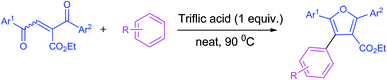Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triflic acid promoted solvent free synthesis of densely functionalized furans†
Pulaganti Vijayaprasad,
Avudoddi Venkanna,
Medi Shanker,
Eslavath Kishan and
Pallapothula Venkateswar Rao *
*
Department of Chemistry, University College of Science, Osmania University, Tarnaka, Hyderabad 500007, India. E-mail: pallapothulavrao@gmail.com
First published on 7th February 2017
Abstract
A simple, efficient and novel methodology has been developed for the synthesis of densely substituted furans mediated by triflic acid. The current protocol proceeds in a domino fashion, the initial step involves Friedel–Crafts arylation, followed by dehydrative cyclization. It is operationally simple and can be carried out under solvent free conditions.
Introduction
Furan and its derivatives constitute a significant class of heterocyclic compounds, they act as key structural units in various natural products, bioactive compounds and agro chemicals.1 They serve as important building blocks in the construction of complex natural molecules.2 Recently the synthesis of densely functionalized furans attained much attention in modern organic chemistry due to their wide range of applications in medicinal chemistry and material sciences.3 Compounds containing the furan moiety were found to demonstrate diverse biological activities such as anticancer,4 antifungal,5 antimicrobial,6 antibacterial,7 and antispasmodic activity.8 Apart from these, furan derivatives also exhibit herbicidal activity.9 Several synthetic strategies have been developed for the synthesis of multi-substituted furans such as functionalization of furans,10 metal catalyzed,11 3 + 2 annulations,12 and intramolecular cyclization reactions.13 Though all these methods were found to be efficient in synthesizing the substituted furan derivatives they suffer from a few drawbacks such as use of expensive metal catalysts, solvents and employing harsh reaction conditions. Therefore, it is enviable to develop simple, cost effective and environment friendly protocols for the synthesis of densely functionalized furan derivatives from the easily accessible starting materials. Herein, we report a novel and versatile strategy for the synthesis of furan derivatives mediated by triflic acid under solvent free conditions.Results and discussion
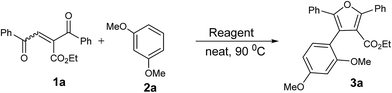
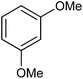
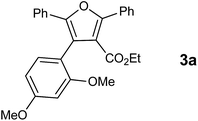
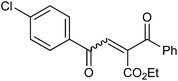
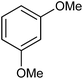
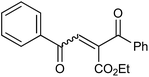
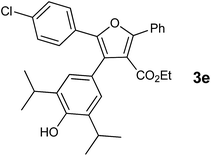
In our preliminary experiment we have carried out the reaction of 1a with 1,3-dimethoxybenzene (2a) in presence of molecular iodine, at 90 °C, the reaction underwent smoothly and afforded the desired furan derivative ethyl 4-(2,4-dimethoxyphenyl)-2,5-diphenylfuran-3-carboxylate (3a) in 55% yield (Scheme 1).The product thus obtained was confirmed by the analysis 1H and 13C NMR spectral data. The result obtained was encouraging and we focused on optimizing the reaction conditions in presence of various Bronsted acids. Accordingly when the reaction was carried out in presence of p-toluenesulfonic acid monohydrate (p-TSA·H2O) and methanesulphonic acid, we were pleased to isolate the product 3a in 82 and 86% yields respectively (Table 1, entry 2 & 3). The similar reaction promoted by trifluoroacetic acid (TFA) provided the product in 84% yield in 4 h (Table 1, entry 4). When we employed triflic acid (TfOH) as a reagent surprisingly, the reaction was completed in 2 h and the product 3a was isolated in 94% yield (Table 1, entry 5).
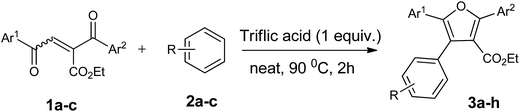
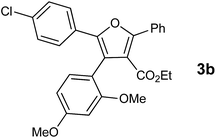
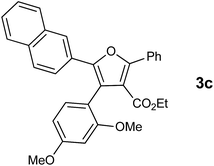
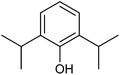
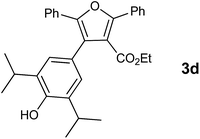
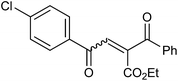
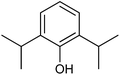
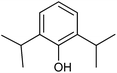
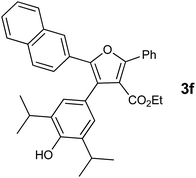
With the optimized reaction conditions, we further proceeded to explore the scope of substrates in the current protocol. Therefore, the starting materials 1a, 1b and 1c were synthesized following the literature procedure.14 Under the standard reaction condition, the compounds 1b and 1c were reacted with 1,3-dimethoxybenzene, the reaction proceeded well and after purification by column chromatography the corresponding furan derivatives 3b and 3c were obtained in 88 and 92% yields (Table 2, entries 2–3). When the reactivity of 2,6-diisopropylphenol was examined with the 1,4-dienones 1a, 1b and 1c, we were pleased to observe that the reactions have undergone smoothly even in presence of bulky iso-propyl groups and gave the desired products in 84, 87 and 82% yields respectively (Table 2, entries 4–6). Furthermore, the reactions of 1a and 1b with phenol in presence of TfOH acid underwent effectively to deliver the desired products in 91 and 81% yields (Table 2, entries 7–8). Purity of all the products was confirmed by the analysis of IR, Mass, 1H and 13C NMR spectral data.
| Entry | 1,4-Dienone | Phenol | Product | Yieldb (%) |
|---|---|---|---|---|
| 6 |  |
 |
 |
82 |
| 7 | 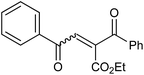 |
 |
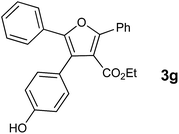 |
91 |
| 8 | 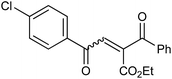 |
 |
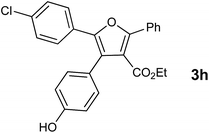 |
81 |
Mechanism
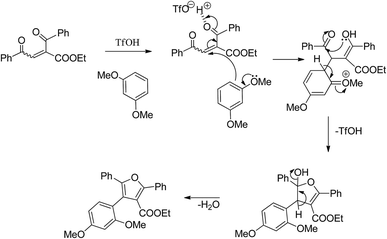
In the initial step of the reaction of 1a with 1,3-dimethoxybenzene in presence of triflic acid, the 1,3-dimethoxybenzene reacts with 1a via Friedel–Crafts reaction to give 1,4-dione intermediate. Thus obtained 1,4-dione, further undergoes the acid-mediated intramolecular cyclization by elimination of water molecule to give the furan derivative 3a (Scheme 2).Conclusion
In conclusion we have developed triflic acid mediated novel, efficient protocol for the synthesis of densely functionalized furan derivatives from the simple starting materials. The current strategy is easy to handle and provides an access to the synthesis of diversely substituted furan derivatives in excellent yields.Experimental section
General information
Triflic acid, 1,4-dienone, 1,3-dimethoxy benzene and other starting materials were purchased from Sigma Aldrich and Fluka were used without purification. All experiments were carried out under air. Column chromatography was carried out with silica gel 60–120 sized mesh using hexane and ethyl acetate as eluents. Analytical TLC was performed with silica gel 60 F254 plates (Merck), and the products were visualized by UV detection. 1H-NMR and 13C-NMR (Avance 300, Innova 400 MHz and Brucker Gemini 200 MHz) spectra were recorded in CDCl3 using TMS as internal standard. Chemical shifts (δ) are reported in ppm, and spin–spin coupling constants (J) are in Hz. Melting points were determined on a Fisher-Johns melting point apparatus. FT-IR and MS were recorded on a Thermo Nicolet Nexus 670 FT-IR spectrometer and Finnegan MAT 1020 mass spectrometer operating at 70 eV.Acknowledgements
The authors thank the University Grants Commission (UGC), New Delhi and Council of scientific and Industrial Research (CSIR), New Delhi for financial assistance.References
- (a) B. H. Lipshutz, Chem. Rev., 1986, 86, 795 CrossRef CAS; (b) M. Gutierrez, T. L. Capson, H. M. Guzman, J. Gonzalez, E. Ortega-Barria, E. Quinoa and R. Riguera, J. Nat. Prod., 2005, 68, 614 CrossRef CAS PubMed; (c) M. C. Sanchez, M. J. Ortega, E. Zubia and J. L. Carballo, J. Nat. Prod., 2006, 69, 1749 CrossRef CAS PubMed; (d) J. Marrero, J. Benitez, A. D. Rodriguez, H. Zhao and R. G. Raptis, J. Nat. Prod., 2008, 71, 381 CrossRef CAS PubMed; (e) M. G. Missakian, B. J. Burresons and P. J. Scheuer, Tetrahedron, 1975, 31, 2513 CrossRef CAS; (f) R. Kazlauskas, P. T. Murphy, R. J. Wells, J. J. Daly and P. Schonholze, Tetrahedron Lett., 1978, 49, 4951 CrossRef; (g) M. V. R. Reddy, S. Lakshman, A. V. R. Rao, Y. Venkateswarlu and V. J. Rao, J. Nat. Prod., 1993, 56, 970 CrossRef CAS; (h) J. Kobayashi, D. Watanabe, N. Kawasaki and M. Tsuda, J. Org. Chem., 1997, 62, 9236 CrossRef CAS; (i) A. Rudi, T. L.-A. Dayan, M. Aknin, E. M. Gaydou and Y. Kashman, J. Nat. Prod., 1998, 61, 872 CrossRef CAS PubMed; (j) Y.-L. Lin, Y.-L. Tsai, Y.-H. Kuo, Y.-H. Liu and M.-S. Shiao, J. Nat. Prod., 1999, 62, 1500 CrossRef CAS PubMed.
- (a) T. Nagata, M. Nakagawa and A. Nishida, J. Am. Chem. Soc., 2003, 125, 7484 CrossRef CAS PubMed; (b) S. F. Kirsch, Org. Biomol. Chem., 2006, 4, 2076 RSC; (c) P. A. Roethle and D. Trauner, Org. Lett., 2006, 8, 345 CrossRef CAS PubMed; (d) T. Montagnon, M. Tofi and G. Vassilikogiannakis, Acc. Chem. Res., 2008, 41, 1001 CrossRef CAS PubMed; (e) D. Kalaitzakis, M. Triantafyllakis, I. Alexopoulou, M. Sofiadis and G. Vassilikogiannakis, Angew. Chem., Int. Ed., 2014, 53, 13201 CrossRef CAS PubMed; (f) K. Tokumaru, T. Ohfusa, S. Arai and A. J. Nishida, J. Antibiot., 2016, 69, 340 CrossRef CAS PubMed; (g) S. F. Martin and P. W. Zinke, J. Org. Chem., 1991, 56, 6600 CrossRef CAS; (h) B. A. Keay, Chem. Soc. Rev., 1999, 28, 209 RSC; (i) X. L. Hou, H. Y. Cheung, T. Y. Hon, P. L. Kwan, T. H. Lo, S. Y. Tong and H. N. C. Wong, Tetrahedron, 1998, 54, 1955 CrossRef CAS.
- (a) A. U. Rao, D. Xiao, X. Huang, W. Zhou, J. Fossetta, D. Lundell, F. Tian, P. Trivedi, R. Aslanian and A. Palani, Bioorg. Med. Chem. Lett., 2012, 22, 1068 CrossRef CAS PubMed; (b) F. Hasegawa, K. Niidome, C. Migihashi, M. Murata, T. Negoro, T. Matsumoto, K. Kato and A. Fujii, Bioorg. Med. Chem. Lett., 2014, 24, 4266 CrossRef CAS PubMed; (c) Y.-S. Li, H. Tian, D.-S. Zhao, D.-K. Hu, X.-Y. Liu, H.-W. Jin, G.-P. Song and Z.-N. Cui, Bioorg. Med. Chem. Lett., 2016, 26, 3632 CrossRef CAS PubMed; (d) S.-Y. Lee, A. Perotti, S. De Jonghe, P. Herdewijn, T. Hanck and C. E. Muller, Bioorg. Med. Chem. Lett., 2016, 24, 3157 CrossRef CAS PubMed; (e) A. E. Shchekotikhin, L. G. Dezhenkova, V. B. Tsvetkov, Y. N. Luzikov, Y. L. Volodina Jr, V. V. Tatarskiy, A. A. Kalinina, M. I. Treshalina, H. M. Treshalina, V. I. Romanenko, D. N. Kaluzhny, M. Kubbutat, D. Schols, Y. Pommier, A. A. Shtil and M. N. Preobrazhenskaya, Eur. J. Med. Chem., 2016, 112, 114 CrossRef CAS PubMed; (f) H. Zhong, C.-H. Wu, C.-Z. Li, J. Carpenter, C.-C. Chueh, J.-Y. Chen, H. Ade and A. K.-Y. Jen, Adv. Mater., 2016, 28, 951 CrossRef CAS PubMed.
- A. Kamal, V. L. Nayak, N. Nagesh, M. V. P. S. Vishnuvardhan and N. V. S. Reddy, Bioorg. Chem., 2016, 66, 124 CrossRef CAS PubMed.
- Z.-N. Cui, Y.-S. Li, D.-K. Hu, H. Tian, J.-Z. Jiang, Y. Wang and X.-J. Yan, Sci. Rep., 2016, 6, 20204 CrossRef PubMed.
- R. Srikanth, A. Sivarajan, C. S. Venkatesan, V. Maheshwaran, P. Sugumar, G. Rajitha, J. C. Varalakshmi and M. N. Ponnuswamy, J. Mol. Struct., 2016, 1125, 481 CrossRef CAS.
- K. Ran, C. Gao, H. Deng, Q. Lei, X. You, N. Wang, Y. Shi, Z. Liu, W. Wei, C. Peng, L. Xiong, K. Xiao and L. Yu, Bioorg. Med. Chem. Lett., 2016, 26, 3669 CrossRef CAS PubMed.
- J. Kobayashi, Y. Ohizumi, H. Nakamura and Y. Hirata, Tetrahedron Lett., 1986, 27, 2113 CrossRef CAS.
- J.-Q. Huo, L.-Y. Ma, Z. Zhang, Z.-J. Fan, J.-L. Zhang, T. V. Beryozkina and V. A. Bakulev, Chin. Chem. Lett., 2016, 27(9), 1547 CrossRef CAS.
- (a) R. Padmavathi, R. Sankar, B. Gopalakrishnan, R. Parella and S. A. Babu, Eur. J. Org. Chem., 2015, 3727 CrossRef CAS; (b) M. Miao, Y. Luo, H. Li, X. Xu, Z. Chen, J. Xu and H. Ren, J. Org. Chem., 2016, 81, 5228 CrossRef CAS PubMed.
- (a) L. Peng, X. Zhang, M. Ma and J. Wang, Angew. Chem., Int. Ed., 2007, 46, 1905 CrossRef CAS PubMed; (b) V. Cadierno, J. Diez, J. Gimeno and N. Nebra, J. Org. Chem., 2008, 73, 5852 CrossRef CAS PubMed; (c) J. Chen and S. Ma, Chem.–Asian J., 2010, 5, 2415 CrossRef CAS PubMed; (d) P. Lenden, D. A. Entwistle and M. C. Willis, Angew. Chem., Int. Ed., 2011, 50, 10657 CrossRef CAS PubMed; (e) Z.-W. Chen, M.-T. Luo, Y.-L. Wen, M. Ye, Z.-G. Zhou and L.-X. Liu, Synlett, 2014, 25, 2341 CrossRef CAS; (f) X. Zhang, W. Dai, W. Wu and S. Cao, Org. Lett., 2015, 17, 2708 CrossRef CAS PubMed; (g) J.-T. Yu, B. Shi, H. Peng, S. Sun, H. Chu, Y. Jiang and J. Cheng, Org. Lett., 2015, 17, 3643 CrossRef CAS PubMed; (h) S. Manna and A. P. Antonchick, Org. Lett., 2015, 17, 4300 CrossRef CAS PubMed; (i) B. V. S. Reddy, V. V. Reddy, G. Karthik and B. Jagadeesh, Tetrahedron, 2015, 71, 2572 CrossRef CAS; (j) X. Cheng, Y. Yu, Z. Mao, J. Chen and X. Huang, Org. Biomol. Chem., 2016, 14, 3878 RSC; (k) L. Wang, X. Liu, M. Wang and J. Liu, Org. Lett., 2016, 18, 2162 CrossRef CAS PubMed.
- (a) S. Ishikawa, Y. Noda, M. Wada and T. Nishikata, J. Org. Chem., 2015, 80, 7555 CrossRef CAS PubMed; (b) C. R. Reddy, S. Z. Mohammed and P. Kumaraswamy, Org. Biomol. Chem., 2015, 13, 8310 RSC.
- (a) Y. Yang, M. Gao, L.-M. Wu, C. Deng, D.-X. Zhang, Y. Gao, Y.-P. Zhu and A.-X. Wu, Tetrahedron, 2011, 67, 5142 CrossRef CAS; (b) X. Yang, F. Hu, H. Di, X. Cheng, D. Li, X. Kan, X. Zoua and Q. Zhang, Org. Biomol. Chem., 2014, 12, 8947 RSC.
- M. Gao, Y. Yang, Y.-D. Wu, C. Deng, L.-P. Cao, X.-G. Meng and A.-X. Wu, Org. Lett., 2010, 12, 1856 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra00489c |
| This journal is © The Royal Society of Chemistry 2017 |