthesized and fully characterized by 1H, 13C NMR and MALDI-TOF. The optical properties of the fullerene C60-azobenzene dyes were studied by UV-vis spectroscopy and compared to those of their precursor malonic azo-dyes without fullerene (9, 10, 11, 12, 13 and 14).Fullerene C60-azobenzene derivatives bearing low dipole moment (15, 16 and 17) in CHCl3 exhibited a maximum absorption band at λmax = 408 nm and a shoulder at 422 nm due to π–π* and n–π* transitions, respectively. The presence of a blue-shifted shoulder at 394 nm revealed the presence of H-aggregates. Fullerene C60-azobenzene derivatives bearing a high dipole moment (18, 19 and 20) showed maximum absorption bands between 448 and 472 nm; the presence of H-aggregates was also detected.
The photoprotonation effect in the fullerene C60-azobenzene derivatives 15 and 19, having the lowest and the highest dipole moment, respectively, was studied by absorption spectroscopy. Compound 15 (lowest dipole moment) reached a photostationary state (PPS). The absorption band at 410 nm decreased drastically in intensity and two absorption bands appeared at 524 and 546 nm. Compound 19 (highest dipole moment) behaved similarly and exhibited the same photochromic effect. It was found that the photoprotonation behavior of these azo-dyes was not perturbed by the incorporation of the fullerene unit.
Photoisomerization experiments were performed with the precursor dyes with low dipole moment (9, 10 and 11) in DMF solution. A typical trans–cis photoisomerization behavior was observed. The absorption band at 410 nm decreased in intensity and a shoulder at 450 nm due to the n–π* transition (cis isomer) appeared. On the contrary, in the UV-vis spectra of the fullerene C60-azobenzenes derivatives (15, 16 and 17) the intensity of the bands did not vary remarkably. It was found that the presence of the fullerene moiety drastically decreases the photoisomerization yield because of a strong quenching effect.
The optical properties of the fullerene C60-azobenzenes derivatives were also studied in cast films, where the presence of J-aggregates was detected. Malonic azo-dyes without fullerene (9, 10 and 11) exhibited typical trans–cis photoisomerization, whereas the fullerene C60-azobenzene derivatives (15, 16, 17) did not since the fullerene moiety acts as quencher.
Acknowledgements
We are grateful to Miguel Angel Canseco for his assistance with UV-vis spectroscopy and Gerardo Cedillo for his help with 1H and 13C NMR spectroscopy. This project was financially supported by PAPIIT (Project IN-100316) and CONACyT (Projects 253155 and 222847).References
- H. W. Kroto, A. W. Allaf and S. P. Balm, C60: Buckminsterfullerene, Chem. Rev., 1991, 91, 1213–1235 CrossRef CAS.
- B. C. Thompson and J. M. J. Fréchet, Organic Photovoltaics Polymer–Fullerene Composite Solar Cells, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- D. M. Guldi and N. Martin, Fullerenes: From Synthesis to Optoelectronic Properties, Kluwer Academic Publishers, Dordrecht, The Netherlands, 2002 Search PubMed.
- F. Wudl, Fullerene materials, J. Mater. Chem., 2002, 12, 1959–1963 RSC.
- A. M. López, A. Mateo-Alonso and M. Prato, Materials chemistry of fullerene C60 derivatives, J. Mater. Chem., 2011, 21, 1305–1318 RSC.
- M. Parto, [60] Fullerene chemistry for materials science applications, J. Mater. Chem., 1997, 7, 1097–1109 RSC.
- F. Wudl, The Chemical Properties of Buckminsterfullerene (C60) and the Birth and Infancy of Fulleroids, Acc. Chem. Res., 1992, 25, 157–161 CrossRef CAS.
- S. A. Backer, K. Sivula, D. F. Kavulak and J. M. J. Fréchet, High Efficiency Organic Photovoltaics Incorporating a New Family of Soluble Fullerene Derivatives, Chem. Mater., 2007, 19, 2927–2929 CrossRef CAS.
- N.-X. Wang, C. H. Sun and W. Liu, Synthesis of fullerene azobenzene derivatives, Fullerene Sci. Technol., 2001, 9, 429–432 CrossRef.
- K.-Y. Kay, K. J. Han, Y. J. Yu and Y. D. Park, Dendritic fullerenes (C60) with Photoresponsive Azobenzene Groups, Tetrahedron Lett., 2002, 43, 5053–5056 CrossRef CAS.
- D. I. Schuster, K. Li, D. M. Guldi, A. Palkar, L. Echegoyen, C. Stanisky, R. J. Cross, M. Niemi, N. V. Tkachenko and H. Lemmetyien, Azobenzene-linked porphyrin fullerene dyads, J. Am. Chem. Soc., 2007, 129, 15973–15982 CrossRef CAS PubMed.
- K. S. Kumar and A. Patnaik, Tunable One-, two-, and three dimensional self assemblies from an acceptor–donor fullerene-N,N-dimethylaminoazobenzene dyad: Interfacial geometry and temporal evolution, Langmuir, 2011, 27, 11017–11025 CrossRef CAS PubMed.
- K. S. Kumar and A. Patmaik, Solvent-polaritydimeric association of a fullerene (C60)-N,N-dimethylaminoazobenzene dyad: Modulated electronic coupling of the azochromophore with a substituted 3D fullerene, Chem.–Eur. J., 2011, 17, 5327–5343 CrossRef CAS PubMed.
- Y. Shirai, T. Sasaki, J. M. Guerrero, B. C. Yu, P. Hodge and J. M. Tour, Synthesis and Photoisomerization of Fullerene and Oligo(phenyleneethynylene) Azobenzene Derivatives, ACS Nano, 2008, 2, 97–106 CrossRef CAS PubMed.
- M. E. El-Khouly, J. H. Kim, K. Y. Kay and S. Fukuzumi, Silicon phthalocyanine-azobenzene-[60] fullerene light harvesting pendant: synthesis, characterization and electron transfer reaction studied by laser flash photolysis, J. Porphyrins Phthalocyanines, 2013, 17, 1055–1063 CrossRef CAS.
- S. Ma, H. Ting, L. Zhang, Y. Ma, L. Zheng, L. Xiao and Z. Chen, Reversible photoinduced bi-state polymer solar cells based on fullerene derivatives with azobenzene groups, Org. Electron., 2015, 23, 1–4 CrossRef CAS.
- M. Wang, E. Chesnut, Y. Sun, M. Tong, M. Guide, Y. Zhang, D. N. Treat, A. Varotto, A. Mayer, L. M. Chabinyc, T.-Q. Nguyen and F. Wudl, PCBM Disperse-Red Ester with Strong Visible-Light Absorption: Implication of Molecular Design and Morphological Control for Organic Solar Cells, J. Phys. Chem. C, 2012, 116, 1313–1321 CAS.
- H. Rau, in Photochemistry and Photophysics, ed. J. K. Rabek, CRC Press. Boca Raton, Florida, 1990, vol. 2, p. 119 Search PubMed.
- A. Natansohn and P. Rochon, Photoinduced Motions in Azo Containing Polymers, Chem. Rev., 2002, 102, 4139–4176 CrossRef CAS PubMed.
- T. Todorov, L. Nikalova and N. Tomova, Polarization Holography. 1: A new high-efficiency organic material with reversible photoinduced birefringence, Appl. Opt., 1984, 23, 4309–4312 CrossRef CAS PubMed.
- S. Xie, A. Natansohn and P. Rochon, Recent Developments in Aromatic Azo Polymers Research, Chem. Mater., 1993, 5, 403–411 CrossRef CAS.
- N. K. Viswanathan, D. Y. Kim, S. Bian, J. Williams, W. Liu, L. Li, L. Samuelson, J. Kumar and S. K. Tripathy, Surface relief structures on azo polymer films, J. Mater. Chem., 1999, 9, 1941–1955 RSC.
- E. Rivera, M. Belletête, A. Natansohn and G. Durocher, Synthesis, characterization, and optical properties of a novel azo-dye bearing an oligo(ethylene glycol) methyl ether side chain in solution and in the solid state, Can. J. Chem., 2003, 81, 1076–1082 CrossRef CAS.
- E. Rivera, M. P. Carreón-Castro, I. Buendía and G. Cedillo, Optical properties and aggregation of novel azo-dyes bearing an end-capped oligo(ethylene glycol) side chain in solution, solid state and Langmuir–Blodgett films, Dyes Pigm., 2006, 68, 217–226 CrossRef CAS.
- E. Rivera, M. P. Carreón-Castro, L. Rodriguez, G. Cedillo, S. Fomine and O. G. Morales-Saavedra, Amphiphilic azo-dyes (RED-PEGM). Part 2: Charge transfer complexes, preparation of Langmuir Blodgett films and optical properties, Dyes Pigm., 2007, 74, 396–403 CrossRef CAS.
- C. Caicedo, E. Rivera, Y. Valdez-Hernández and M. P. Carreón-Castro, Synthesis and characterization of novel liquid-crystalline azo-dyes bearing two amino-nitro substituted azobenzene units and a well-defined, oligo(ethylene glycol) spacer, Mater. Chem. Phys., 2011, 130, 471–480 CrossRef CAS.
- T. García, M. P. Carreón-Castro, A. Gelover-Santiago, P. Ponce, M. Romero and E. Rivera, Synthesis and Characterization of Novel Amphiphilic Azo-Polymers Bearing Well-Defined Oligo(Ethylene Glycol) Spacers, Des. Monomers Polym., 2012, 15, 159–174 CrossRef.
- F. Tapia, J. M. Reyna-González, G. Huerta, S. Almeida and E. Rivera, Synthesis and characterization of novel polythiophenes bearing oligo(ethylene glycol) segments and azobenzene units, Polym. Bull., 2010, 64, 581–594 CrossRef CAS.
- J. Ortíz-Palacios, E. Rodríguez-Alba, G. Zaragoza-Galán, J. R. León-Carmona, A. Martínez and E. Rivera, Incorporation of novel azobenzene dyes bearing oligo(ethylene glycol) spacers into first generation dendrimers, Dyes Pigm., 2015, 116, 1–12 CrossRef.
- E. Rivera, M. P. Carreón-Castro, R. Salazar, G. Huerta, C. Becerril and L. Rivera, Preparation and characterization of novel grafted polyethylene based azo-polymers bearing oligo(ethylene glycol) spacers, Polymer, 2007, 48, 3420–3428 CrossRef CAS.
- D. Felder, M. Gutiérrez-Nava, M. P. Carreón-Castro, J. F. Eckert, M. Luccisano, C. Schall, P. Masson, J. L. Gallani, B. Heinrich and D. Gillonand, Synthesis of amphiphilic fullerene derivatives and their incorporation in Langmuir and Langmuir–Blodgett films, Helv. Chim. Acta, 2002, 85, 288–319 CrossRef CAS.
- X. Camps and A. Hirsch, Efficient cyclopropanation of C60 starting from malonates, J. Chem. Soc., Perkin Trans. 1, 1997, 1595 RSC.
- J. Ortíz-Palacios, E. Rodríguez-Alba, M. Avelar, A. Martínez, M. P. Carreón-Castro and E. Rivera, Synthesis and characterization of novel dendrons bearing amino-nitro-substituted azobenzene units and oligo(ethylene glycol) spacers: thermal, optical properties, Langmuir Blodgett films and liquid-crystalline behaviour, Molecules, 2013, 18, 1502–1527 CrossRef PubMed.
- C. J. Hawker and J. M. J. Fréchet, Preparation of Polymers with Controlled Molecular Architecture. A new Convergent Approach to Dendritic Macromolecules, J. Am. Chem. Soc., 1990, 112, 7638–7647 CrossRef CAS.
- J. Ortíz-Palacios, E. Rodríguez-Alba, G. Zaragoza-Galán and E. Rivera, Fréchet-type dendrons bearing azobenzene units and flexible oligo(ethylene glycol) spacers: synthesis, characterization, thermal and optical properties, Des. Monomers Polym., 2013, 16, 578–591 CrossRef.
- M. Kasha, Energy transfer mechanisms and the molecular exciton model for molecular aggregates, Radiat. Res., 1963, 20, 55–71 CrossRef CAS PubMed.
- K. S. Kumar and A. Patnaik, Tunable electronic properties of a proton-responsive N,N-dimethylaminoazobenzene fullerene (C60) dyad, ChemPhysChem, 2010, 11, 3645–3655 CrossRef CAS PubMed.
- S. K. Lin, L. L. Shiu, K. M. Chien, T. L. Luh and T. I. Lin, Fluorescence of Fullerene Derivatives at Room Temperature, J. Phys. Chem., 1995, 99, 105–111 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00413c |
| This journal is © The Royal Society of Chemistry 2017 |