Open Access Article
Open Access ArticleMultifunctional NaYF4:Yb3+,Er3+@SiO2@Au heterogeneous nanocomposites for upconversion luminescence, temperature sensing and photothermal conversion
Dongdong Lia,
Wen-Yong Lai *ab,
Qiyue Shao*c and
Wei Huang
*ab,
Qiyue Shao*c and
Wei Huang ab
ab
aKey Laboratory for Organic Electronics and Information Displays (KLOEID), Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China. E-mail: iamwylai@njupt.edu.cn
bKey Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (NanjingTech), 30 South Puzhu Road, Nanjing 211816, China
cJiangsu Key Laboratory of Advanced Metallic Materials, Department of Materials Science and Engineering, Southeast University, Nanjing 211189, People's Republic of China. E-mail: qiyueshao@seu.edu.cn
First published on 14th February 2017
Abstract
NaYF4:Yb3+,Er3+@SiO2@Au heterogeneous nanostructures, with the ability of integrating upconversion luminescence (UCL) and photothermal therapy as well as real-time temperature monitoring, have been successfully prepared. The UCL intensities of NaYF4:Yb3+,Er3+ nanocrystals showed an obvious reduction after gold nanoparticle attachment and gold shell coating, but it was found that they were independent of the SiO2 spacer layer thicknesses. The presence of gold nanostructures can significantly improve the photothermal conversion properties of NaYF4:Yb3+,Er3+ nanocrystals with an increased temperature up to 39 °C, while retaining their temperature sensing properties, manifesting their great potential as diagnostic and therapeutic tools for use in biomedical fields.
Introduction
Multifunctional nanostructures that are capable of combining various intrinsic properties in a single construct have received considerable attention due to their potential applications in multimodality imaging, biosensing, disease diagnosis, and therapy.1–3 Lanthanide-doped upconversion nanoparticles (UCNPs), which are able to emit high-energy photons under excitation by near-infrared (NIR) light, have emerged as attractive materials to achieve these applications in vitro and in vivo.4–8 Compared with the traditionally used down conversion fluorescent organic dyes and quantum dots, the use of NIR light as the excitation source provides UCNPs with many unique advantages such as low photobleaching, minimized autofluorescence and photodamage, and high penetration depth in biospecies, making them suitable for use as imaging, sensing and theranostic tools in biomedical fields.9–11Among various UCNPs, hexagonal phase (β-)NaYF4:Yb3+,Er3+ nanoparticles have been demonstrated to be the most efficient upconversion materials.12 However, their absolute quantum yields remain still low and are usually less than 1%.13 Most of absorption energy has dissipated to crystal lattice as heat. Based on this reason, β-NaYF4:Yb3+,Er3+ UCNPs are potential novel photothermal therapy tools for clinical applications. Nevertheless, most previous efforts have been made to improve the UCL efficiency, but little has been attempted to utilize the heat energy generated from the photothermal conversion process. The use of NaGdF4:Yb3+,Er3+ nanocrystals alone is able to offer some heat energy, but the finite temperature increase (≤10 °C) in most cases was insufficient for photothermal therapy application.14 The introduction of noble metal nanoparticles, especially gold and silver nanomaterials, is attractive to improve this performance, due to their strong optical absorption in visible and NIR range. Improved photothermal conversion properties have been achieved by constructing UCNPs@Au (or Ag) heterogeneous nanostructures.15,16 However, their photothermal conversion efficiency is still unsatisfactory and only limited temperature rise no higher than 25 °C was recorded even at a high excitation power density.16 Further optimization is highly desirable for realizing efficient photothermal conversion process.
In this work, NaYF4:Yb3+,Er3+@SiO2@Au heterogeneous nanostructures were designed, synthesized and efficient photothermal conversion was achieved by precisely controlling the particle size of Au nanoparticles and the thickness of Au shell. In order to clarify the controversial mechanism between noble metals and UCNPs, the influences of gold nanostructures (Au particles and Au shell) on the UCL properties were studied by accurately regulating SiO2 isolation layer thicknesses. In addition, the temperature sensing application for the resulting heterogeneous nanostructures was also investigated. The results reveal that the presence of gold nanostructures can significantly improve the photothermal conversion properties of NaYF4:Yb3+,Er3+ nanocrystals with the increased temperature up to 39 °C, while retaining their temperature sensing properties.
Experimental
Sample preparation
The chemical reagents were all purchased from Sigma-Aldrich, and used without further purification. NaYF4:Yb3+,Er3+ UCNPs, silica shell, gold nanoparticles, and gold shell were synthesized using modified procedures as previously reported.17–19In a typical synthesis of NaYF4:Yb3+,Er3+ UCNPs, 1.0 mmol rare-earth acetates (Y/Yb/Er = 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with 6 mL of oleic acid and 15 mL of 1-octadecene were added to a flask under vigorous stirring. The solution was heated to 100 °C under vacuum conditions to remove oxygen and residual water. After cooling to 50 °C, a methanol solution (10 mL) containing 4.0 mmol of NH4F and 2.5 mmol of NaOH was added. The resulting solution was kept at 50 °C for 30 min. After methanol was evaporated, the solution was heated to 300 °C under an argon atmosphere for 90 min. The nanoparticles were precipitated by addition of ethanol and isolated via centrifugation.
2) with 6 mL of oleic acid and 15 mL of 1-octadecene were added to a flask under vigorous stirring. The solution was heated to 100 °C under vacuum conditions to remove oxygen and residual water. After cooling to 50 °C, a methanol solution (10 mL) containing 4.0 mmol of NH4F and 2.5 mmol of NaOH was added. The resulting solution was kept at 50 °C for 30 min. After methanol was evaporated, the solution was heated to 300 °C under an argon atmosphere for 90 min. The nanoparticles were precipitated by addition of ethanol and isolated via centrifugation.
For the formation of 10 nm silica shell, 0.5 mmol of NaYF4:Yb3+,Er3+ UCNPs in 20 mL of cyclohexane were mixed with 1 mL of CO-520 and stirred for 10 min. Then, 4 mL of CO-520 and 0.8 mL of concentrated ammonia were added and sonicated for 20 min. After that, 0.2 mL of tetraethyl orthosilicate (TEOS) was slowly added, and the mixture was stirred for 48 h at room temperature. The nanoparticles were precipitated with acetone and washed 3 times with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume of ethanol/water solution. The silica thicknesses were adjusted by varying the contents of TEOS (0.02 mL of TEOS for 2 nm SiO2 shell and 0.4 mL of TEOS for 18 nm SiO2 shell).
1 volume of ethanol/water solution. The silica thicknesses were adjusted by varying the contents of TEOS (0.02 mL of TEOS for 2 nm SiO2 shell and 0.4 mL of TEOS for 18 nm SiO2 shell).
For the amine functionalization of NaYF4:Yb3+,Er3+@SiO2 surface, 0.5 mmol of NaYF4:Yb3+,Er3+@SiO2 nanoparticles were dispersed in 20 mL of ethanol by sonication. Then, 1.3 mL of concentrated ammonia was added and stirred for 10 min under a sealed condition. After that, 1.0 mL of 3-aminopropyltriethoxysilane was slowly added and stirred for 24 h under a sealed condition. In order to improve the modification effect, the solution was then stirred at 70 °C for 2 h in water bath. The nanoparticles were centrifuged at 8000 rpm and washed 2 times with ethanol. The final products were dispersed in 40 mL of de-ionized (DI) water.
For the formation of gold seeds, 0.1 g of NaOH with 12 μL of 80% tetrakis (hydroxymethyl) phosphonium chloride were added to 45 mL of DI water under vigorous stirring. Then, 2 mL of 1% chloroauric acid (HAuCl4) was quickly added. The solution was stirred for 3 h in darkroom, and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Gold seeds were washed 2 times with ethanol before dispersed in 10 mL of DI water. The formation procedure of gold nanoparticles was the same as that of gold seeds, except that the content of HAuCl4 was 4 mL. After the synthesis of gold seeds (or gold nanoparticles), 10 mL of amine-modified NaYF4:Yb3+,Er3+@SiO2 aqueous solution was slowly dropped into 5 mL of gold seeds (or gold nanoparticles) solution. With 6 h of curing, gold seeds (or gold nanoparticles) were attached onto the surface of NaYF4:Yb3+,Er3+@SiO2 nanoparticles. The solution was centrifuged at 5500 rpm and washed 2 times with ethanol. The as-prepared NaYF4:Yb3+,Er3+@SiO2@Au nanoparticles were then dispersed in 10 mL of DI water.
000 rpm. Gold seeds were washed 2 times with ethanol before dispersed in 10 mL of DI water. The formation procedure of gold nanoparticles was the same as that of gold seeds, except that the content of HAuCl4 was 4 mL. After the synthesis of gold seeds (or gold nanoparticles), 10 mL of amine-modified NaYF4:Yb3+,Er3+@SiO2 aqueous solution was slowly dropped into 5 mL of gold seeds (or gold nanoparticles) solution. With 6 h of curing, gold seeds (or gold nanoparticles) were attached onto the surface of NaYF4:Yb3+,Er3+@SiO2 nanoparticles. The solution was centrifuged at 5500 rpm and washed 2 times with ethanol. The as-prepared NaYF4:Yb3+,Er3+@SiO2@Au nanoparticles were then dispersed in 10 mL of DI water.
For gold shell growth, 0.025 g of potassium carbonate was dissolved in 100 mL of DI water. After 10 min, 1.5 mL of 1% HAuCl4 was added. The solution was aged for 24 h in darkroom. Then, 10 mL of above solution was mixed with 5 mL of gold seeds attached NaYF4:Yb3+,Er3+@SiO2 nanoparticles, and stirred for 10 min. A reducing agent formaldehyde (35%, 36 μL) was then introduced dropwise. Under vigorous stirring, a black precipitate was observed few minutes later. The solution was centrifuged at 5500 rpm and washed 2 times with ethanol. The NaYF4:Yb3+,Er3+@SiO2@Au core–shell nanostructures were dispersed in 10 mL DI water for further study.
Characterization
The nanoparticle morphology was recorded on a Tecnai G2 transmission electron microscopy (TEM). A temperature-controlled heating system was used for temperature-dependent UCL spectra measurement. Room-temperature and temperature-dependent UCL spectra were measured by a portable spectrometer (Maya 2000, Ocean Optics Co.) using a continuous 975 nm diode laser as the excitation source. An excitation power of about 120 mW was used, corresponding to a power density of 3 W cm−2. For the measurement of temperature-dependent UCL spectra of solution samples, a heating platform was used for heating the solution and the temperature was monitored by a thermocouple immersed into the solution. Absorption spectra of solution samples were measured by a Cary 5000 UV-Vis-NIR spectrophotometer. For measurement of the photothermal conversion properties of UCNPs in solution, the 975 nm laser was used as the radiation source, and the temperature was monitored by a thermocouple immersed into the solution.Results and discussion
Structure and morphology
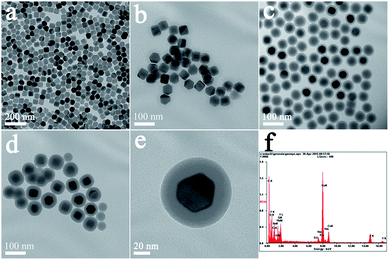
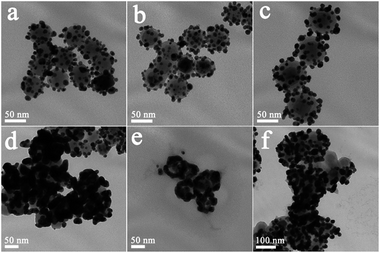
Fig. 1(a–d) shows typical TEM images of NaYF4:Yb3+,Er3+ UCNPs before and after SiO2 shell growth. It can be seen that the uncoated NaYF4:Yb3+,Er3+ UCNPs are monodispersed and can self-assemble onto the surface of copper film (Fig. 1a). After SiO2 shell growth, the nanoparticles could be re-dispersed in aqueous solution with a high monodispersity, which is favorable for their further biomedical applications. The average diameter of the NaYF4:Yb3+,Er3+@SiO2 nanoparticles increased by 4, 20, and 36 nm compared to the uncoated NaYF4:Yb3+,Er3+ core (Fig. 1b–d), indicating the successful coating of 2, 10, and 18 nm SiO2 shells around the NaYF4:Yb3+,Er3+ core. Fig. 1(e) shows a clear NaYF4:Yb3+,Er3+@SiO2 core–shell nanostructures. The corresponding energy-dispersive X-ray analysis (EDX) proves the existence of Si and O elements (Fig. 1(f)), indicating the formation of SiO2 shell around NaYF4:Yb3+,Er3+ core.After amine modification, gold nanoparticles with average diameter of ∼9.3 nm were attached onto the surface of NaYF4:Yb3+,Er3+@SiO2 nanoparticles. As shown in Fig. 2(a–c), NaYF4:Yb3+,Er3+@SiO2@Au nanostructures with different SiO2 spacer layer thicknesses were constructed after the gold nanoparticles adsorption. Taking advantage of NaYF4:Yb3+,Er3+@SiO2 nanoparticles attached with gold seeds as the precursors, gold shells were then successfully obtained by introducing additional HAuCl4 and reductant into the solution. It can be seen from Fig. 2(d–f) that continuous gold shells with the average thickness of 15 nm have grown on the surface of NaYF4:Yb3+,Er3+@SiO2 nanoparticles. The coated gold shell reduced the monodispersity of NaYF4:Yb3+,Er3+@SiO2@Au nanostructures in aqueous solution, and even presented a black precipitate in final products, indicating the strong absorption of visible light.
Upconversion luminescence properties
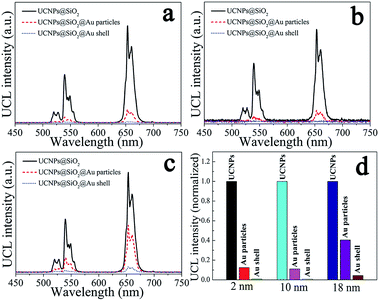
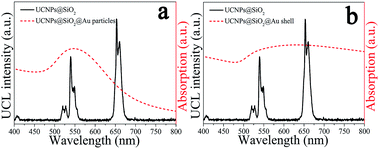
The upconversion emission spectra of NaYF4:Yb3+,Er3+@SiO2@Au nanostructures were collected in aqueous solution under 975 nm laser excitation. It can be seen from Fig. 3(a–c) that green and red upconversion emissions centered at around 525, 545, and 660 nm are observed for all samples, corresponding to the 2H11/2 → 4I15/2, 4S3/2 → 4I15/2, and 4F9/2 → 4I15/2 transitions of Er3+ ions, respectively. With gold nanoparticles attachment, an obvious reduction in UCL intensity can be found when 2 nm thickness of SiO2 was used as the spacer layer (Fig. 3a). The UCL intensity will further attenuate with the formation of gold shell, e.g., the integral UCL intensities decreased over 85% and 98% after the gold nanoparticles adsorption and gold shell coating, respectively (Fig. 3d). It is well known that there exits an interaction between noble metals and UCNPs, depending on the distance of UCNPs from the metal surface.20 Therefore, we attempt to further regulate the dominant effect of the gold nanostructures on UCL properties by increasing the SiO2 spacer layer thicknesses. Fig. 3(b) and (c) show the UCL intensities of NaYF4:Yb3+,Er3+@SiO2@Au nanostructures with increasing the SiO2 spacer layer thicknesses to 10 and 18 nm, respectively. It can be found that significant UCL quenching effect still exists as the presence of gold nanoparticles and gold shell (Fig. 3d). It means that the existence of gold nanostructures (including gold particles and gold shell) led to an obvious quenching in UCL intensities, and the spacer layer thickness was not the dominant factor that governed the UCL intensities of NaYF4:Yb3+,Er3+@SiO2@Au nanostructures.Three interactions between metal nanostructures and UCNPs can influence the UCL intensities of UCNPs: (a) an increase of the excitation rate by local field enhancement (LFE); (b) an increase of the emission rate by surface-plasmon-coupled emission (SPCE); (c) energy transfer from UCNPs to metal nanostructures (ET).21 LFE and SPCE factors account for the metal-induced UCL enhancement.22 However, the energy transfer from UCNPs to metal nanostructures would result in an obvious quenching in UCL intensities.23 In order to understand the interaction between gold nanostructures and UCNPs, we further characterized the UV-Vis-NIR absorption spectra of gold nanoparticles and gold shell. As shown in Fig. 4(a), a surface plasmon resonance (SPR) peak centered at around 550 nm can be observed for gold nanoparticles, which overlays well with the upconversion emission peaks at around 525 and 545 nm, and partially overlays with the emission peak at around 660 nm. Given that UCL was suppressed rather than enhanced by gold nanoparticles, it is reasonable to believe that ET instead of SPCE plays the dominant role in regulating the UCL intensities of NaYF4:Yb3+,Er3+ UCNPs. Compared with the sharp absorption peak of gold nanoparticles, a wide absorption band from visible to NIR region can be observed for gold shell (Fig. 4b). Due to the strong absorption of all upconversion emissions as well as the 975 nm excitation light, the UCL was quenched after the gold shell growth. The obvious enhancement in photothermal conversion properties in the presence of gold nanostructures (will be discussed later) also proved the huge energy extraction from upconversion emission, and consequently led to a significant reduction in UCL intensities. Previous study on enhancing the UCL by gold shell tuned the SPR peak to the NIR region (900 nm) by reducing the shell thickness to 3 nm.24 We thus believe that a thin gold shell is necessary for the enhancement of UCL.
Temperature sensing and photothermal properties
Due to the narrow energy gap between 2H11/2 and 4S3/2 energy levels of Er3+ ions, a thermal equilibrium exists between them.25 Therefore, the intensity ratio (RHS) of 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of Er3+ ions is sensitive to temperature. According to Boltzmann's distribution, the intensity ratio RHS as a function of temperature can be expressed as:26| RHS = RHS(0)exp(−ΔE/kT) | (1) |
Fig. 5(a) shows the dependence of ln(RHS) on the inverse temperature for NaYF4:Yb3+,Er3+ UCNPs with and without gold nanoparticles and gold shell. In accordance with eqn (1), all the samples show a good linear relation of ln(RHS) versus 1/T. It means that the temperature can be accurately measured from the intensity ratio of 525 and 545 nm emissions. In addition, almost the same relations of RHS vs. 1/T are obtained for three samples, indicating that the existence of gold nanostructures has little effects on the temperature sensing properties of NaYF4:Yb3+,Er3+ UCNPs. Therefore, NaYF4:Yb3+,Er3+@SiO2@Au nanostructures can be used as the temperature sensing probes for real-time temperature monitoring, without altering the temperature sensing properties of UCNPs.
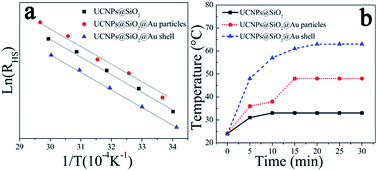 | ||
| Fig. 5 (a) Plots of ln(RHS) vs. 1/T and (b) photothermal conversion properties of NaYF4:Yb3+,Er3+@SiO2, NaYF4:Yb3+,Er3+@SiO2@Au nanoparticles and NaYF4:Yb3+,Er3+@SiO2@Au shell. | ||
To assess the photothermal conversion properties for possible biomedical applications, the change of the temperature of NaYF4:Yb3+,Er3+@SiO2@Au nanostructures in aqueous solution were measured under the exposure to 975 nm laser with the pump power of 1.73 W. As depicted in Fig. 5(b), the temperature of NaYF4:Yb3+,Er3+ UCNPs increased ∼9 °C within 10 min. This temperature rises can be attributed to the low quantum yields of UCNPs, which lead to most of the energy consumed in the form of heat. Therefore, the temperature of NaYF4:Yb3+,Er3+ UCNPs can rise rapidly using 975 nm NIR light as the radiation source. As the presence of gold nanostructures, the photothermal conversion properties of NaYF4:Yb3+,Er3+ UCNPs are dramatically improved. For instance, the temperature increased by ∼24 and ∼39 °C with gold nanoparticles attachment and gold shell coating, respectively. This photothermal conversion results outperform the previous records reported in literature,16 suggesting great promise for further clinical applications. The improved photothermal conversion properties can be attributed to the precise position and high intensity of the SPR peak (Fig. 4) regulated by the appropriate dimension of metal nanostructures, including the size of metal nanoparticles and the thickness of metal shells. The gold nanostructures can absorb energy from UCNPs, and then converts it into heat.
The above results suggest that NaYF4:Yb3+,Er3+@SiO2@Au nanostructures would be ideal diagnostic and therapeutic tool to be used in vitro and in vivo, due to their ability of integrating UCL, temperature sensing, and photothermal therapy simultaneously. Considering the big particle size and the low dispersion in aqueous solution of gold shell coated NaYF4:Yb3+,Er3+@SiO2@Au nanostructures, we believe that gold nanoparticles coated NaYF4:Yb3+,Er3+@SiO2@Au nanostructures with excellent photothermal conversion properties and high dispersion in aqueous solution are preferable as multifunctional nanomaterials in biomedical fields.
Conclusions
In summary, NaYF4:Yb3+,Er3+@SiO2@Au heterogeneous nanostructures have been successfully synthesized, and their multifunctional properties including UCL, temperature sensing and photothermal conversion were systemically studied. The results show that the NaYF4:Yb3+,Er3+@SiO2@Au nanostructures are capable of simultaneously providing UCL, photothermal therapy, and real-time temperature monitoring. The presence of gold nanostructures has dramatically improved the photothermal conversion properties of NaYF4:Yb3+,Er3+ UCNPs without altering their temperature sensing properties. The temperature increased by ∼39 °C with gold shell coating, representing the best photothermal conversion result among UCNPs. In addition, the UCL intensities of NaYF4:Yb3+,Er3+ UCNPs exhibit an obvious attenuation with gold nanoparticles attachment and gold shell coating, and it was found that they were independent of the SiO2 spacer layer thicknesses, due to the efficient energy transfer from UCNPs to the gold nanostructures.Acknowledgements
The authors acknowledge financial support from the National Key Basic Research Program of China (973 Program, 2014CB648300), the National Natural Science Foundation of China (21422402, 21674050, 61136003), the Natural Science Foundation of Jiangsu Province (BK20160073, BK20140060, BK20130037, BM2012010), Program for Jiangsu Specially-Appointed Professors (RK030STP15001), Program for New Century Excellent Talents in University (NCET-13-0872), Specialized Research Fund for the Doctoral Program of Higher Education (20133223110008), the Synergetic Innovation Center for Organic Electronics and Information Displays, the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), the NUPT “1311 Project”, the Six Talent Plan (2012XCL035), the 333 Project (BRA2015374) and the Qing Lan Project of Jiangsu Province. This work was also sponsored by NUPTSF (NY216025).Notes and references
- M. K. Tsang, G. X. Bai and J. H. Hao, Chem. Soc. Rev., 2015, 44, 1585–1607 RSC.
- C. X. Li and J. Lin, J. Mater. Chem., 2010, 20, 6831–6847 RSC.
- Y. Sun, X. J. Zhu, J. J. Peng and F. Y. Li, ACS Nano, 2013, 7, 11290–11300 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. G. Li, X. Zeng, M. W. Shao, S. T. Lee and Z. Liu, Biomaterials, 2012, 33, 2215–2222 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. G. Li, J. H. Chen, C. Wang, M. W. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 123, 7523–7528 CrossRef.
- S. S. Han, X. Qin, Z. F. An, Y. H. Zhu, L. L. Liang, Y. Han, W. Huang and X. G. Liu, Nat. Commun., 2015, 7, 13059 CrossRef PubMed.
- X. G. Liu, C. H. Yan and J. A. Capobianco, Chem. Soc. Rev., 2015, 44, 1299 RSC.
- W. Wei, T. C. He, X. Teng, S. X. Wu, L. Ma, H. Zhang, J. Ma, Y. H. Yang, H. Y. Chen, Y. Han, H. D Sun and L. Huang, Small, 2012, 8, 2271–2276 CrossRef CAS PubMed.
- H. Y. Xing, W. B. Bu, S. J. Zhang, X. P. Zheng, M. Li, F. Chen, Q. J. He, L. P. Zhou, W. J. Peng, Y. Q. Hua and J. L. Shi, Biomaterials, 2012, 33, 1079–1089 CrossRef CAS PubMed.
- F. Vetrone, R. Naccache, A. Zamarrón, A. J. de la Fuente, F. S. Rodríguez, L. M. Maestro, E. M. Rodriguez, D. Jaque, J. G. Solé and J. A. Capobianco, ACS Nano, 2010, 4, 3254–3258 CrossRef CAS PubMed.
- D. M. Yang, P. A. Ma, Z. Y. Hou, Z. Y. Cheng, C. X. Li and J. Lin, Chem. Soc. Rev., 2015, 44, 1416–1448 RSC.
- C. R. Lecuna, R. M. Rodríguez and R. Valiente, Chem. Mater., 2011, 23, 3442–3448 CrossRef.
- J. C. Boyer and F. C. J. M. van Veggel, Nanoscale, 2010, 2, 1417–1419 RSC.
- Q. Y. Shao, L. L. Ouyang, L. F. Jin and J. Q. Jiang, Opt. Express, 2015, 23, 30057–30066 CrossRef PubMed.
- B. Dong, S. Xu, J. Sun, S. Bi, D. Li, X. Bai, Y. Wang, L. P. Wang and H. W. Song, J. Mater. Chem., 2011, 21, 6193–6200 RSC.
- Y. Song, G. X. Liu, X. T. Dong, J. X. Wang, W. S. Yu and J. M. Li, RSC Adv., 2014, 4, 62802–62808 RSC.
- H. S. Qian and Y. Zhang, Langmuir, 2008, 24, 12123–12125 CrossRef CAS PubMed.
- L. Li, K. Green, H. Hallen and S. F. Lim, Nanotechnology, 2015, 26, 025101 CrossRef CAS PubMed.
- H. Zhang, Y. J. Li, I. A. Ivanov, Y. Q. Qu, Y. Huang and X. F. Duan, Angew. Chem., Int. Ed., 2010, 49, 2865–2868 CrossRef CAS PubMed.
- M. Saboktakin, X. C. Ye, S. J. Oh, S. H. Hong, A. T. Fafarman, U. K. Chettiar, N. Engheta, C. B. Murray and C. R. Kagan, ACS Nano, 2012, 6, 8758–8766 CrossRef CAS PubMed.
- P. Y. Yuan, Y. H. Lee, M. K. Gnanasammandhan, Z. P. Guan, Y. Zhang and Q. H. Xu, Nanoscale, 2012, 4, 5132–5137 RSC.
- W. Deng, L. Sudheendra, J. B. Zhao, J. X. Fu, D. Y. Jin, I. M. Kennedy and E. M. Goldys, Nanotechnology, 2011, 22, 325604 CrossRef PubMed.
- Z. Q. Li, L. M. Wang, Z. Y. Wang, X. H. Liu and Y. J. Xiong, J. Phys. Chem. C, 2011, 115, 3291–3296 CAS.
- A. Priyam, N. M. Idris and Y. Zhang, J. Mater. Chem., 2012, 22, 960–965 RSC.
- J. N. Shan, W. J. Kong, R. Wei, N. Yao and Y. G. Ju, J. Appl. Phys., 2010, 107, 054901 CrossRef.
- M. D. Shinn, W. A. Sibley, M. G. Drexhage and R. N. Brown, Phys. Rev. B: Condens. Matter Mater. Phys., 1983, 27, 6635–6648 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |