Open Access Article
Open Access ArticleSynthesis and organic field effect transistor properties of isoindigo/DPP-based polymers containing a thermolabile group†
Mohamed Shaker‡
ab,
Byoungwook Park‡a,
Jong-Hoon Lee‡a,
Wonbin kima,
Cuc Kim Trinha,
Hong-Joon Leea,
Jin woo Choia,
Heejoo Kima,
Kwanghee Lee*a and
Jae-Suk Lee *a
*a
aSchool of Materials Science & Engineering, Research Institute for Solar and Sustainable Energies (RISE), Heeger Center for Advanced Materials (HCAM), Advanced Photonics Research Institute, Gwangju Institute of Science and Technology, Gwangju 500-712, Korea. E-mail: jslee@gist.ac.kr
bChemistry Department, Faculty of Science, Tanta University, Tanta 31527, Egypt
First published on 14th March 2017
Abstract
(E)-6,6′-Dibromo-1,1-bis(2-octyldodecyl)-(3,3′-biindolinylid-ene)-2,2′-dione and/or 2,5-bis(2-octyldodecyl)-3,6-di(5-bromothien-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione and their tBoc-counterparts were propagated with 2,5-bis(tributylstannyl)thiophene in a molar ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to release P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) as a new series of random conjugated polymers (RCPs) bearing a large number of octyldodecyl chains to ensure solubility and a small number of thermocleavable tBoc function to cast H-bonding upon heating up to 220 °C. All new polymers were synthesised via Pd catalysed Stille cross-coupling methodology in high yields and reasonable average molecular weights. The cast polymer films exhibited considerable red-shifted UV-vis absorption spectra and a further red-shift was also obtained in the thermal annealed films (at 220 °C for 30 min), which reflected the increasing of crystalline structure. The formation of H-bonding in these polymers was investigated using X-ray diffractometry (XRD) measurements. The field-effect mobilities of these polymers were investigated in the configuration of bottom-gate and bottom-contact (BGBC) field-effect transistors (FETs). The results from FETs indicated that the crystalline structure of RCPs exhibited reasonable FET mobilities with 1.17 × 10−3 cm2 V−1 s−1 for P(ODDPPT·BID) and 1.41 × 10−3 cm2 V−1 s−1 for P(ODDPPT·BDPP).
1.0 to release P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) as a new series of random conjugated polymers (RCPs) bearing a large number of octyldodecyl chains to ensure solubility and a small number of thermocleavable tBoc function to cast H-bonding upon heating up to 220 °C. All new polymers were synthesised via Pd catalysed Stille cross-coupling methodology in high yields and reasonable average molecular weights. The cast polymer films exhibited considerable red-shifted UV-vis absorption spectra and a further red-shift was also obtained in the thermal annealed films (at 220 °C for 30 min), which reflected the increasing of crystalline structure. The formation of H-bonding in these polymers was investigated using X-ray diffractometry (XRD) measurements. The field-effect mobilities of these polymers were investigated in the configuration of bottom-gate and bottom-contact (BGBC) field-effect transistors (FETs). The results from FETs indicated that the crystalline structure of RCPs exhibited reasonable FET mobilities with 1.17 × 10−3 cm2 V−1 s−1 for P(ODDPPT·BID) and 1.41 × 10−3 cm2 V−1 s−1 for P(ODDPPT·BDPP).
Introduction
For the past decade, isoindigo (ID) and diketopyrrolopyrrole (DPP) based π conjugated polymers (CPs) have attracted much attention as electronic materials for solution processing polymer solar cells (PSCs)1,2 and organic field-effect transistors (OFETs).1–3 As is well known, ID and DPP possess interesting properties, such as a highly fused conjugated structure, electron-deficient nature and easy synthesis. These characteristics are reflected in their polymers, which show low bandgaps, strong π–π interactions, high crystallinity and high charge carrier mobility. In addition, ID and DPP based polymers have alkyl chains on their N atoms to induce solubility.However, CPs based on the two units exhibit different optical and electrochemical properties, such as the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO) and band gaps. To date, the donor–acceptor (D–A) system, the alternative linkage between electron-rich and electron-deficient aromatic units, is one of the important protocols to design low bandgap electron donating materials in organic photovoltaic cells (OPVs),4 as well as high mobility CPs for OFETs.5–7 Careful design and the matching between the selected donor and acceptor molecular units help us to tune the HOMO and LUMO energy levels as well as the bandgap of synthesized CPs, owing to the HOMO and LUMO energy levels being centered on the donor and acceptor moieties, respectively.8 For example, isoindigo-based low bandgap polymers, containing thiophene as a donor moiety (PT-ID1), were synthesized and the isoindigo monomer was branched with a 2-octyldodecyl side chain to improve the solubility.9 The HOMO and LUMO energy levels of PT-ID1 were −5.49 and −3.91 eV, respectively. A photovoltaic device with PT-ID1/PC71BM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio as the active layer (70 nm) gave an open circuit voltage (Voc) value of 0.87 V, a fill factor (FF) of 60%, and a short circuit current (Jsc) value of 1.76 mA cm−2, resulting in a power conversion efficiency (PCE) value of 0.92%.9 The same polymer structure on the application of OFETs showed a hole-mobility (μh) of 0.019 cm2 V−1 s−1, but has a HOMO/LUMO level of −5.8/−3.8 eV.10,11
2 ratio as the active layer (70 nm) gave an open circuit voltage (Voc) value of 0.87 V, a fill factor (FF) of 60%, and a short circuit current (Jsc) value of 1.76 mA cm−2, resulting in a power conversion efficiency (PCE) value of 0.92%.9 The same polymer structure on the application of OFETs showed a hole-mobility (μh) of 0.019 cm2 V−1 s−1, but has a HOMO/LUMO level of −5.8/−3.8 eV.10,11
Similarly, an alkylated DPP/thiophene based CP (PDPP3T) showed the onset of optical absorption at 1.30 eV as a small band-gap polymer; its HOMO/LUMO levels were found to be −5.17 and −3.61 eV. The optimized cells provided a PCE value of 4.7% and gave μh = 0.05 cm2 V−1 s−1 and μe = 0.008 cm2 V−1 s−1.12 A PCE of 6.71% was recorded for PDPP3T by using the optimal composition of ternary solvent.13 Moreover, Leclerc et al. investigated the alternative combination between ID and DPPDT structure moiety-based CPs under Suzuki coupling conditions. This polymer showed HOMO/LUMO levels at −5.3/−4.0 eV with a 1.3 eV bandgap. After annealing at 200 °C, ID/DPPDT based CP demonstrated an electron mobility of 1.6 × 10−4 cm2 V−1 s−1.14 In parallel, random copolymers with different molar ratios of DPP to isoindigo (DPP/isoindigo = 0.3/0.7; 0.5/0.5; 0.7/0.3) with thiophene were synthesized and offered moderate properties between PT-ID1 and PDPP3T. The equal amounts of DPP and isoindigo in the copolymer absorbed a wide range of the solar spectrum from 600 to 900 nm with a low HOMO level, which is essential for high performance PSCs. These classes of random polymers have bandgaps in the range from 1.38 to 1.46 eV and have semi-crystalline structure characteristics, which may facilitate the hole transport affording high Jsc = 13.52 mA cm−2 as well as high PCE, up to 6%.15 The hole mobility of the 0.5/0.5 random copolymer is 0.102 cm2 V−1 s−1, which is higher than the corresponding alternative.15 All the previous CPs contain alkyl side chains for solubility.
On the other hand, the thermolabile tert-butoxy carbonyl (Boc) protecting group is a convenient group for protecting indigo and/or DPP imide N–H function during the coupling reactions. These groups can be removed by heat treatment to recover the N–H group when desired. The story of Boc-indigo and Boc-DPP started in the 1990s,16,17 but in the field of organic electronics, the Boc group has made a small contribution and has been employed to allow solution processing.18 Boc-indigo/fluorine based CP was synthesized and underwent a clean deprotection reaction and showed promising physical properties.19 To the best of our knowledge, organic macromolecule materials, such as CPs and small molecules based on Boc-isoindigo (Boc-ID), were rarely released.20
Besides our interest in CP synthesis and characterization,21–24 recently, we have synthesized several novel organic materials based on isoindigo and achieved PCE = 1.6 up to 3.2%.25,26
In this work, we introduce Boc-ID and Doc-DPP along the backbone structure of a new series of random CPs based on octyldodecyl-alkylated ID and dithienyl-DPP (DTDPP). N-Protected electron deficient monomers participate in a small molar ratio on the alternative condensation reaction of alkylated ID and/or DPP with thiophene under Stille coupling polymerization conditions. The new random CPs are designed to exhibit high crystalline structure after the thermal deprotection of Boc and release of the N–H function, and help to form H bonding, which is important for long range ordering in the solid state. The formation of H bond interactions between polymer chains has been investigated, and their impact on the optical and electrochemical properties has been studied.
Experimental
Synthetic details
Co-monomers, (E)-6,6′-dibromo-1,1-bis(2-octyldodecyl)-(3,3′-biindolinylid-ene)-2,2′-dione (1) 2,5-bis(2-octyldodecyl)-3,6-di(5-bromothien-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione (3) and 2,5-bis(tert-butyloxycarbonyl)-3,6-di(5-bromothien-2-yl)pyrrolo[3,4-c] pyrrole-1,4-(2H,5H)-dione (4) were synthesized as per the published literature.1–3General procedures for Pd catalyzed Stille coupling arylation for the synthesis of the new polymers
Under highly dry conditions, a mixture of 5-bis(tributylstannyl)thiophene (5) (0.66 g, 1.00 mmol), the desired alkylated dibromo-derivative of ID/DPP materials (1 and/or 3) (0.8 mmol), the desired Boc-derivative of ID/DPP materials (2 and/or 4) (0.2 mmol) and Pd(PPh3)4 (0.058 g, 0.05 mmol) in dry DMF (20 mL) was degassed with nitrogen for 20 min then heated under reflux for 24–48 h at a temperature of 120 °C. The reaction mixture was allowed to return to room temperature and then poured into methanol. The crude polymer was collected by filtration and washed repeatedly with methanol. The residual solid was loaded into an extraction thimble and washed with methanol (24 h) followed by acetone (24 h).P(ODIDT-BID) dark violet color. 1H NMR (CDCl3, 400 MHz), δ (ppm): 9.27–8.92 (br, 4H), 7.49–6.48 (br, 10H), 3.81–3.33 (br, 2H), 2.72–2.49 (br, 4H) 2.06–1.78 (br, 22H), 1.58–1.05 (br, 76H), 1.02–0.72 (br, 18H).
P(ODIDT·BDPP) dark violet color. 1H NMR (CDCl3, 400 MHz), δ (ppm): 9.31–8.94 (br, 4H), 7.47–6.51 (br, 10H), 3.89–3.48 (br, 2H), 2.70–2.58 (br, 4H) 2.07–1.81 (br, 22H), 1.50–1.04 (br, 76H), 0.96–0.71 (br, 18H).
P(ODDPPT·BID) dark violet color. 1H NMR (CDCl3, 400 MHz), δ (ppm): 9.00–8.92 (br, 4H), 7.55–6.79 (br, 10H), 4.14–3.99 (br, 2H), 2.00–1.95 (br, 4H), 1.82–1.59 (br, 22H), 1.59–1.19 (br, 76H), 0.98–0.71 (br, 18H).
P(ODDPPT·BDPP) dark violet color. 1H NMR (CDCl3, 400 MHz), δ (ppm): 9.09–8.76 (br, 4H), 7.40–6.73 (br, 10H), 4.06–3.37 (br, 2H), 2.08–1.84 (br, 4H), 1.73–0.95 (br, 98H), 0.93–0.71 (br, 18H).
Device fabrication
The bottom-gate/bottom-contact (BGBC) OFET devices were fabricated using n++-Si/SiO2 (200 nm) substrates. The Au electrodes (40 nm) for the source and drain were thermally evaporated with a length of 50 μm and a width of 1000 μm on the SiO2 surface under high vacuum (∼10−6 Torr). The substrates were subjected to cleaning by ultra-sonication in deionized water, acetone, and isopropanol each for 20 min. The cleaned substrates were dried in an oven at 80 °C for 2 h. The substrates were UV/ozone treated for 20 min and then transferred into a nitrogen-filled glovebox. The thin films of the random CPs were spin-cast on the substrates with the random CP solutions (1 wt% in chloroform) at 3000 rpm for 30 s. As-prepared films were annealed at 200 °C for 10 min.Results and discussion
Molecular design and synthesis
Random donor–acceptor polymers based on diketopyrrolopyrrole/isoindigo-thiophene were selected to be the semiconducting backbones for several reasons, such as the excellent device performance that has been shown in organic electronic devices and the easy functionalization of the nitrogen atom on the isoindigo or diketopyrrolopyrrole units. To ensure high solubility a large number of t-Boc and octyldedocyl groups were attached. In previous work, the thermolabile random copolymerization strategy showed good solubility with high stacking order in the annealed solid state but had a possible low impact on the electronic performance.20(E)-6,6′-Dibromo-1,1-bis(2-octyldodecyl)-(3,3′-biindolinylid-ene)-2,2′-dione (1) was reported in our previous work (Fig. 1).26 In this work, we introduce (E)-6,6′-dibromo-1,1-bis(tert-butyloxycarbonyl)-(3,3′-biindolinylid-ene)-2,2′-dione (Boc ID) (2) by the modification of the reported procedure:27 treatment of (E)-6,6′-dibromo-1H,1′H-[3,3′]biindolylidene-2,2′-dione with 3 equivalents of tert-butyl dicarbonate (tBoc2O) in tetrahydrofuran (THF) at room temperature resulted in over 80% pure product. Two precursor comonomers, 2,5-bis(2-octyldodecyl)-3,6-di(5-bromothien-2-yl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione (3) and 2,5-bis(tert-butyloxycarbonyl)-3,6-di(5-bromothien-2-yl)pyrrolo[3,4-c]-pyrrole-1,4-(2H,5H)-dione (4), were synthesized as per the published literature,28,18e (Fig. 1). Moreover, lithiation of thiophene by n-BuLi at −80 °C followed by quenching with tributyltinchloride gave the corresponding 2,5-bis(tributylstannyl)thiophene (5). All materials were extracted with organic solvents and highly purified via column chromatography.
Synthesis of random copolymers
Based on Stille coupling conditions, the polymerization reactions were done in degassed anhydrous N,N-dimethylformamide (DMF) as a solvent in the presence of catalytic amounts of Pd(PPh3)4 under N2 atmosphere at 80 °C using thermal methodology. The alkylated acceptors and t-butyloxy carbonyl moieties were mixed with 2,5-bis(tributylstannyl) thiophene (5) in a molar ratio of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.
New random conjugated polymers were designed to obtain large numbers of octyldodecyl comonomers to insure the solubility for film casting. Polymers P(ODIDT-BID) and P(ODIDT·BDPP) were obtained via Stille coupling reaction, in which comonomers 1 and distannyl derivative 5 were mixed with Boc monomers 2 or 4, respectively, in good yields (77% and 73% respectively) and acceptable average molecular weights (Mn) and reasonable polydispersity index (PDI) values (Table 1). Similarly, comonomers 3 and distannyl derivative 5 were reacted with Boc monomers 2 or 4 under Stille coupling conditions in DMF to afford P(ODDPPT·BID) and P(ODDPPT·BDPP) random copolymers in 75% and 73% yields, respectively, and showed reasonable molecular weights (Table 1). The synthetic route to the polymers is shown in Scheme 1.
| Polymer | Mnb (kg mol−1) | PDI (Mw/Mn)b | Yieldc (%) | Tdd (°C) |
|---|---|---|---|---|
| a All polymerizations were carried out using the Stille cross-coupling method.b Calculated from GPC (eluent, CHCl3; 30 °C; polystyrene standards).c Based on the weight of pure polymer obtained.d Determined by DSC under nitrogen atmosphere at a heating rate of 10 °C min−1. | ||||
| P(ODIDT·BID) | 20.2 | 1.81 | 77.0 | 338.4 |
| P(ODIDT·BDPP) | 19.7 | 1.77 | 73.0 | 390.3 |
| P(ODDPPT·BID) | 16.5 | 1.69 | 75.0 | 436.2 |
| P(ODDPPT·BDPP) | 17.2 | 1.71 | 73.0 | 391.9 |
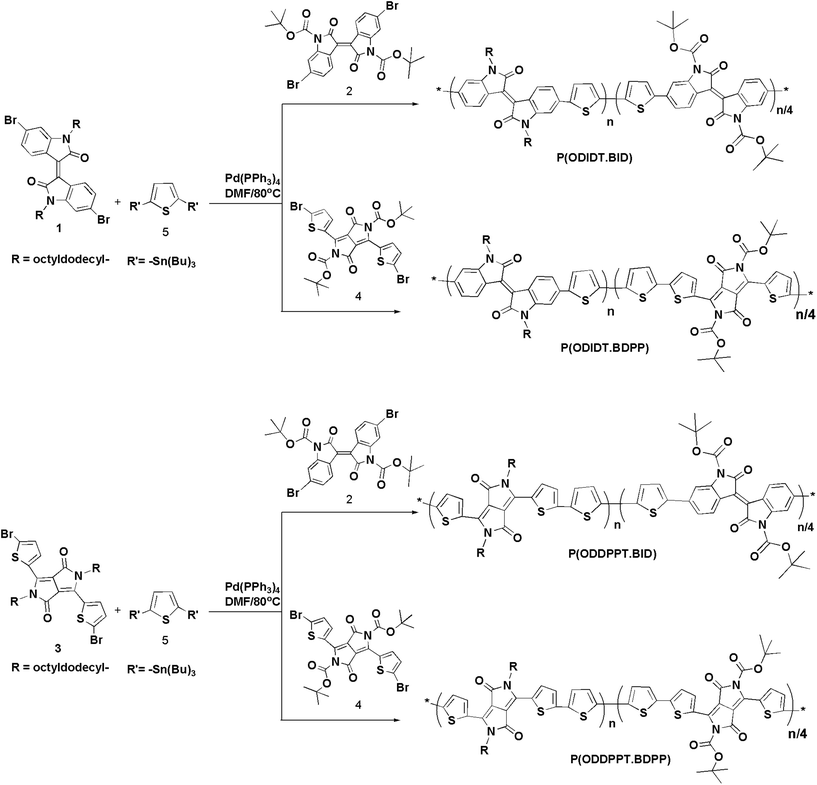 | ||
| Scheme 1 Synthetic routes of all new random conjugated polymers P(ODIDT·BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP). | ||
All copolymers showed good solubility at room temperature in most common organic solvents. After purification by Soxhlet extraction with methanol and acetone repeatedly to remove the by-products and the oligomers, all polymer structures were confirmed by 1H NMR and elemental analyses. All the data were inserted with the proposed structures (see the ESI† for the 1H NMR spectra).
Thermal properties
Under nitrogen atmosphere, thermogravimetric analysis (TGA) was used to investigate the thermal properties of the four new CPs. From TAG analysis, all polymers retained 50% residual weights when the temperature increased up to 800 °C, except P(ODIDT·BDPP), which lost 60% of its total weight at 750 °C. All polymers showed two decomposition steps. The thermal decomposition related to isolation of the tBoc function appeared at Td 98.8–99.8 wt% residues in the range of 180–220 °C.The second thermal decomposition temperatures (Td, 92–84 wt% residues) were in the range of 388–436 °C, related to the decomposition of the long alkyl chains during heating,29 which confirmed the high thermal stability of all polymer products. The thermogravimetric analysis diagrams of the synthesized random copolymers are shown in the ESI (Fig. S1†).
To provide the formation of H-bonds after the thermal elimination of the t-Boc groups, Fourier transform infrared (FT-IR) spectroscopy was used (Fig. S2†). In general in the annealed films, a weak band was found at around 3450 cm−1, which was assigned to the lactam structure N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding of the isoindigo and DPP units. In addition, the stretching vibration band of C
C hydrogen bonding of the isoindigo and DPP units. In addition, the stretching vibration band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at ∼1700 cm−1 disappeared, which clearly confirmed the complete decomposition of t-Boc groups, and the lactam moiety C
O at ∼1700 cm−1 disappeared, which clearly confirmed the complete decomposition of t-Boc groups, and the lactam moiety C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifted to slightly smaller wavenumber.
O shifted to slightly smaller wavenumber.
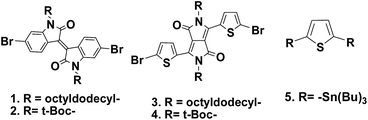
Optical properties
Fig. 2 shows the solution and film absorption spectra of the new polymers. In dilute solutions of polymers in chloroform, P(ODDPPT·BID) and P(ODDPPT·BDPP) offered one absorption band, which indicates the intermolecular charge transfer (ICT) between donor and acceptor units, with maxima at 612 and 616 nm, respectively.However, P(ODIDT-BID) and P(ODIDT·BDPP) showed two major absorption bands in dilute solutions, the shorter wavelength absorption bands at 451 and 454 nm for P(ODIDT-BID) and P(ODIDT·BDPP), respectively, are indicated from the π–π* transition and the longer absorption bands at wavelength 646 nm and 620 nm, respectively, representing the ICT at the donor–acceptor composition.
In solid films, polymers containing ODDPP showed broader absorbance than ODID-based polymers, which reflects the high π-stacking ordering in the case of ODDPP based polymers. Corresponding to the absorption edges, polymers based on ODID represent a slight red shift in solid state film compared to in dilute solutions. P(ODIDT-BID) showed λonset = 823 nm in film and λonset = 796 nm in solution. Similarly, P(ODIDT·BDPP) exhibits absorption edges at 850 nm in film and 792 nm in solution. In contrast, random CPs based on ODDPP offer long range red shift and broadness compared to their corresponding absorptions in solution, reflecting the increasing interchain interaction and ordering along the polymer backbones as well as the extended π-conjugation length in the solid state. The onset absorptions of P(ODDPPT·BID) and P(ODDPPT·BDPP) are 1036 nm and 980 nm, respectively, more red shifted by approximately 200 nm than the absorptions edges in solution. The optical bandgaps of all polymers, which were calculated from λonset in the solid state [Eopg (eV) = 1240/λonset (nm)], are 1.51, 1.46, 1.20 and 1.26 eV for P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP), respectively (Table 2). The new random CPs possess lower optical band gaps and broader absorption windows than their corresponding CPs not containing N-block tBoc-acceptors along their backbone structures.9,15 Interestingly, in the as-cast films, P(ODDPPT·BID) and P(ODDPPT·BDPP) offered second short absorption bands, which indicate the π–π* transition, at 423 nm and 420 nm, respectively.
| Polymer | UV-vis absorption | |||
|---|---|---|---|---|
| Solution | In film | |||
| λmax (nm) | λmax (nm) | λonset (nm) | Eopg (eV) | |
| P(ODIDT-BID) | 641 | 641 | 791 | 1.57 |
| P(ODIDT·NHID) | 641 | 801 | 1.55 | |
| P(ODIDT·BDPP) | 617 | 680 | 801 | 1.55 |
| P(ODIDT·NHDPP) | 684 | 807 | 1.54 | |
| P(ODDPPT·BID) | 614 | 839 | 1019 | 1.22 |
| P(ODDPPT·NHID) | 782 | 1038 | 1.20 | |
| P(ODDPPT·BDPP) | 618 | 751 | 955 | 1.30 |
| P(ODDPPT·NHDPP) | 744 | 955 | 1.30 | |
Furthermore, after annealing polymer films up to 220 °C to ensure the recovery of the N–H function and liberation of the tBoc group, all polymers showed two major absorption bands and induced a slight red shift, which indicates a great increase of long range ordering and the π-stacking orientation of the semi-crystalline polymer structures.
Electrochemical properties
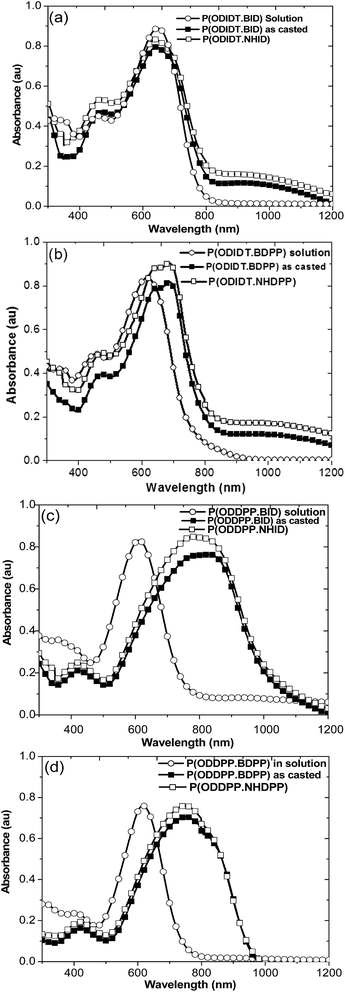
The electrochemical properties of the polymers were explored by cyclic voltammetry (CV). The cyclic voltammograms of the P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) films are shown in Fig. 3.The HOMO and LUMO energy levels of the polymers were calculated according to the equations: EHOMO = −(Eox + 4.71) eV and ELUMO = −(Ered + 4.71) eV, where Eox and Ered are the onset oxidation and reduction potentials, respectively, of the polymers vs. Ag/AgCl (saturated KCl) as a reference electrode. The HOMO energy levels moved gradually deeper in the order [P(ODIDT-BID) > P(ODIDT·BDPP) > P(ODDPPT·BID) > P(ODDPPT·BDPP)] and showed −5.44, −5.65, −5.68 and −5.69 eV, respectively. However, there are contrasts in the LUMO energy levels of the polymers. The random CPs based on the same structure of electron accepting units offered higher LUMO energy levels than the other containing a mixed (ID/DPP) backbone structure. The LUMO energy level of P(ODDPPT·BDPP) was the highest at −3.89 eV, thus P(ODIDT-BID) and P(ODIDT·BDPP) exhibited LUMO energy levels at −3.97 and −4.13 eV, respectively. However, the LUMO energy level of P(ODDPPT·BID) was the lowest at −4.17 eV. Moreover, the electrochemical bandgaps (Eg = ELUMO − EHOMO) of P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) were 1.47, 1.52, 1.51 and 1.80 eV, respectively.
In the case of decarboxylated polymers (NH-forms), the improvement of π–π packing did not show a significant change to the HOMO and LUMO energy levels. However, in the case of P(ODIDT-NHID), it showed a deeper HOMO level at −5.62 eV than its corresponding P(ODIDT-BID) polymer.
In many aspects, the new synthesized random CPs showed low band-gaps closely matched the ideal low band gaps of the polymers used for organic electronic applications.
Crystalline structure of polymers
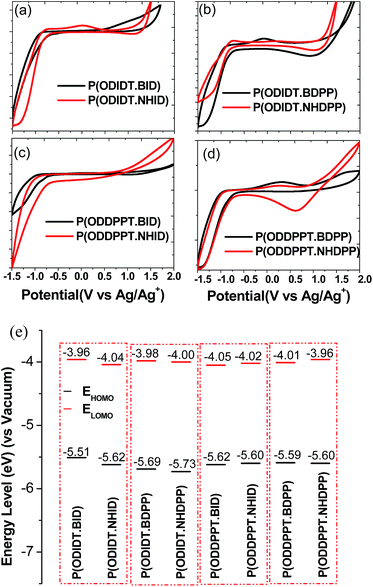
Charge carrier transport strongly depends on microstructure in CPs. To explain that, the X-ray diffractometry (XRD) measurements of the as-spun films and the films annealed at 200 °C are shown in Fig. 4. The as-spun films of P(ODDPPT·BID) and P(ODDPPT·BDPP) showed diffraction peaks at 2θ = 6.65° and 6.51°, which corresponded to d-spacing of 13.3 Å and 13.6 Å, respectively. However, P(ODIDT-BID) and P(ODIDT·BDPP) exhibited week arrangement of diffraction peaks at 2θ = 4.00° and 3.87°, proportional to d-spacing of 22.1 Å and 22.8 Å, respectively, considered to reflect the inter-lamellar distance induced by octyldodecyl chains (Fig. 4a). After annealing at 200 °C for 10 min to remove the tBoc function, the molecular ordering was improved by thermal annealing as well as the formation of H bonding across polymer chains. As a result, two new sharp and intensive diffraction peaks were found after the annealing process. The diffraction peaks at 2θ = 8.52°, 8.56°, 8.74° and 8.67°, for P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) films, respectively, considering the d-spacing of 10.0–10.4 Å confirming much stacking between the alkyl chains, owing to the formation of H bonding between polymer chain upon the removal by thermocleavage of the tBoc function during heating, which resulted in the shortening of the interlayer distance.The other diffraction peaks at 2θ = 17.28°, 17.32°, 17.98° and 17.39° for P(ODIDT-BID), P(ODIDT·BDPP), P(ODDPPT·BID) and P(ODDPPT·BDPP) films, respectively, corresponding to d-spacing of 5.0–5.2 Å, indicating that it adopted the highest degree of π–π stacking, suggested that these peaks did not only originate from crystallites formed by a combination of edge-to-edge and face-to-face packing but also from localized aggregation. These localized aggregates could readily form in synthesized polymers because of the enhanced backbone planarity and the incorporated successive H-bonding, which exhibit greatly reduced steric hindrance compared with tBoc units.
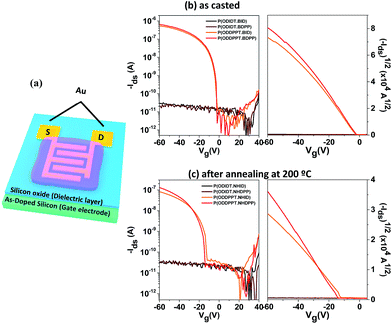
Field-effect properties of polymers
To investigate the charge transport properties of the synthesized random CPs, we fabricated bottom-gate and top-contact (BGTC) OFETs with them.30,31 Fig. 5b presents the transfer curves of the OFETs with the random CPs. There are no field-effect characteristics in the devices with P(ODIDT-BID) and P(ODIDT·BDPP), while the P(ODDPPT·BID) and P(ODDPPT·BDPP) show fine field-effect characteristics. The field-effect mobilities were extracted from the saturation region in the transfer curves by using the following equation: IDS = (W/2L)Cμ(VGS − VT)2 (Table 3), where W, L, C, μ, IDS, VGS, and VT are channel width, channel length, gate dielectric capacitance per unit area, charge carrier mobility, drain-source current, gate-source voltage, and the threshold voltage, respectively.| Polymer | Mobility (cm2 V−1 s−1) | On/off ratio | VT (V) |
|---|---|---|---|
| P(ODIDT·BID) | — | — | — |
| P(ODIDT·NHID) | — | — | — |
| P(ODIDT·BDPP) | — | — | — |
| P(ODIDT·NHDPP) | — | — | — |
| P(ODDPPT·BID) | 1.17 × 10−3 | 4.24 × 104 | −2.6 |
| P(ODDPPT·NHID) | 2.07 × 10−4 | 2.02 × 103 | −10.1 |
| P(ODDPPT·BDPP) | 1.41 × 10−3 | 1.01 × 105 | −2.3 |
| P(ODDPPT·NHDPP) | 3.91 × 10−4 | 6.80 × 103 | −13.9 |
As summarized in Table 3, as-spun films of P(ODIDT-BID) and P(ODIDT·BDPP) exhibited relatively lower hole mobilities of 2.26 × 10−8 cm2 V−1 s−1 and 6.68 × 10−8 cm2 V−1 s−1, respectively, while higher hole mobilities were obtained in the as-spun films of P(ODIDT-BID) and P(ODIDT·BDPP) (1.17 × 10−3 cm2 V−1 s−1 and 1.41 × 10−3 cm2 V−1 s−1, respectively); these results are in good agreement with the observations from the XRD analysis. However, after the films were annealed at 200 °C, poor performance with relatively lower μ and negatively-shifted VT were observed, which can be ascribed to the interruption of charge carrier injection owing to the morphology change during thermal annealing.
Surface morphologies
Therefore, we examined the surface morphologies of the films before and after annealing with tapping mode atomic force microscopy (AFM).As shown in Fig. 6, the pristine films of P(ODIDT-BID) and P(ODIDT·BDPP) showed smooth surface morphology, but that did not reveal any structural features. However, densely-packed large domains but rough morphologies were observed in P(ODDPPT·BID) and P(ODDPPT·BDPP) films owing to relatively strong inter-lamellar stacking. After annealing, it no significant change was observed in the morphology of the films of P(ODIDT-NHID) and P(ODIDT·NHDPP), compared to those of P(ODIDT-BID) and P(ODIDT·BDPP) films. Note that P(ODDPPT·NHID) and P(ODDPPT·NHDPP) films exhibited smaller aggregates or domains in the surface morphology. These phenomenon agreed with the XRD results, whereby aggregated crystallites were formed by H bonding across polymer chains. Removing tBoc-units by thermal annealing results in molecular ordering within small aggregates having disconnected morphology. This kind of molecular ordering leads to difficulties in charge carrier mobility in OFET devices, which require lateral transfers, but this may have better performance in vertical transfer or in other future devices.
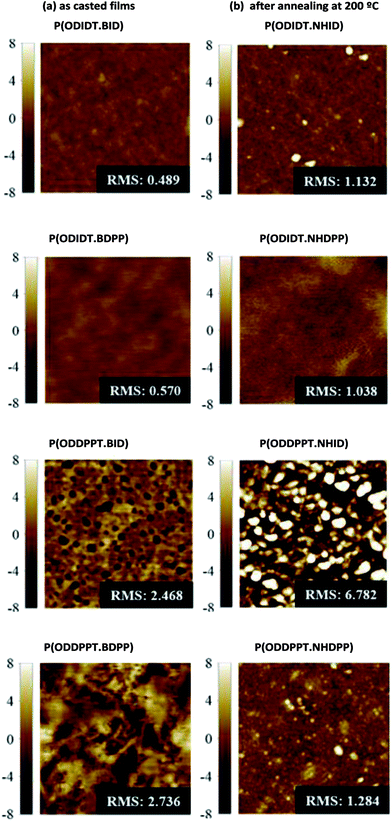 | ||
| Fig. 6 Tapping-mode atomic force microscopy images of synthesized new polymers: (a) as casted films and (b) after annealing treatment at 200 °C. | ||
Conclusions
Narrow bandgap random co-polymeric semiconductors, comprising alkyl substituted diketopyrrolopyrrole (DPP) and isoindigo (ID) and tert-butoxycarbonyl (t-BOC)-protected DPP/ID, were synthesized and used in organic field-effect transistors (OFETs). The polymer films were prepared by solution deposition and thermal annealing of precursors featuring thermally labile t-BOC groups. The effects of the thermal cleavage on the molecular packing structure in the polymer thin films were investigated using thermogravimetric analysis (TGA), UV-vis spectroscopy, and X-ray diffraction (XRD) analysis. By utilizing t-BOC thermolysis at 220 °C, the BOC films were directly converted to deprotected films that can potentially possess a hydrogen-bonded network. Finally, we fabricated OFET devices with these random CPs. The trend of field-effect mobility of the as-spun OFET corresponds to that of the XRD data. However, excessive aggregation of the random CP films during thermal annealing results in the performance degradation in the OFET devices.Acknowledgements
The research was supported by the Basic Science Research Program though the National Research Foundation (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A2A1A01002493); and by the GIST Research Institute (GRI) project through a grant provided by GIST in 2017.Notes and references
- (a) P. Qi, Z. Wang, Z. Liu, S. Yang, Y. Yang, J. Yao, G. Zhang and D. Zhang, Polym. Chem., 2016, 7, 3838 RSC; (b) P. Deng and Q. Zhang, Polym. Chem., 2014, 5, 3298 RSC; (c) J.-R. Pouliot, B. Sun, M. Leduc, A. Najari, Y. Li and M. Leclerc, Polym. Chem., 2015, 6, 278 RSC.
- R. Stalder, J. Mei, K. R. Graham, L. A. Estrada and J. R. Reynolds, Chem. Mater., 2014, 26, 664 CrossRef CAS.
- S. Holliday, J. E. Donaghey and I. McCulloch, Chem. Mater., 2014, 26, 647 CrossRef CAS.
- J.-M. Jiang, M.-C. Yuan, K. Dinakaran, A. Hariharan and K.-H. Wei, J. Mater. Chem. A, 2013, 1, 4415 CAS.
- H.-W. Lin, W.-Y. Lee and W.-C. Chen, J. Mater. Chem., 2012, 22, 2120 RSC.
- Y. N Li, P. Sonar, S. P. Singh, W. J. Zeng and M. S. Soh, J. Mater. Chem., 2011, 21, 10829 RSC.
- J. Fan, J. D. Yuen, M. F. Wang, J. Seifter, J.-H. Seo, A. R. Mohebbi, D. Zakhidov, A. J. Heeger and F. Wudl, Adv. Mater., 2012, 24, 2186 CrossRef CAS PubMed.
- S. C. Lan, P. A. Yang, M. J. Zhu, C. M. Yu, J. M. Jiang and K. H. Wei, Polym. Chem., 2013, 4, 1132 RSC.
- G. Zhang, Y. Fu, Z. Xie and Q. Zhang, Macromolecules, 2011, 44, 1414 CrossRef CAS.
- T. Lei, Y. Cao, Y. Fan, C.-J. Liu, S.-C. Yuan and J. Pei, J. Am. Chem. Soc., 2011, 133, 6099 CrossRef CAS PubMed.
- T. Lei, Y. Cao, X. Zhou, Y. Peng, J. Bian and J. Pei, Chem. Mater., 2012, 24, 1762 CrossRef CAS.
- J. C. Bijleveld, A. P. Zoombelt, S. G. J. Mathijssen, M. M. Wienk, M. Turbiez, D. M. de Leeuw and R. A. J. Janssen, J. Am. Chem. Soc., 2009, 131, 16616 CrossRef CAS PubMed.
- L. Ye, S. Zhang, W. Ma, B. Fan, X. Guo, Y. Huang, H. Ade and J. Hou, Adv. Mater., 2012, 24, 6335 CrossRef CAS PubMed.
- F. Grenier, P. Berrouard, J.-R. Pouliot, H.-R. Tseng, A. J. Heeger and M. Leclerc, Polym. Chem., 2013, 4, 1836 RSC.
- J. W. Jung, F. Liu, T. P. Russell and W. H. Jo, Energy Environ. Sci., 2013, 6, 3301 CAS.
- J. S. Zambounis, Z. Hao and A. Iqbal, Nature, 1997, 388, 131 CrossRef CAS.
- U. Schaedeli, J. S. Zambounis, A. Iqbal, Z. Hao and H. Dubas, EP 0654711, 1993.
- (a) C. Lee, Y. Seo and S. Lee, Macromolecules, 2004, 37, 4070 CrossRef CAS; (b) T. L. Chen, J. J.-A. Chen, L. Catane and B. Ma, Org. Electron., 2011, 12, 1126 CrossRef CAS; (c) H. Yanagisawa, J. Mizuguchi, S. Aramaki and Y. Sakai, Jpn. J. Appl. Phys., 2008, 47, 4728 CrossRef CAS; (d) Y. Suna, J. Nishida, Y. Fujisaki and Y. Yamashita, Chem. Lett., 2011, 40, 822 CrossRef CAS; (e) Y. Suna, J. Nishida, Y. Fujisaki and Y. Yamashita, Org. Lett., 2012, 14, 3356 CrossRef CAS PubMed; (f) L. Yang, Y. Yu, Y. Gong, J. Li, F. Ge, L. Jiang, F. Gao and Y. Dan, Polym. Chem., 2015, 6, 7005 RSC.
- H. Fukumoto, H. Nakajima, T. Kojima and T. Yamamoto, Materials, 2014, 7, 2030 CrossRef CAS.
- (a) Z.-H. Guo, N. Ai, C. R. McBroom, T. Yuan, Y.-H. Lin, M. Roders, C. Zhu, A. L. Ayzner, J. Pei and L. Fang, Polym. Chem., 2016, 7, 648 RSC; (b) C. Liu, S. Dong, P. Cai, P. Liu, S. Liu, J. Chen, F. Liu, L. Ying, T. P. Russell, F. Huang and Y. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 9038–9051 CrossRef CAS PubMed; (c) J. Lee, A. R. Han, J. Hong, J. H. Seo, J. H. Oh and C. Yang, Adv. Funct. Mater., 2012, 22, 4128 CrossRef CAS; (d) E. Wang, W. Mammo and M. R. Andersson, Adv. Mater., 2014, 26, 1801 CrossRef CAS PubMed; (e) L. Dou, J. You, Z. Hong, Z. Xu, G. Li, R. A. Street and Y. Yang, Adv. Mater., 2013, 25, 6642 CrossRef CAS PubMed; (f) L. Fang, Y. Zhou, Y.-X. Yao, Y. Diao, W.-Y. Lee, A. L. Appleton, R. Allen, J. Reinspach, S. C. B. Mannsfeld and Z. Bao, Chem. Mater., 2013, 25, 4874 CrossRef CAS.
- A. A. El-Shehawy, N. I. Abdo, A. A. El-Barbary and J.-S. Lee, Eur. J. Org. Chem., 2011, 25, 4841 Search PubMed.
- N. I. Abdo, A. A. El-Shehawy, A. A. El-Barbary and J.-S. Lee, Eur. J. Org. Chem., 2012, 5540 CrossRef CAS.
- (a) A. A. El-Shehawy, N. I. Abdo, A. A. El-Barbary and J.-S. Lee, Tetrahedron Lett., 2010, 51, 4526 CrossRef CAS; (b) N. I. Abdo, J. Ku, A. A. El-Shehawy, H.-S. Shim, J.-K. Min, A. A. El-Barbary, Y. H. Jang and J.-S. Lee, J. Mater. Chem. A, 2013, 1, 10306 RSC.
- M. Shaker, C. K. Trinh, W. Kim, H. Kim, K. Lee and J.-S. Lee, New J. Chem., 2015, 39, 4957 RSC.
- (a) W. Elsawy, C.-L. Lee, S. Cho, S.-H. Oh, S.-H. Moon, A. Elbarbary and J.-S. Lee, Phys. Chem. Chem. Phys., 2013, 15, 15193 RSC; (b) W. Elsawy, H. Kang, K. Yu, A. Elbarbary, K. Lee and J.-S. Lee, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2926 CrossRef CAS.
- M. Shaker, J.-H. Lee, C. K. Trinh, W. Kim, K. Lee and J.-S. Lee, RSC Adv., 2015, 5, 66005 RSC.
- E. D. Glowacki, G. Voss, K. Demirak, M. Havlicek, N. Sünger, A. C. Okur, U. Monkowius, J. Gasiorowski, L. Leonat and N. S. Sariciftci, Chem. Commun., 2013, 49, 6063 RSC.
- Y. Deng, Y. Chen, X. Zhang, H. Tian, C. Bao, D. Yan, Y. Geng and F. Wang, Macromolecules, 2012, 45, 8621 CrossRef CAS.
- K. Godula and D. Sames, Science, 2006, 312, 67 CrossRef CAS PubMed.
- J. Zaumseil, K. W. Baldwin and J. A. Rogers, J. Appl. Phys., 2003, 93, 6117 CrossRef CAS.
- B. H. Lee, G. C. Bazan and A. J. Heeger, Adv. Mater., 2016, 28, 57 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Thermogravimetry analysis, FT-IR spectra and characterization data (1H and 13C NMR spectra). See DOI: 10.1039/c7ra01726j |
| ‡ Mohamed Shaker contributed equally with Byoungwook Park and Jong-Hoon Lee to this work. The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. |
| This journal is © The Royal Society of Chemistry 2017 |