Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A solvent free method of analysis to rapidly determine trace levels of ten medium and low brominated diphenyl ethers in soil pore water
Hu Chengab,
Yongrong Bian *a,
Yang Songa,
Wenxiang Hec,
Chenggang Gua,
Fang Wanga,
Xinglun Yanga,
Mao Yea,
Rongting Jiab and
Xin Jiang*a
*a,
Yang Songa,
Wenxiang Hec,
Chenggang Gua,
Fang Wanga,
Xinglun Yanga,
Mao Yea,
Rongting Jiab and
Xin Jiang*a
aKey Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, PR China. E-mail: yrbian@issas.ac.cn; jiangxin@issas.ac.cn; Tel: +86 25 86881195
bUniversity of the Chinese Academy of Sciences, Beijing 100049, PR China
cCollege of Resources and Environment, Northwest A&F University, Yangling 712100, PR China
First published on 10th March 2017
Abstract
A solvent free method of analysis for rapid simultaneous determination of ten medium and low brominated diphenyl ethers at trace levels in soil pore water by headspace solid phase microextraction coupled with gas chromatography equipped with an electron capture detector was developed. Extraction conditions such as desorption time, polydimethylsiloxane thickness, ionic strength, temperature, stirring and extraction time were carefully studied and optimized. In addition, the effects of pH and dissolved organic matter on headspace solid phase microextraction were thoroughly studied. For the ten medium and low brominated diphenyl ethers in this study, the limits of detection ranged from 0.04 to 1.20 ng L−1, and the relative standard deviations were lower than 10.00%. Soil pore water from red soil, yellow-brown soil and black soil was quantitatively analyzed with recoveries ranging from 80.40% to 108.90%, and from 85.9% to 108.45% at 50 ng L−1 and 200 ng L−1, respectively.
1. Introduction
Recent studies have shown that polybrominated diphenyl ethers (PBDEs), which are one of the three major flame retardants in the world and widely embedded in products such as building materials, electrical appliances, plastics compounds and textiles, are semi-volatile organic compounds (SVOCs) with the potential to cause harm to the environment and human health.1,2 In 2004, PBDEs were banned in Europe, and in 2009 they were listed as persistent organic pollutants (POPs) by the Stockholm Convention because of their persistence, bioaccumulation and toxicity. Unfortunately, the degree of PBDEs pollution is still increasing because of their continued use in Asia,3 as well as their persistence and transference in ecosystems. Medium and low brominated diphenyl ethers (MALBDEs) have much higher bioavailability and can easily undergo long-range transport in the environment and move up food chains4 because of their physicochemical properties, such as lower vapor pressure. Some toxicological studies have also revealed that MALBDEs had seriously adverse effects on animal health. Chevrier et al.5 reported that verbal comprehension and working memory were lower in children with early-life exposure to higher BDE-99 concentrations in indoor environments. Moreover, the European Chemical Bureau (ECB) has reported that high brominated congeners are less toxic than low brominated congeners of PBDEs. In addition, recent findings have indicated that high brominated congeners can be metabolically transformed and degraded to low and medium brominated congeners through reductive debromination.6 As a result, the possibility of their risk to human health and the environment is comparably higher, indicating that the environmental effects of MALBDEs should be focused on.Soil, which is a major environmental reservoir for MALBDEs7 and natural habitat of animals and plants, has been severely polluted through human activities. Li et al.8 reported that the total concentrations of PBDEs in the topsoil in Shandong province ranged from 17.0 to 146 μg g−1 dry weight (dw), with a mean value of 58.7 μg g−1 (dw), while Ming-Hong Wu9 also showed that the concentrations of PBDEs ranged from 4.68 to 174.7 ng g−1 dry weight (dw) in Shanghai soil, possibly because of the high number of electronics factories in these regions. Soils have also been found to be secondary emission sources of MALBDEs, as these compounds can constantly be desorbed into soil pore water and then be absorbed by plants or animals. Accordingly, a great quantity of MALBDEs could be transferred to humans through food chains, which threatens human health. Many studies10 have shown that freely dissolved part are the most bioavailable fraction of POPs in soil and sediment, and this fraction of POPs is commonly used to assess whether soils are at risk of bioconcentration or not. However, the concentration of MALBDEs in soil pore water is often at trace levels that are too low to detect because of the lipophilicity with low solubility and the relatively small amount of free MALBDEs, which has hindered research. According to Carter,11 the higher concentration of contaminants was set in his study because trace level of POPs were hard to detect in the soil pore water. Thus, detection of the trace quantity of MALBDEs in soil pore water is important for monitoring and assessing the risk of MALBDEs in soil.
Nevertheless, few studies have investigated methods of measuring MALBDEs in soil pore water. Conventional techniques including liquid–liquid extraction (LLE), accelerated solvent extraction (ASE), Soxhlet extraction and solid phase extraction (SPE) may enable determination of MALBDEs in soil pore water. However, these techniques are hindered by many practical issues, such as time-consuming, the need for large sample volumes, the occurrence of secondary pollution because of the use of organic solvents, and difficulties associated with purification and enrichment. Moreover, most MALBDEs in soil pore water are found at the nanograms per liter level, which is below the limit of detection for conventional methods.12
Solid phase microextraction (SPME)13–15 technology is a solvent-free technology that integrates sampling, concentration, and clean-up has rapidly developed in recent years and has the potential to overcome the aforementioned problems.16 In addition, headspace solid phase microextraction (HS-SPME)17 technology is the optimum mode of SPME for interference-free sampling. In this method, the extraction of analytes avoids direct contact by coating fibers with matrixes, which can prolong the lifespan of SPME fiber, remove purification process.18 HS-SPME coupled with an ECD detector has been implemented to reduce the cost relative to mass spectrometry.
Here, a solvent free method of analysis, head space-solid phase microextraction coupled with GC-ECD, was established for the simultaneous determination of trace levels of ten MALBDEs in soil pore water.
2. Materials and methods
2.1 Materials and reagents
Ten analytes including 2,4-dibromodiphenyl ether (BDE-7, 98.5% purity), 4,4′-dibromodiphenyl ether (BDE-15, 98.5% purity), 2,4,4′-tribromodiphenyl ether (BDE-28, 97.9% purity), 3,3′,4-tribromodiphenyl ether (BDE-35, 98.5% purity), 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47, 98.5% purity), 2,4,4′,6-tetrabromodiphenyl ether (BDE-75, 98.5% purity), 2,2′,3,4,4′-pentabromodiphenyl ether (BDE-85, 98.5% purity), 2,3,4′,5,6-pentabromodiphenyl ether (BDE-117, 98.5% purity), 2,2′,3,4,4′,5′-hexabromodiphenyl ether (BDE-138, 98.5% purity), and 2,2,4,4,5,5-hexabromo-diphenyl ether (BDE-153, 98.6% purity) were stocked in isooctane at 50 mg L−1 and used for identification, quantification and investigation of the HS-SPME method. All of these analytes were purchased from Accustandard Inc. (New Haven, CT, USA). The basic physicochemical properties of the above analytes are shown in Table 1. Working standard solutions for the external standard curve of analytes and investigation of the HS-SPME method were prepared with n-hexane by serially diluting stock solutions from 50 to 1000 μg L−1. All stock and working standards were stored at 4 °C until analysis. Chromatographic grade acetone and n-hexane were obtained from Merck KGaA (Darmstadt, Germany). Technical grade humic acid was purchased from Sigma-Aldrich (Saint Louis, MO, USA). Highly pure water (R ≥ 18 MΩ) used throughout the study was prepared using a Millipore water purification system (MilliQ Water, Molsheim, France). Soils were obtained from a depth of 10–20 cm in Heilongjiang, Henan and Jiangxi provinces and the basic physicochemical properties of the three test soils were shown in Table 2. The polydimethylsiloxane (PDMS) coating of fiber, as a nonpolar substance, has been used for extracting hydrophobic organic contaminants (HOCs) and the manual holder, used in this study for extraction MALBDEs, were purchased from Supelco (Bellefonte, PA, USA). MS400 magnetic agitator was obtained from Bante (Shanghai, China).| Medium and low brominated diphenyl ethers | Structural formula | lgHa37 | Kowb | Swc (298 K) (mg L−1) |
|---|---|---|---|---|
| a Henry's law constant.b Octanol–water partition coefficients.c Aqueous solubility. | ||||
| 2,4-Dibromo diphenyl ether | 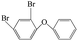 |
9.343 | — | — |
| 4,4′-Dibromo diphenyl ether | 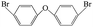 |
5.978 | 5.55 (ref. 38) | 0.216 ± 0.03 (ref. 39) |
| 2,4,4′-Tribromo diphenyl ether | 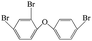 |
4.816 | 5.94 ± 0.15 (ref. 40) | 0.07 ± 0.01 (ref. 38) |
| 3,3′,4-Tribromo diphenyl ether | 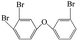 |
6.647 | — | — |
| 2,2′,4,4′-Tetrabromo diphenyl ether | 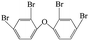 |
0.843 | 6.81 ± 0.08 (ref. 40) | 1.47 × 10−2 ± 0.09 (ref. 39) |
| 2,4,4′,6-Tetrabromo diphenyl ether | 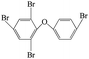 |
3.002 | — | — |
| 2,2′,3,4,4′-Pentabromo diphenyl ether | 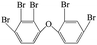 |
3.414 | 7.37 ± 0.12 (ref. 40) | 0.006 ± 0.0001 (ref. 38) |
| 2,3,4′,5,6-Pentabromo diphenyl ether | 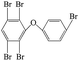 |
3.868 | — | — |
| 2,2′,3,4,4′,5′-Hexabromo diphenyl ether | 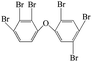 |
2.914 | 7.91 (ref. 38) | — |
| 2,2′,4,4′,5,5′-Hexabromo diphenyl ether | 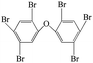 |
0.348 | 7.90 ± 0.14 (ref. 40) | 5.04 × 10−5 ± 0.27 (ref. 39) |
| Red soil | Yellow-brown soil | Black soil | |
|---|---|---|---|
| pH | 5.11 | 8.05 | 6.26 |
| Organic matter (%) | 1.78 | 2.54 | 3.14 |
| Total C (%) | 1.03 | 1.47 | 1.82 |
| Total N (%) | 0.1 | 0.12 | 0.15 |
| Total P (%) | 0.07 | 0.08 | 0.05 |
| Total K (%) | 0.86 | 1.04 | 0.75 |
| Clay (%) | 52.9 | 22.9 | 30.3 |
| Silt (%) | 23.8 | 31.4 | 21.6 |
| Sand (%) | 23.3 | 45.7 | 48.1 |
| Pore water dissolved organic carbon (mg L−1) | 23.1 | 30.6 | 17.1 |
2.2 Preparation of soil pore water
Testing soils were air-dried, gently ground and sieved to 1 mm to ensure homogeneity, then stored at 4 °C prior to use. The soil pore water was collected using a modified method.19,20 In brief, 50 g of soil (dry weight) was spiked with 50 mL of highly pure water containing 0.01 M calcium chloride and 10 mM sodium azide in a 250 mL conical flask covered with aluminum foil to avoid photolysis, then mixed at 160 rpm for 48 h using a mechanical shaker. Next, the soil slurry was homogenized, placed in centrifuge tubes and centrifuged at 1448 g for 30 minutes using a Sigma 2–16 K centrifugal machine obtained from Germany. Collecting the supernatant from two-phase sample, filtered with 0.45 μm pore diameter polycarbonate membranes using a vacuum pump purchased from GAST (Chicago, IL, USA) and stored at 4 °C before analysis. A sub-sample of 30 mL soil pore water was spiked with the ten MALBDEs to determine the recovery of real samples by the HS-SPME method. Another sub-sample of 20 mL of soil pore water was used to measure the dissolved organic carbon (DOC) level, which was accomplished with a Multi N/C 3100(Jena, Germany).21 All treatments were repeated three times.2.3 Process of headspace solid phase microextraction
The polydimethylsiloxane (PDMS) coating of fiber and the manual holder used to acquire the proper exposure depth were assembled according to the device description of manual holder, after which the fibers were conditioned with nitrogen protection in the pre-injector of an Agilent 6890 at 280 °C for 30 minutes. Following conditioning, the steel tube of the fiber, to protect the PDMS coating from mechanical damage, was inserted into a 20 mL amber vial that sealed with a polyethylene and silicone polytetrafluoroethylene (PTFE)-faced septum cap (CNW Technologies; Düsseldorf, Germany) and containing a magnetic rotor covered with PTFE for stirring the sample solution. Then the PDMS coating was exposed to the surface samples at fixed temperature and agitation for a time. Finally, the fiber bound with the PDMS coating contracted in the steel tube, at which time it was quickly removed from the amber vial and directly inserted into the injection port of the Agilent 6890 to induce thermal desorption at 280 °C.2.4 Instrumentation and conditions
An Agilent 6890 (Santa Clara, CA, USA) gas chromatograph equipped an electron capture detector (GC-ECD) and an HP 7683 auto-sampler was used for the qualitative and quantitative analysis of PBDEs. A HP-5MS capillary column (30.0 m length × 0.25 mm inside diameter × 0.25 μm film thickness) coated with 5% phenyl methyl siloxane was used for the separation of selected MALBDEs. Ultra-pure nitrogen (purity > 99.999%) was applied as carrier and makeup gas at a flow rate of 2.9 mL min−1 and 60 mL min−1, respectively. The injection port was maintained at 280 °C, and then switched into splitless mode. The oven temperature was programmed as follows: initial temperature 60 °C (3 min), followed by an increase to 240 °C at a rate of 30 °C min−1, where it was held for 1 min, then increased to 260 °C at a rate of 10 °C min−1, where it was held for 1 min, followed by a further increase to 280 °C at a rate of 5 °C min−1, where it was held for 1 min, and then finally an increase to 300 °C at a rate of 15 °C min−1, which was held for 11 min. The temperature of the ECD was fixed at 300 °C.3. Results and discussion
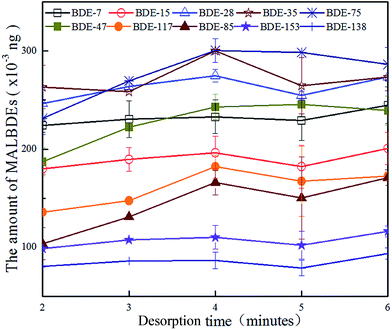
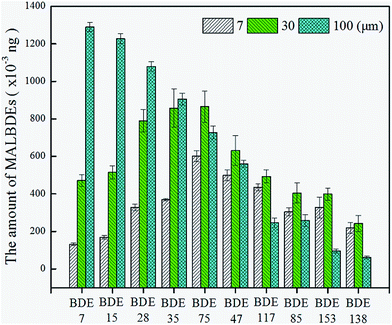
3.1 Multivariate optimization of headspace solid phase microextraction
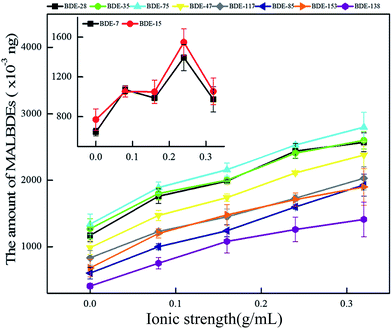
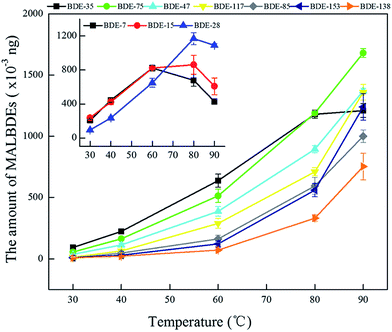
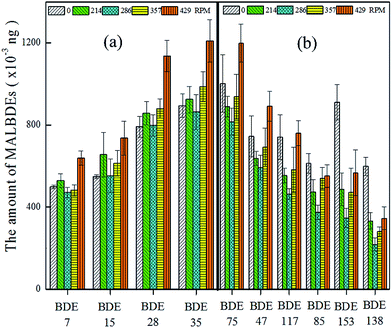
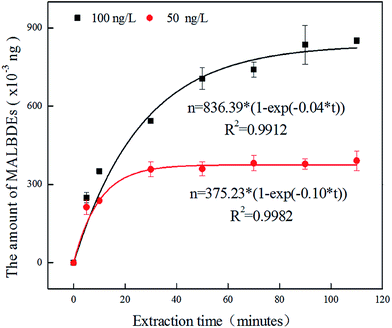
3.2 Effect of interference factors on headspace solid phase microextraction
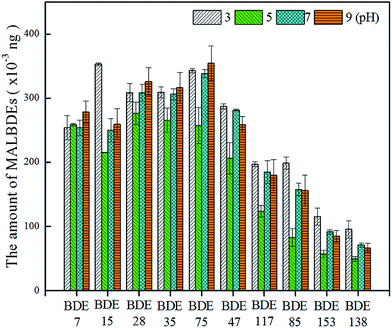
3.3 Evaluation of method performance
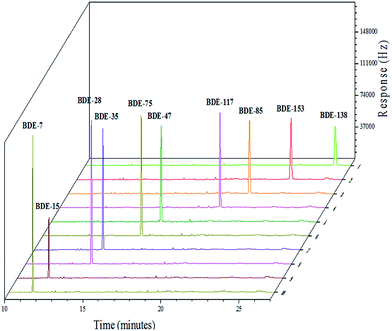
A series of experiments were conducted under the optimum conditions established for the HS-SPME-GC-ECD process to validate the performance of the proposed method based on the linear dynamic range (LDR), limit of detection (LOD), limit of quantification (LOQs), and repeatability. Results obtained and summarized are listed in Table 3. Investigation of the LDR by extracting the spiked MALBDEs with ultrapure water at concentrations of 5–200 ng L−1 revealed good linearity (R2 > 0.9960). Fig. 8 shows the chromatogram of 1.0 mg L−1 MALBDEs. The LOD values ranged from 0.04 to 1.2 ng L−1 (calculated on the basis of 3 times of signal-to-noise ratio), while the LOQ values ranged from 0.13 to 4.02 ng L−1 (calculated on the basis of 10 times of signal-to-noise ratio). Moreover, good reproducibility was obtained for samples spiked with 10 ng L−1 using a single fiber, with relative standard deviations (RSDs) found to range from 1.60 to 10.00%. Overall, these findings indicate that the proposed method has excellent reproducibility and high sensitivity for simultaneous determination of the trace levels of the ten investigated MALBDEs.| Compound | Linear range (ng L−1) | R2 | LOD (ng L−1) | LOQ (ng L−1) | RSD% (c = 10 ng L−1, n = 6) |
|---|---|---|---|---|---|
| BDE-7 | 5–200 | 0.9986 | 0.39 | 1.29 | 6.5 |
| BDE-15 | 5–200 | 0.9980 | 0.30 | 1.01 | 10.0 |
| BDE-28 | 5–200 | 0.9964 | 0.04 | 0.13 | 2.2 |
| BDE-35 | 5–200 | 0.9971 | 0.05 | 0.16 | 1.6 |
| BDE-75 | 5–200 | 0.9980 | 0.68 | 2.28 | 4.3 |
| BDE-47 | 5–200 | 0.9979 | 0.99 | 3.29 | 3.6 |
| BDE-117 | 5–200 | 0.9988 | 0.49 | 1.63 | 4.3 |
| BDE-85 | 5–200 | 0.9998 | 0.23 | 0.76 | 5.8 |
| BDE-153 | 5–200 | 0.9963 | 1.20 | 4.02 | 7.0 |
| BDE-138 | 5–200 | 0.9960 | 0.50 | 1.66 | 8.7 |
3.4 Comparison with previous methods
In this study, the method developed based on HS-SPME-GC-ECD was compared with other methods for determination of MALBDEs (Table 4). As shown in Table 4, measurement of BDE-28, BDE-47, BDE-85, BDE-138 and BDE-153 has been investigated using different methods, but no methodological investigations of BDE-7, BDE-15, BDE-35, BDE-75 and BDE-117 have been reported. Moreover, the LODs of MALBDEs obtained from the proposed method were lower than those based on TA-IL-DLLME-HPLC,32 SFOME-HPLC,33 DLLME-HPLC,12 LC/NI-APPI/MS/MS,34 SBSE-GC-MS4 and MWCNT-HS-SPME-GC-ECD,35 but slightly higher than that observed for SPE-DLLME-GC-ECD.36 This is because the proposed method was applied to simultaneously determine ten kinds of MALBDEs. Namely, the LOD of this method could be further reduced still by adjusting the conditions investigated in this study when the measured kinds of MALBDEs were less. Moreover, when compared to SPE-DLLME-GC-ECD, the developed method did not use organic solvent, and consisted of a simple process with smaller labour and time costs.| Methods | Analytes (ng L−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BDE-7 | BDE-15 | BDE-28 | BDE-35 | BDE-47 | BDE-75 | BDE-85 | BDE-117 | BDE-138 | BDE-153 | |
| a Temperature-assisted ionic liquid dispersive liquid–liquid microextraction-high performance liquid chromatography.b Solidified floating organic drop microextraction-high performance liquid chromatography.c Dispersive liquid–liquid microextraction-phase-high performance liquid chromatography.d Solid-phase extraction-dispersive liquid–liquid microextraction-gas chromatography-electron capture detector.e Liquid chromatography-negative ion atmospheric pressure photoionization tandem mass spectrometry.f Stir bar sorptive extraction-gas chromatography-mass spectrometry.g Multiwalled carbon nanotube fibers-headspace solid phase microextraction-gas chromatography with electron-capture detection. | ||||||||||
| TA-ILDLLME-HPLCa | — | — | — | — | 100 | — | — | — | — | — |
| SFOME-HPLCb | — | — | 10 | — | 10 | — | — | — | — | — |
| DLLME-HPLCc | — | — | 12.4 | — | 55.6 | — | — | — | — | — |
| SPE-DLLME-GC-ECDd | — | — | 0.03 | — | 0.06 | — | 0.15 | — | — | 0.13 |
| LC/NI-APPI/MS/MSe | — | — | — | — | 10.9 | — | — | — | 0.5 | |
| SBSE-GC-MSf | — | — | 0.4 | — | 0.3 | — | 2.4 | — | 3.3 | 5.4 |
| MWCNT-HS-SPME-GC-ECDg | 1.1 | 16 | ||||||||
| PDMS-HS-SPME-GC-ECD | 0.39 | 0.30 | 0.04 | 0.05 | 0.99 | 0.68 | 0.23 | 0.49 | 0.50 | 1.20 |
3.5 Analysis of real samples
The proposed method for MALBDEs was applied to the detection of soil pore water of red soil, yellow-brown soil and black soil collected on a large scale (E126°48′N45°49′44-E117°09′N28°04′) in China. No target analytes were detected in these soil pore water. The actual samples were then spiked with 50 ng L−1 and 200 ng L−1 of MALBDEs, respectively, and analysed using the developed HS-SPME method. As shown in Table 5, the recovery of MALBDEs at 50 ng L−1 and 200 ng L−1 varied from 80.40% to 108.90% and from 85.90% to 108.45%, respectively, while the relative standard deviation of the developed method was lower than 18.36% and 11.93%, respectively.| Sample | Spiked (ng L−1) | Detected (μg L−1)/recovery (% RSD, n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-7 | BDE-15 | BDE-28 | BDE-35 | BDE-75 | |||||||
| Red soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 48.84 | 97.67(5.86) | 48.77 | 97.54(8.45) | 43.19 | 86.37(4.65) | 52.14 | 104.27(4.53) | 44.44 | 88.88(3.23) | |
| 200 | 184.62 | 92.31(4.61) | 177.10 | 88.55(6.91) | 177.91 | 88.96(2.13) | 182.54 | 91.27(2.30) | 176.74 | 88.37(6.64) | |
| Yellow-brown soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 42.31 | 84.61(8.32) | 54.46 | 108.90(3.30) | 41.28 | 82.56(9.30) | 50.22 | 100.44(9.58) | 43.02 | 86.04(9.58) | |
| 200 | 213.10 | 106.55(0.27) | 179.60 | 89.80(4.00) | 196.18 | 98.09(5.75) | 200.51 | 100.25(5.56) | 182.69 | 91.35(5.68) | |
| Black soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 47.56 | 95.12(4.36) | 50.78 | 101.56(11.50) | 40.37 | 82.60(0.02) | 50.18 | 100.35(6.98) | 40.33 | 82.10(0.40) | |
| 200 | 213.65 | 106.80(0.00) | 181.92 | 90.96(11.00) | 206.03 | 103.02(6.96) | 209.31 | 104.65(6.46) | 196.38 | 98.19(7.18) | |
| Sample | Spiked (ng L−1) | Detected (μg L−1)/recovery (% RSD, n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BDE-47 | BDE-117 | BDE-85 | BDE-153 | BDE-138 | |||||||
| Red soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 41.86 | 83.72(2.32) | 50.63 | 101.26(15.52) | 43.27 | 86.54(5.67) | 50.24 | 100.48(11.66) | 50.95 | 101.90(18.36) | |
| 200 | 177.52 | 88.76(6.21) | 181.71 | 90.85(5.36) | 199.96 | 99.98(4.13) | 216.90 | 108.45(11.93) | 214.94 | 107.47(8.35) | |
| Yellow-brown soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 45.01 | 90.02(10.06) | 51.92 | 103.85(12.25) | 51.43 | 102.85(0.42) | 47.50 | 95.00(16.32) | 52.60 | 105.20(16.05) | |
| 200 | 181.68 | 90.84(4.96) | 184.70 | 92.35(3.67) | 203.05 | 101.53(5.31) | 208.10 | 104.00(14.00) | 171.80 | 85.90(0.74) | |
| Black soil | 0 | ND | ND | ND | ND | ND | |||||
| 50 | 40.18 | 80.40(0.00) | 52.85 | 105.70(3.96) | 43.47 | 86.93(1.48) | 48.86 | 97.72(4.25) | 53.73 | 107.50(3.50) | |
| 200 | 195.89 | 97.94(6.78) | 201.61 | 100.81(4.48) | 222.24 | 108.10(2.00) | 208.48 | 104.20(2.1) | 217.23 | 108.60(0.40) | |
4. Conclusions
A green HS-SPME method combined with GC-ECD for rapidly simultaneous determination of ten trace medium and low brominated diphenyl ethers in soil pore water was successfully developed. Many extraction conditions of the developed method were optimized, including desorption time, coating thickness, ionic strength, temperature, and stirring and extraction time. The pH and DOM also were also thoroughly investigated as potential interference factors. The method developed for determination of trace levels of MALBDEs has the advantages of free-pre-treatment, free-solvent, low limit of detection and excellent recovery and reproducibility, while avoiding direct contact of the fiber coating with the matrix. However, the LOD of this method could be further reduced still by adjusting the conditions investigated in this study.Acknowledgements
This study was financially supported by the National Key Basic Research Program of China (2014CB441105), the National Natural Science Foundation of China (41271464, 41671236 and 21377138), Natural Science Foundation of Jiangsu Province (BK20131463), and the “135” Plan and Frontiers Program of the Institute of Soil Science, Chinese Academy of Sciences (ISSASIP1614 and ISSASIP1618).References
- M. Frederiksen, K. Vorkamp, M. Thomsen and L. E. Knudsen, Int. J. Hyg. Environ. Health, 2009, 212, 109–134 CrossRef CAS PubMed.
- A. P. Vonderheide, K. E. Mueller, J. Meija and G. L. Welsh, Sci. Total Environ., 2008, 400, 425–436 CrossRef CAS PubMed.
- Y. Li, T. Lin, L. Hu, J. Feng and Z. Guo, Environ. Int., 2016, 92–93, 507–514 CrossRef CAS PubMed.
- A. Bacaloni, L. Callipo, E. Corradini, P. Giansanti, R. Gubbiotti, R. Samperi and A. Lagana, J. Chromatogr. A, 2009, 1216, 6400–6409 CrossRef CAS PubMed.
- C. Chevrier, C. Warembourg, G. Le Maner-Idrissi, A. Lacroix, V. Dardier, S. Le Sourn-Bissaoui, F. Rouget, C. Monfort, E. Gaudreau, F. Mercier, N. Bonvallot, P. Glorennec, G. Muckle, B. Le Bot and S. Cordier, Neurotoxicology, 2016, 54, 81–88 CrossRef CAS PubMed.
- Y. Pan, D. C. W. Tsang, Y. Wang, Y. Li and X. Yang, Chem. Eng. J., 2016, 297, 74–96 CrossRef CAS.
- I. T. Cousinsa, A. J. Beckb and K. C. Jonesa, Sci. Total Environ., 1999, 228, 5–24 CrossRef.
- Y. Li, S. Niu, R. Hai and M. Li, Environ. Sci. Pollut. Res. Int., 2015, 22, 1133–1143 CrossRef CAS PubMed.
- M. H. Wu, J. C. Pei, M. Zheng, L. Tang, Y. Y. Bao, B. T. Xu, R. Sun, Y. F. Sun, G. Xu and J. Q. Lei, Chemosphere, 2015, 118, 87–95 CrossRef CAS PubMed.
- Y. Hong, D. Wetzel, E. L. Pulster, P. Hull, D. Reible, H. M. Hwang, P. Ji, E. Rifkin and E. Bouwer, Environ. Monit. Assess., 2015, 187, 646 CrossRef PubMed.
- L. J. Carter, E. Harris, M. Williams, J. J. Ryan, R. S. Kookana and A. B. Boxall, J. Agric. Food Chem., 2014, 62, 816–825 CrossRef CAS PubMed.
- Y. Li, G. Wei, J. Hu, X. Liu, X. Zhao and X. Wang, Anal. Chim. Acta, 2008, 615, 96–103 CrossRef CAS PubMed.
- M. Polo, G. Gomez-Noya, J. B. Quintana, M. Llompart, C. Garcia-Jares and R. Cela, Anal. Chem., 2004, 76, 1054–1062 CrossRef CAS PubMed.
- L. Sanchez-Prado, C. Gonzalez-Barreiro, M. Lores, M. Llompart, C. Garcia-Jares and R. Cela, Chemosphere, 2005, 60, 922–928 CrossRef CAS PubMed.
- J. X. Wang, D. Q. Jiang, Z. Y. Gu and X. P. Yan, J. Chromatogr. A, 2006, 1137, 8–14 CrossRef CAS PubMed.
- É. A. Souza-Silva, R. Jiang, A. Rodríguez-Lafuente, E. Gionfriddo and J. Pawliszyn, TrAC, Trends Anal. Chem., 2015, 71, 224–235 CrossRef.
- A. R. Ghiasvand and S. Hajipour, Talanta, 2016, 146, 417–422 CrossRef CAS PubMed.
- D. A. Lambropoulou, I. K. Konstantinou and T. A. Albanis, J. Chromatogr. A, 2007, 1152, 70–96 CrossRef CAS PubMed.
- M. T. O. Jonker, S. A. Van der heijden, J. P. Kreitinger and S. B. Hawthorne, Environ. Sci. Technol., 2007, 41, 7472–7478 CrossRef CAS PubMed.
- T. L. Terlaak, S. O. Agbo, A. Barendregt and J. L. M. Hermens, Environ. Sci. Technol., 2006, 40, 1307–1313 CrossRef CAS.
- F. Jia, X. Cui, W. Wang, L. Delgado-Moreno and J. Gan, Environ. Pollut., 2012, 167, 34–40 CrossRef CAS PubMed.
- L. Wang, Z. Zhang, X. Xu, D. Zhang, F. Wang and L. Zhang, Talanta, 2015, 142, 97–103 CrossRef CAS PubMed.
- M. Xie, Z. Y. Yang, L. J. Bao and E. Y. Zeng, J. Chromatogr. A, 2009, 1216, 4553–4559 CrossRef CAS PubMed.
- Z. Y. Yang, E. Y. Zeng, H. Xia, J. Z. Wang, B. X. Mai and K. A. Maruya, J. Chromatogr. A, 2006, 1116, 240–247 CrossRef CAS PubMed.
- S. Huang, F. Zhu, R. Jiang, S. Zhou, D. Zhu, H. Liu and G. Ouyang, Talanta, 2015, 136, 198–203 CrossRef CAS PubMed.
- M. Mei, X. Huang, J. Yu and D. Yuan, Talanta, 2015, 134, 89–97 CrossRef CAS PubMed.
- M. Kazemipour, M. Behzadi and R. Ahmadi, Microchem. J., 2016, 128, 258–266 CrossRef CAS.
- H. Lord and J. Pawliszyn, J. Chromatogr. A, 2000, 902, 17–63 CrossRef CAS PubMed.
- M. Zhang, Q. Zhen, H. Wang, M. Guo, S. Zhou, X. Wang and X. Du, Talanta, 2016, 158, 214–221 CrossRef CAS PubMed.
- A. M. Gioacchini, M. Menotta, E. Polidori, G. Giomaro and V. Stocchi, J. Mass Spectrom., 2002, 37, 1229–1235 CrossRef CAS PubMed.
- C. T. Huang, Y. Y. Su and Y. Z. Hsieh, J. Chromatogr. A, 2002, 977, 9–16 CrossRef CAS PubMed.
- A. Zhao, X. Wang, M. Ma, W. Wang, H. Sun, Z. Yan, Z. Xu and H. Wang, Microchim. Acta, 2012, 177, 229–236 CrossRef CAS.
- H. Liu, M. Zhang, X. Wang, Y. Zou, W. Wang, M. Ma, Y. Li and H. Wang, Microchim. Acta, 2011, 176, 303–309 CrossRef.
- X. Liu, J. Li, Z. Zhao, W. Zhang, K. Lin, C. Huang and X. Wang, J. Chromatogr. A, 2009, 1216, 2220–2226 CrossRef CAS PubMed.
- M. K. Tian and X. L. Feng, Chin. J. Chem., 2008, 26, 1251–1256 CrossRef CAS.
- J. Llorca-Porcel, G. Martínez-Sánchez, B. Álvarez, M. A. Cobollo and I. Valor, Anal. Chim. Acta, 2006, 569, 113–118 CrossRef CAS.
- H. Y. Xu, J. W. Zou, Q. S. Yu, Y. H. Wang, J. Y. Zhang and H. X. Jin, Chemosphere, 2007, 66, 1998–2010 CrossRef CAS PubMed.
- S. A. Tittlemier, T. Halldorson, G. A. Stern and G. T. Tomy, Environ. Toxicol. Chem., 2002, 21, 1804–1810 CrossRef CAS PubMed.
- H. Kuramochi, K. Maeda and K. Kawamoto, Chemosphere, 2007, 67, 1858–1865 CrossRef CAS PubMed.
- E. Braekevelt, S. A. Tittlemier and G. T. Tomy, Chemosphere, 2003, 51, 563–567 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |