Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pyridinium 5-aminothiazoles: specific photophysical properties and vapochromism in halogenated solvents†
Kirara
Yamaguchi
 a,
Toshiaki
Murai
a,
Toshiaki
Murai
 *a,
Yuki
Tsuchiya
*a,
Yuki
Tsuchiya
 a,
Yohei
Miwa
a,
Yohei
Miwa
 a,
Shoichi
Kutsumizu
a,
Shoichi
Kutsumizu
 a,
Takahiro
Sasamori
a,
Takahiro
Sasamori
 b and
Norihiro
Tokitoh
b and
Norihiro
Tokitoh
 b
b
aDepartment of Chemistry and Biomolecular Science, Faculty of Engineering, Gifu University, Yanagido, Gifu 501-1193, Japan. E-mail: mtoshi@gifu-u.ac.jp; Fax: +81-58-293-2614; Tel: +81-58-293-2614
bInstitute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan
First published on 24th March 2017
Abstract
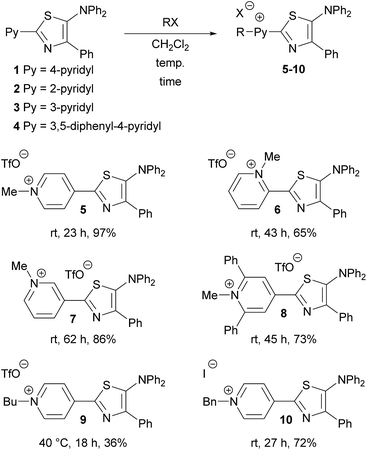
Treatment of pyridyl-5-aminothiazoles (1–4) with alkyl triflates or benzyl iodide afforded the corresponding pyridinium 5-aminothiazoles (5–10), which exhibited bathochromically shifted absorption and fluorescence spectra relative to those of 1–4. Moreover, the vapochromic properties of 5 specific to halogenated solvents were examined by powder X-ray diffraction analysis. Pmma films containing 5 can thus be used to detect halogenated solvents, especially CH2Cl2.
Molecular scaffolds consisting of a combination of electron-donating (D) and -accepting (A) moieties via azole rings are of great interest. In particular, the introduction of electron-donating substituents at the 5-position of the 2-pyridinium azole provides conformationally flexible D–A systems1 that find applications in organic electronics,2 chemosensors,3 biosensors,4 and as potential drug candidates.5 We have recently reported the first thiazoles that contain a strongly electron-donating diarylamino group at the 5-position, and pyridyl groups at the 2-position.6 A further alkylation of the pyridine moiety was expected to enhance the acceptor character to provide molecules showing bathochromically shifted absorption and emission spectra. Herein, we report the synthesis and photophysical properties of pyridinium 5-aminothiazoles. Moreover, their vapochromic properties7,8 specific to halogenated solvents, were examined by powder X-ray diffraction studies.
We initially treated 5-aminothiazoles 1–4 with either methyl or butyl triflate, or benzyl iodide to afford the desired pyridinium salts (5–10) in moderate to high yields (Fig. 1). The alkylation and benzylation occurred selectively at the nitrogen atom of the pyridine ring, even though 1–4 contain three nitrogen atoms. Pyridinium thiazole 8 was also obtained by this method, despite the steric hindrance of the nitrogen atom of the pyridine moiety.
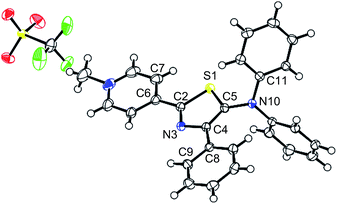
Single crystals of 5 were obtained from a CH2Cl2 solution of 5 upon adding hexane. The molecular structure of 5 was determined by a single-crystal X-ray diffraction analysis (Fig. 2 and Table S1†), which revealed a significantly twisted 5-diphenylamino group similarly to that in 1. The methylation of thiazole 1 induced a decrease of the dihedral angles at the 2- and 4-positions, under a concomitant slight planarization of the thiazole core and the substituents at these positions. Single crystals of 5 were also obtained from a THF solution of 5 upon adding hexane, and the structure obtained from these crystals is virtually identical to that extracted from the crystals from CH2Cl2 (Fig. S1 and Table S2†).
The photophysical properties of 1–10 were measured in solution (CHCl3 and THF) and in the solid state (Fig. 3 and S2, Table S3†). The absorption and fluorescence maxima of 1–4 shifted bathochromically upon conversion into the pyridinium salts (5–10). For example, the absorption maximum of 5 (λabs = 510 nm) in CHCl3 was bathochromically shifted (Δλ5/1 = 116 nm) relative to that of 1 (λabs = 394 nm) (Fig. 3a). The absorption wavelengths of 6 (Δλ6/2 = 96 nm), 7 (Δλ7/3 = 72 nm), and 8 (Δλ8/4 = 113 nm) in CHCl3 were also bathochromically shifted relative to the neutral starting materials. Thus, among the three regioisomers, i.e., the isomers that contain 2-, 3-, or 4-pyridyl substituents at the 2-positions of the thiazoles, the alkylation of the 4-pyridyl group had the most profound impact on the photophysical properties. The pyridinium thiazoles with a butyl group (9; λabs = 510 nm) or a benzyl group (10; λabs = 517 nm) on the pyridyl nitrogen atoms showed similar absorption spectra in CHCl3 to that of 5 with a methyl group. This result indicates that the nature of the substituents on the nitrogen atoms of the pyridinium thiazoles have little impact on the photophysical properties. Additionally, 5, 9, and 10 showed virtually identical fluorescence emissions (λabs = 650 nm) in CHCl3 (Fig. 3b). All alkylated thiazoles (5–10) showed longer-wavelength emissions than those of the corresponding thiazoles (1–4). Although their fluorescence quantum yields (ΦF) were very low in solution, 5–8 showed higher ΦF values in the solid state than in solution (Fig. S3 and Table S3†).
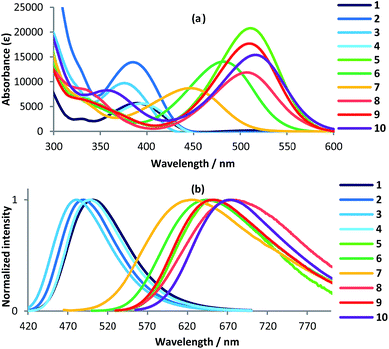 | ||
| Fig. 3 (a) UV-vis absorption spectra of 1–10; (b) emission spectra of 1–10; [1–10] = 1 × 10−5 M in CHCl3. | ||
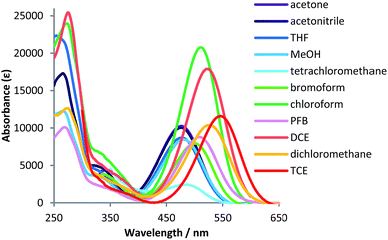
The absorption spectra of 5–10 were also examined in various other solvents, which revealed characteristic differences between halogen-free (acetone, acetonitrile, and methanol) and halogenated solvents [tetrachloromethane, bromoform, pentafluorobutane (PFB), dichloromethane, dichloroethane (DCE), and tetrachloroethane (TCE)] (Table 1).9 The maximum absorption wavelength of 5 in halogen-free solvents (λabs = ∼480 nm) was bathochromically shifted in fluorinated, brominated, and chlorinated solvents.10 In halogen-free solvents, a 1 × 10−5 M solution of 5 is pale yellow (Fig. 4), while it is orange, pink, and purple in fluorinated, brominated, and chlorinated solvents, respectively. These results indicate that the solvent polarity is not the major factor influencing the spectra. For example, the longest absorption maxima of 5 were observed at 510 nm (CHCl3), 526 nm (CH2Cl2), and 547 nm (TCE), although the polarity parameter ET(30)11 of these solvents increases in the order CHCl3 (39.1) < TCE (39.4) < CH2Cl2 (40.7) (Fig. 5). However, the magnitude of the bathochromic shift appeared to be governed by the number of halogen atoms per solvent molecule, e.g., solvents with two halogen atoms shifted the absorption maximum of 5 to longer wavelengths than solvents with one halogen atom. Depending on the solvent, the fluorescence spectra of 5 in halogenated solvents exhibited emissions at λem = 596–723 nm (Fig. S4†). The Stokes shifts of 5 in halogenated solvents were also affected by the number of halogen atoms per solvent carbon atom, i.e., the fluorescence emissions of 5 in CH2Cl2, DCE, and TCE occurred at longer wavelengths than those in CCl4 and CHBr3.
| Solvent | E T(30) | UV-vis | Fluorescence | |||
|---|---|---|---|---|---|---|
| λ abs/nm | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε ε |
λ em a/nm | Φ F b | Stokes shift/cm−1 | ||
| a Excited at the excitation wavelengths. b Absolute fluorescence quantum yield. | ||||||
| Acetone | 42.2 | 476 | 4.01 | — | — | — |
| CH3CN | 45.6 | 476 | 4.00 | — | — | — |
| MeOH | 55.4 | 482 | 3.93 | — | — | — |
| CCl4 | 32.4 | 484 | 3.39 | 596 | 0.07 | 3713 [108 nm] |
| CHBr3 | 37.7 | 501 | 3.90 | 649 | 0.07 | 4552 [148 nm] |
| PFB | — | 510 | 3.94 | — | — | — |
| CH2Cl2 | 40.7 | 526 | 4.02 | 711 | 0.02 | 4947 [185 nm] |
| DCE | 41.3 | 524 | 4.07 | 723 | 0.02 | 5253 [199 nm] |
| TCE | 39.4 | 547 | 4.25 | 712 | 0.03 | 4237 [165 nm] |
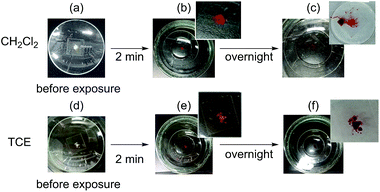
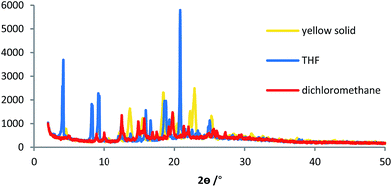
Interestingly, solid 5 exhibited reversible vapochromism toward halogenated solvents (Fig. 6 and Tables S5 and S6†).11 When yellow solid 5 was exposed to CH2Cl2 vapor in a sealed glass case, its color quickly (2 min) changed to red. Opening the glass case and releasing the solvent vapor induced a rapid (∼5 s) retroconversion to the yellow solid. When this red solid was exposed to CH2Cl2 vapor overnight, it changed to a red solution, which suggests that the solvent molecules may be intercalated into 5, and that electrostatic interactions between the positively charged pyridinium moieties and halogen atoms may be present. This phenomenon was also observed for PMMA films prepared from 5 and PMMA pellets (Fig. S6†). After short (2 min) exposure to CH2Cl2 vapor, PMMA films containing 5 clearly turned red, and rapidly retroconverted to yellow upon opening the glass case. Exposure of 5 to TCE vapor also induced a similar color change from a yellow solid to a red solid (2 min), or to a red solution (overnight). However, in contrast to CH2Cl2 after opening the glass case and releasing the TCE vapor, the red solution converted only slowly (>2 min) back to the original yellow solid, while the color change is clearly visible with CH2Cl2. The discrepancy with respect to the rate of retroconversion should most likely be attributed to the difference in boiling point of the solvents. Nevertheless, dropping a CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution (1
H2O solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) on to a PMMA film containing 5 led to a swift color change, i.e., such films can easily detect these solvents. In contrast, 5 did not show vapochromism in the presence of CCl4 and PFB under similar conditions (Table S5†). When THF, MeOH, or DCE were used, the yellow solid of 5 turned to an orange solid overnight. Vapors of CHCl3 and CHBr3 changed the yellow solid of 5 into a red solid.
1, v/v) on to a PMMA film containing 5 led to a swift color change, i.e., such films can easily detect these solvents. In contrast, 5 did not show vapochromism in the presence of CCl4 and PFB under similar conditions (Table S5†). When THF, MeOH, or DCE were used, the yellow solid of 5 turned to an orange solid overnight. Vapors of CHCl3 and CHBr3 changed the yellow solid of 5 into a red solid.
To compare the effects of halogenated and halogen-free solvents, the absorption spectra of solutions of 5 were measured. When 0.1–1.5 mL of CHCl3 were added to a THF solution of 5 ([5] = 1 × 10−5 M, 2.5 mL), the longest absorption maximum shifted to a longer wavelength (Δλabs = 18 nm) (Fig. S7†), while the addition of 0.1–1.5 mL of THF to a solution of 5 in CHCl3 (2.5 mL) shifted the longest absorption maximum to shorter wavelengths (Δλabs = 11 nm) (Fig. S8†). As the quantities of CHCl3 and THF added are almost equivalent, CHCl3 seems to change the photophysical properties more efficiently compared to THF.
Subsequently, we measured powder X-ray diffraction patterns of 5 in order to determine solvent-specific packing structures (Fig. 7, S9, and S10; Tables S7 and S8†). Column chromatography furnished 5 as a yellow solid, which was used after drying under reduced pressure. Samples containing THF and CH2Cl2 were generated by a solution-cast method followed by drying under atmospheric conditions. The thus obtained solid samples showed different patterns in the low-angle region. These results suggest that their packing structures are very different from each other. Therefore, packing structures of solid 5 should vary with the absorbed solvent. Based on the powder X-ray diffraction analysis, 5 crystallizes in the absence of any solvents in the relatively simple P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. S11†), whereas in the presence of THF, 5 includes THF molecules and crystallizes in the C2/c (#15) space group (Fig. S12†). Moreover, the distance between the thiazole molecules shortened in the presence of THF.
space group (Fig. S11†), whereas in the presence of THF, 5 includes THF molecules and crystallizes in the C2/c (#15) space group (Fig. S12†). Moreover, the distance between the thiazole molecules shortened in the presence of THF.
In conclusion, pyridinium 5-aminothiazoles bearing methyl, butyl, or benzyl groups on the pyridyl nitrogen atoms (5–10) were synthesized for the first time. These pyridinium thiazoles showed absorption in the visible region and red fluorescence in solution and in the solid state. In particular, the longest-wavelength absorption maximum of 5–10 exhibited specific bathochromic shifts in halogenated solvents. Moreover, 5 showed a reversible vapochromism upon exposure to vapors of CH2Cl2 and TCE. Powder X-ray diffraction patterns revealed that the packing structures of 5 change depending on the nature of the solvent molecules incorporated into the crystals. Furthermore, the addition of CHCl3 to a THF solution of 5 caused a stronger shift of its absorption maximum than the addition of THF to a CHCl3 solution of 5 under otherwise similar conditions. This phenomenon and the reversible vapochromism observed in PMMA films containing 5 should find applications in the detection of halogenated solvents, especially CH2Cl2.12 Further studies on the applications of pyridinium 5-N-arylaminothiazoles that are responsive towards metals and solvents are currently in progress in our laboratory.
Notes and references
- (a) V. V. Volchkov, G. H. B. Hoa, J. A. Kossanyi, S. P. Gromov, M. V. Alfimov and B. M. Uzhinov, J. Phys. Org. Chem., 2005, 18, 21–25 CrossRef CAS; (b) A. V. V. Volchkov, G. V. Dem'yanov, M. V. Rusalov and T. I. Syreishchikova, Russ. J. Gen. Chem., 2005, 75, 790–794 CrossRef.
- D. Svechkarev, D. Kolodezny, S. Mosquera-Vázquez and E. Vauthey, Langmuir, 2014, 30, 13869–13876 CrossRef CAS PubMed.
- (a) M.-H. Zheng, J.-Y. Jin, W. Suna and C.-H. Yan, New J. Chem., 2006, 30, 1192–1196 RSC; (b) L.-L. Li, H. Sun, C.-J. Fang, J. N. Xu, J.-Y. Jin and C.-H. Yan, J. Mater. Chem., 2007, 17, 4492–4498 RSC; (c) Z.-X. Li, W. Sun, Y.-F. Yue, M.-H. Zheng, C.-H. Xu, J.-Y. Jin, C.-J. Fang and C.-H. Yan, Tetrahedron Lett., 2007, 48, 7675–7679 CrossRef CAS; (d) Z.-X. Li, C.-H. Xu, W. Sun, Y.-C. Bai, C. Zhang, C.-J. Fanga and C.-H. Yan, New J. Chem., 2009, 33, 853–859 RSC; (e) S. Yamaguchi, H. Hosoi, M. Yamashita, P. Sen and T. Tahara, J. Phys. Chem. Lett., 2010, 1, 2662–2665 CrossRef CAS; (f) S. Pajk, Tetrahedron Lett., 2014, 55, 6044–6047 CrossRef CAS; (g) X. Ma, Y. Wang, T. Zhao, Y. Li, L.-C. Su, Z. Wang, G. Huang, B. D. Sumer and J. Gao, J. Am. Chem. Soc., 2014, 136, 11085–11092 CrossRef CAS PubMed.
- (a) Q. Wang, E. I. Zimmerman, A. Toutchkine, T. D. Martin, L. M. Graves and D. S. Lawrence, ACS Chem. Biol., 2010, 5, 887–895 CrossRef CAS PubMed; (b) C. W. Cunningham, A. Mukhopadhyay, G. H. Lushington, B. S. J. Blagg, T. E. Prisinzano and J. P. Krise, Mol. Pharm., 2010, 7, 1301–1310 CrossRef CAS PubMed; (c) B. Dumat, G. Bordeau, E. Faurel-Paul, F. Mahuteau-Betzer, N. Saettel, M. Bombled, G. Metgé, F. Charra, C. Fiorini-Debuisschert and M.-P. Teulade-Fichou, Biochimie, 2011, 93, 1209–1218 CrossRef CAS PubMed; (d) K. Zhou, H. Liu, S. Zhang, X. Huang, Y. Wang, G. Huang, B. D. Sumer and J. Gao, J. Am. Chem. Soc., 2012, 134, 7803–7811 CrossRef CAS PubMed; (e) Q. Wang, M. A. Priestman and D. S. Lawrence, Angew. Chem., Int. Ed., 2013, 52, 2323–2325 CrossRef CAS PubMed; (f) R. Schneider, A. Gohla, J. R. Simard, D. B. Yadav, Z. Fang, W. A. L. van Otterlo and D. Rauh, J. Am. Chem. Soc., 2013, 135, 8400–8408 CrossRef CAS PubMed; (g) B. Dumat, E. Faurel-Paul, P. Fornarelli, N. Saettel, G. Metgé, C. Fiorini-Debuisschert, F. Charra, F. Mahuteau-Betzer and M.-P. Teulade-Fichou, Org. Biomol. Chem., 2016, 14, 358–370 RSC; (h) O. Brun, J. Agramunt, L. Raich, C. Rovira, E. Pedroso and A. Grandas, Org. Lett., 2016, 18, 4836–4839 CrossRef CAS PubMed.
- (a) M. Kitamatsu, T. Yamamoto, M. Futami and M. Sisido, Bioorg. Med. Chem. Lett., 2010, 20, 5976–5978 CrossRef CAS PubMed; (b) Y. Martín-Cantalejo, B. Sáez, M. I. Monterde, M. T. Murillo and M. F. Braña, Eur. J. Med. Chem., 2011, 46, 5662–5667 CrossRef PubMed; (c) M. Manal, K. Arul, A. K. Anjana and K. Remya, Int. J. Curr. Pharm. Res., 2014, 6, 22–26 Search PubMed; (d) K. Yokoo, K. Yamawaki, Y. Yoshida, S. Yonezawa, Y. Yamano, M. Tsuji, T. Hori, R. Nakamura and K. Ishikura, Eur. J. Med. Chem., 2016, 124, 698–712 CrossRef CAS PubMed.
- (a) K. Yamaguchi, T. Murai, S. Hasegawa, Y. Miwa, S. Kutsumizu, T. Maruyama, T. Sasamori and N. Tokitoh, J. Org. Chem., 2015, 80, 10742–10756 CrossRef CAS PubMed; (b) K. Yamaguchi, T. Murai, J.-D. Guo, T. Sasamori and N. Tokitoh, ChemistryOpen, 2016, 5, 434–438 CrossRef CAS PubMed.
- (a) O. S. Wenger, Chem. Rev., 2013, 113, 3686–3733 CrossRef CAS PubMed; (b) C. Reus and T. Baumgartner, Dalton Trans., 2016, 45, 1850–1855 RSC.
- (a) R. Matsushima, N. Nishimura and Y. Kohno, Chem. Lett., 2003, 32, 260–261 CrossRef CAS; (b) R. Matsushima, N. Nishimura, K. Goto and Y. Kohno, Bull. Chem. Soc. Jpn., 2003, 76, 1279–1283 CrossRef CAS; (c) M. Taneda, H. Koyama and T. Kawato, Chem. Lett., 2007, 36, 354–355 CrossRef CAS; (d) E. Takahashi, H. Takaya and T. Naota, Chem.–Eur. J., 2010, 16, 4793–4802 CrossRef CAS PubMed; (e) S. Yamada, A. Katsuki, Y. Nojiri and Y. Tokugawa, CrystEngComm, 2015, 17, 1416–1420 RSC; (f) H. Naito, Y. Morisaki and Y. Chujo, Angew. Chem., Int. Ed., 2015, 54, 5084–5087 CrossRef CAS PubMed; (g) P. S. Hariharan, D. Moon and S. P. Anthony, J. Mater. Chem. C, 2015, 3, 8381–8388 RSC; (h) C. Peebles, C. D. Wight and B. L. Iverson, J. Mater. Chem. C, 2015, 3, 12156–12163 RSC; (i) A. Sakon, A. Sekine and H. Uekusa, Cryst. Growth Des., 2016, 16, 4635–4645 CrossRef CAS.
- (a) A. Laguna, T. Lasanta, J. M. López-de-Luzuriaga, M. Monge, P. Naumov and M. E. Olmos, J. Am. Chem. Soc., 2010, 132, 456–457 CrossRef CAS PubMed; (b) G. Park, H. Yang, T. H. Kim and J. Kim, Inorg. Chem., 2011, 50, 961–968 CrossRef CAS PubMed; (c) H. Imoto, S. Tanaka, T. Kato, T. Yumura, S. Watase, K. Matsukawa and K. Naka, Organometallics, 2016, 35, 3647–3650 CrossRef CAS.
- (a) Y. Ooyama, K. Kushimoto, Y. Oda, D. Tokita, N. Yamaguchi, S. Inoue, T. Nagano, Y. Harima and J. Ohshita, Tetrahedron, 2012, 68, 8577–8580 CrossRef CAS; (b) Y. Ooyama, Y. Oda, T. Mizumo and J. Ohshita, Tetrahedron, 2013, 69, 1755–1760 CrossRef CAS.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, Wiley-VCH, Weinheim, Germany, 1988 Search PubMed.
- The importance of the development of the chemosensors to detect halogenated solvents has been reported elsewhere. For examples, see: (a) A. M. Alhuthali and I.-M. Low, Polym.-Plast. Technol. Eng., 2013, 52, 921–930 CrossRef CAS; (b) T. Permpool, A. Sirivat, D. Aussawasathien and L. Wannatong, Mater. Res., 2013, 16, 1020–1029 CrossRef CAS; (c) L. Dai, D. Wu, Q. Qiao, W. Yin, J. Yin and Z. Xu, Chem. Commun., 2016, 52, 2095–2098 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H- and 13C-NMR spectra, elemental analysis data, X-ray crystallographic data of reaction products. CCDC 1530518 and 1530519. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra01896g |
| This journal is © The Royal Society of Chemistry 2017 |