Open Access Article
Open Access ArticleEvaluation of biodegradability and biotoxicity of surfactants in soil
Guixiang Liab,
Guihong Lan *ab,
Yongqiang Liuc,
Chen Chenab,
Lin Leiab,
Jiao Duab,
Yingchun Luab,
Qiang Lid,
Guoyong Duab and
Jihong Zhange
*ab,
Yongqiang Liuc,
Chen Chenab,
Lin Leiab,
Jiao Duab,
Yingchun Luab,
Qiang Lid,
Guoyong Duab and
Jihong Zhange
aKey Laboratory of Oil & Gas Applied Chemistry of Sichuan Province, Southwest Petroleum University, No. 8 Xindu Avenue, Xindu District, Chengdu 610500, PR China. E-mail: guihonglan416@sina.com; Fax: +86 02883037306; Tel: +86 02883037306
bCollege of Chemistry and Chemical Engineering, Southwest Petroleum University, Chengdu 610500, PR China
cFaculty of Engineering and the Environment, University of Southampton, Southampton SO17 1BJ, UK
dDevelopment and Application of Rural Renewable Energy, Ministry of Agriculture, PR China
eXinjiang Oilfield Company, No. 1 Gas Production Plant, PR China
First published on 15th June 2017
Abstract
In this study, the biodegradability and biotoxicity of four surfactants, i.e. modified heterogeneous alcohol ether with an 8–12 carbon alkylic chain, fatty acid methyl ester ethoxylates, Tween-80 and rhamnolipid (a kind of biosurfactant), under natural soil conditions were investigated. Batch experiments of degradation with an initial concentration of surfactants of 120 mg kg−1 in soil were carried out at room temperature varying from 15 °C to 22 °C. The concentrations of surfactants over time were measured and metabolites of the surfactants were characterized by gas chromatography-mass spectrometry. In addition, the amount of microorganisms in soil over time was measured by using agar plates. The results showed that fatty acid methyl ester ethoxylates had the highest biodegradation rate followed by rhamnolipid, Tween-80 and modified heterogeneous alcohol ether. A biotoxicity assay based on a photobacterium revealed that these surfactants had low toxicity. It is concluded that the fatty acid methyl ester ethoxylates are the most environmentally friendly surfactants among the four surfactants studied.
Introduction
A surfactant is a kind of substance containing hydrophobic and hydrophilic groups, which is capable of decreasing the surface tension between water and air. Owing to their unique characteristic, surfactants have been extensively applied in our daily lives, such as in washing detergents and by the food industry.1In addition, a great amount of surfactants was used in the oil industry, such as SDS, Brij 35, and NEPOn,2–4 to wash crude oil-contaminated soil to remove the most of the oil or organic contaminants. Nevertheless, the most common use of surfactants was bioremediation in several environmental fields. The use of surfactants as enhancers for the removal of heavy metals5,6 and organic contaminations, such as ethylbenzene, DDT and polycyclic aromatic hydrocarbons,6–8 has been widely reported.
The worldwide production of surfactants reached 1.78 million tons in 2015, with an annual increasing rate increase of 6% predicted.
Because of the extensive production and consumption of these compounds, some parts of them can get into the environment via the discharge of wastewater after their primary use in aqueous solutions. The compounds which cannot be easily degraded in the environment would accumulate in sediments and soil and leak into groundwater and wastewater.9 As reported, after application these chemicals are disposed of down the drain to sewers, where it is estimated that 50% by volume is degraded, with 25% absorbed as suspended solids and 25% dissolved.10 Different surfactants vary in their behavior and fate in the environment.
To overcome the pollution problem caused by surfactants, a broad range of chemical and biological methods have been applied to mitigate their effects.11,12 The most common chemical treatment of a surfactant is adsorption by means of activated carbon and advanced oxidation such as the H2O2/UV-C process and photo-Fenton processes which have been applied in degradation treatments of surfactants in higher concentration.13,14 However, for lower concentrations, chemical treatments not only operate poorly with high cost but also cause secondary pollutants, such as Fe2+ in the photo-Fenton oxidation treatment process. Therefore, the biodegradation treatment of surfactant-contaminated soil was considered to be a promising and cost-effective method for degrading surfactants when environmental conditions were optimal for performing biodegradation reactions. In addition, surfactants as a species of organic compounds can be used as a carbon source for the growth of various kinds of bacteria. Linear alkylbenzene sulphonic acid can be easily eliminated in natural conditions, with a half-life time range from 7 to 33 days, reported by John Jensen in 1999.15 It has also been demonstrated that microorganisms can consume cationic surfactant quaternary ammonium compounds under aerobic conditions. However, the degradation process was complex and depended on the structure of the carbon chain.16 The non-ionic surfactant branched chain nonyl phenol ethoxylate can be degraded by an ozone-induced biodegradability process.17 Considering the contamination caused by surfactants and the biodegradability potential of some types of surfactants, it is better to evaluate the degradability and toxicity of surfactants before they are put into applications to minimize their negative effects on the environment. In this study, we investigated one type of biosurfactant represented by rhamnolipid and three widely used synthetic nonionic surfactants. The synthetic surfactants are modified heterogeneous alcohol ether (MHAE) with the structure of a fatty alcohol containing several oxyethyl groups, fatty alcohol methyl esters of ethoxylate (FMEE) consisting of a fatty acid methyl ester, and Tween-80 whose main component is the sorbitol ester. All of them have a surface tension value, such as 29.5 mN m−1, 33.6 mN m−1 and 37.8 mN m−1, respectively, at each of their critical micelle concentrations (CMC), which is relatively lower than most other types of surfactants (Table 1). Thus, they are widely used in different areas. However, the biodegradation and toxicity information about these surfactants is lacking, which results in a surfactant selection based only on their function rather than on both function and environmental and environmental protection. It is well known that the discharge of surfactant contaminated water may cause serious damage to the water body and soil environment around us. An excess of surfactant can change the microbial community of the surroundings, and its toxicity may affect the growth of plants.
| Surfactant | CMC (mg L−1) | Surface tension (mN m−1) |
|---|---|---|
| MHAE | 14 | 29.5 |
| FMEE | 80 | 33.6 |
| Rhamnolipid | 15 | 31.5 |
| Tween-80 | 14 | 37.8 |
This research aimed to evaluate the potential bioconversion process and the biodegradation mechanism of three synthetic nonionic surfactants and rhamnolipid in soil. The environmental effect that each surfactant may have on its surroundings has also been determined to predict its safety and to avoid the potential risk that surfactants may bring about in the future.
Materials and methods
Chemicals preparation
All of the reagents used in this work were analytical grade unless specially mentioned. The modified heterogeneous alcohol ether (MHAE), fatty alcohol methyl esters of ethoxylate (FMEE) and Tween-80 with 98% purity were supplied by Kelong (Chengdu. China). The rhamnolipid used in this research was produced by Pseudomonas SWP-4.18Table 1 lists the critical micelle concentration (CMC) and the surface tension at CMC of each surfactant. And deionized water (>18.25 Mcm) was used to make the solutions. Stock solutions of each of the studied compounds at a concentration of 1000 mg L−1 were prepared in deionized water and stored at 4 °C.
Working solutions were diluted with the stock solutions in deionized water according to different concentration levels.
Characterization of soil samples
Soil samples were collected from agricultural land at latitude 30.84° north, and longitude 104.19° east in China, in which all the surroundings were without any pollution sources. Before the experiment, soil was air dried and sieved through a 2 mm screening mesh. The collected air-dried soil was put in a refrigerator at a temperature of −4 °C until analysis. Prior to the application of the soil, its properties, including pH, total potassium, total phosphorus, total organic carbon content (TOC) and total nitrogen (TN), were investigated and the results are shown in Table 2.| Parameter | Value |
|---|---|
| pH | 6.89 |
| Coars sand (%) | 39.1 |
| This sand (%) | 4.0 |
| Silt (%) | 5.3 |
| Clay (%) | 51.5 |
| Total phosphorus (% P2O5) | 0.14 |
| Total organic carbon (%) | 1.15 |
| Nitrogen (% TKN) | 0.17 |
| Total potassium (% K2O) | 0.43 |
Biodegradation test of surfactants in soil
Batch experiments were carried out in 3 L pots with 2.5 kg of soil and 120 mL of surfactant solution with a concentration of 1000 mg L−1, resulting in 120 mg of surfactant per kilogram of dry soil. All of these soil samples dosed with surfactants were homogenized by hand before and after being used to fill pots to ensure a homogeneous distribution of surfactants in the soil. We firstly investigated the degradation rates of four surfactants in the collected soil. Each surfactant solution was added into soil in duplicate. The other factors were adjusted as follows: the room temperature in this period varied from 15 °C to 22 °C. In order to archive a stable condition, deionized water was needed to maintain the moisture content at around 30%. The biodegradation experiments were carried out for a period of 75 days. Samples were collected periodically for analysis.Monitoring microorganism amount during the surfactant biodegradation period
To investigate the influence of surfactants on microorganisms in the soil, the number of microorganisms was counted based on the method reported by Jackson.19 2.5 gram of soil sample was placed into a centrifuge tube. Sterilized physiological saline (20 mL) was added into the centrifuge tube and the tube was shaken in a shaker for 10 min at a speed of 200 rpm. The water extract was serially diluted and then 100 μL of the higher dilutions (10−2 to 10−5) were plated on nutrient agar which was made up of peptone, beef extract, deionized water, sodium chloride and agar. After incubation over 48 hours at 35 °C, independent colonies which had emerged in the cultures were counted.Analysis of surfactants and metabolites
All four surfactants were extracted from soil samples by sonication-assisted extraction, as described in a previously reported method.20 The concentrations of nonionic-surfactants were measured by the KI–I2 spectrophotometric method because of the color reaction of nonionic surfactant and KI–I2.21 The concentration of biosurfanctant rhamnolipid was determined by the anthrone–sulfuric acid colorimetric method which based on the color reaction between glycolipid and anthrone–sulfuric acid.22,23 The metabolites in soil were detected by gas chromatography-mass spectrometry (GC-MS), and solid-phase extraction (SPE) of surfactants was adopted for the concentration and purification of samples for GC-MS analysis, according to the method described by Castillo et al.24 The silylation derivation process was carried out by adding a mixture of ethyl acetate and BSTFA/TMCS (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and then heating at 60 °C for 30 minutes before the GC-MS procedure.25 Once the derivation process was complete, 1 μL of the reaction mixture was injected into an Agilent gas chromatography-mass spectrometer (GC-MS) (7890A/5975C, USA) without splitting. The study of all these extracts was conducted by the same temperature programming: the initial temperature was 120 °C; then it was raised at a rate of 15 °C min−1 to 230 °C; a second ramp was then applied at 30 °C min−1 to 260 °C, at which it was maintained for 8 minutes.
1, v/v) and then heating at 60 °C for 30 minutes before the GC-MS procedure.25 Once the derivation process was complete, 1 μL of the reaction mixture was injected into an Agilent gas chromatography-mass spectrometer (GC-MS) (7890A/5975C, USA) without splitting. The study of all these extracts was conducted by the same temperature programming: the initial temperature was 120 °C; then it was raised at a rate of 15 °C min−1 to 230 °C; a second ramp was then applied at 30 °C min−1 to 260 °C, at which it was maintained for 8 minutes.
Acute toxicity assay
The acute toxicity of all the surfactants was assayed by a photobacterium, using the method reported by Chongjian Tang.11 1000 mg L−1 of stored solutions of surfactants were diluted 5 to 20 times. At the same time, a preparation for the determination of the toxicity of soil contaminated with surfactant was obtained by dissolving it into deionized water at the rate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). Samples were analyzed according to the method of Jiao.12 And 10 μL of stored Photobacterium phosphereum solution were added to each of the treated samples, and the luminous power of these samples was measured by a DXY-2 instrument (DXY-2, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China). The relative luminosity (X) was calculated according to eqn (1):
1 (w/w). Samples were analyzed according to the method of Jiao.12 And 10 μL of stored Photobacterium phosphereum solution were added to each of the treated samples, and the luminous power of these samples was measured by a DXY-2 instrument (DXY-2, Institute of Soil Science, Chinese Academy of Sciences, Nanjing, China). The relative luminosity (X) was calculated according to eqn (1):
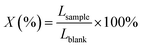 | (1) |
Results and discussion
Characterization of the soil
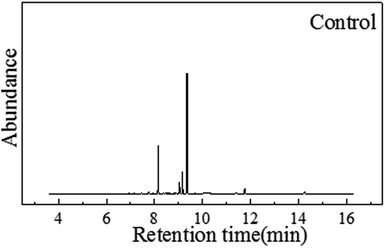
From Table 2, it can be inferred that the soil was a loamy soil type, with a clay content of 51.5%, coarse sand: 39.1%, thin sand: 4.0%, silt: 5.3% and organic carbonate: 1.15%. The soil itself was enriched with organic compounds, which were mainly hexadecanoic acid, butyric acid, 4-methoxy, octadecanoic acid, hexadecanoic acid and propyl ester, as listed in Table 3. The GC-MS profiles are shown in Fig. 1.| Sample number | Compounds |
|---|---|
| 1 | Hexadecanoic acid, trimethylsilyl ester |
| 2 | Butyric acid, 4-methoxy-, trimethylsilyl ester |
| 3 | Octadecanoic acid, trimethylsilyl ester |
| 4 | 1,8-Octanediylbis(trimethylsilane) |
| 5 | Hexadecanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester |
| 6 | Octadecanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester |
Bacteria growth in surfactant contaminated soils
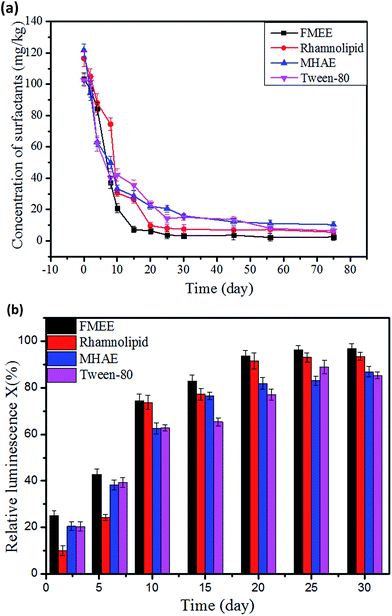
Added surfactants could be an additional source of carbon for bacteria in the soils. The growth characteristics of aerobic bacteria in soil contaminated by different surfactants were investigated. As shown in Fig. 2, the growth profiles with surfactants and the control sample showed a similar pattern. Firstly, all of them underwent a latent phase for around 10 days, and the amount of bacteria was not statistically significant in the first 10 days. Subsequently, after two weeks' incubation, it can be observed that the highest CFU value found in the soil with added FMEE (5.6 in 108 CFU g−1) on the 15th day and the time point for the highest CFU value in the soil was also earlier than for the others. Under the same surfactant concentration, bacteria can gain a relatively quicker growth in the FMEE environment, attaining maximum growth for 30 days and then declining. After almost the 35th day, the bacteria count rose a little all of a sudden. This may explained by secondary growth, because the bacteria use other substances as nutrients. The CFU values in soil with added rhamnolipid, MHAE and Tween-80 have the same tendency but at a much lower level.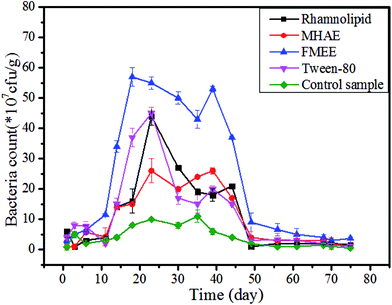 | ||
| Fig. 2 Bacterial growth profile in soil contaminated by different surfactants during the degradation period, “control” means the soil without a surfactant. | ||
Surfactant degradation in the soils
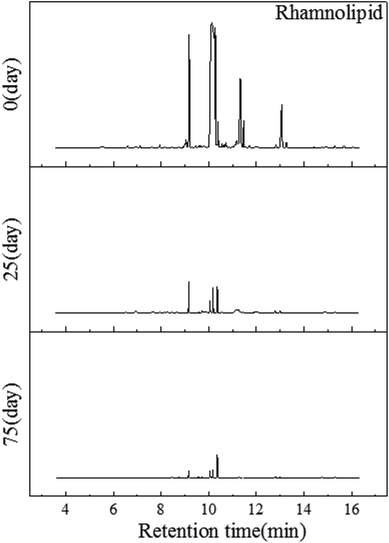
It can be seen from Fig. 3(a) that, after surfactants had been applied onto the soil, the initial concentration of FMEE, MHAE, rhamnolipid and Tween-80 in the soils were 103.29 mg kg−1, 110.5 mg kg−1, 116.45 mg kg−1 and 102.55 mg kg−1, respectively. At the beginning of the experiment, the concentration of MHAE was lower than the added concentration, namely, 120 mg kg−1. This indicated that MHAE was adsorbed into the soil more easily than the others. Finally, the concentrations of these surfactants decreased to 2.5 mg kg−1, 10.35 mg kg−1, 6.34 mg kg−1 and 6.37 mg kg−1, respectively, after 75 days of the degradation process. The rate for FMEE is 6.4 mg (kg day)−1 faster than for the others in the first 15 days, which may because of the increase in the number of microorganisms and greater microbial population in this period. Rhamnoplid reached the same relative degradation rate 25 days later. Thirty days later, the decrease in degradation rate may be because of the degradation-resistant group in the surfactants and also a decrease in the number of microorganisms.Metabolites in surfactants biodegradation process
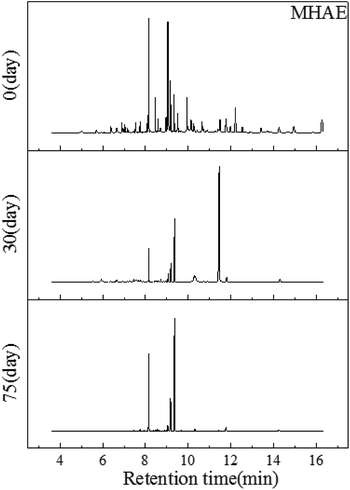 | ||
| Fig. 4 GC-MS profiles of the MHAE obtained from soil on the first day, the 30th day and the 75th day, respectively. | ||
| Number | Compounds |
|---|---|
| 1 | Dibutyl phthalate |
| 2 | 2-Butoxyethoxy acid, trimethylsilane |
| 3 | Hexadecanoic acid, trimethylsilyl ester |
| 4 | Octadecanoic acid, trimethylsilyl ester |
| 5 | Octadecanoic acid, trimethylsilyl ester |
| 6 | C8 alcohol ether |
| 7 | C8 alcohol ether |
| 8 | Bicyclo[3.2.0]hepta-3,6-diene-1-carbonitrile |
| 9 | Eicosenoic acid, trimethylsilyl ester |
| 10 | C8 alcohol ether |
| 11 | C12 alcohol ether |
| 12 | Pyrrole |
| 13 | Docosenoic acid, trimethylsilyl ester |
| 14 | C8 alcohol ether |
| 15 | Cycloheptatriene carbonitrile |
| 17 | C4 alcohol ether |
| 18 | Octadecanoic acid, trimethylsilyl ester |
| 19 | C8 alcohol ether |
With the degradation proceeding, the C12 alcohol ether can be consumed in the later process. 75 days later, compared with Fig. 1 there were almost no telling changes between the control sample and the 75th day's sample. And this may be explained by the fact that MHAE had been degraded completely. As Carolina C Ang reported, MHAE with different amounts of carbon can be almost totally used up by bacteria in the soil.27 This means that the MHAE can be mineralized in soil by microorganisms.
Fig. 5 shows the GC-MS characteristics of the extractions from soil contaminated FMEE after different degradation times. The components detected after FMEE was added into the soil are methyl ester with 16 and 18 carbon chains and aliphatic acids (Table 5). While after 15 days of degradation, most of the methyl ester cannot be detected except for C16 methyl ester. The by-products remaining in the soil were not only parts of the hexadecanoic acid and octadecanoic acid, but some alkanes were generated, such as heneicosane, tetracosane, octacosane and hexacosene. This may because of the consumption of the terminal methyl ester group and microorganism metabolism. Finally, only some fatty acids were left, such as octadecanoic acid and hexadecanoic acid, which inhabited the soil.
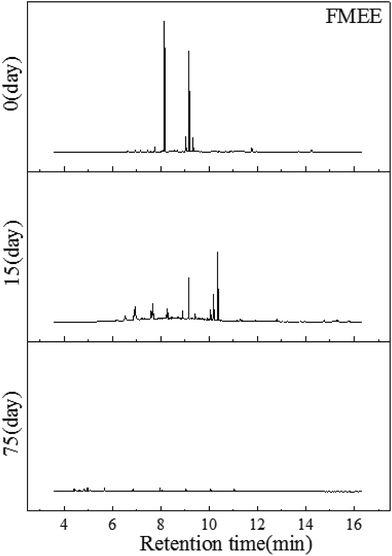 | ||
| Fig. 5 GC-MS profiles of the FMEE extracted from soil on the first day, the 15th day and the 75th day, respectively. | ||
| Number | Compounds |
|---|---|
| 1 | Tetradecanoic acid, trimethylsilyl ester |
| 2 | C16 methyl ester |
| 3 | C16 methyl ester |
| 4 | C18 methyl ester |
| 5 | trans-13-Octadecanoic acid, trimethylsilyl ester |
| 6 | Octadecanoic acid, trimethylsilyl ester |
| 7 | 1-Pentamethyldisilyloxyoctadecane |
At the end of this experiment, only a little of the hexadecanoic acid and octadecanoic acid were found and there was no methyl ester in the soil. Matthew J. Scott had produced a review that explained that the degradation of fatty alcohol ethoxylates begins with the cleavage of the bond between the hydrophobe and hydrophile.26 We can infer from Table 4 that the FMEEs with different carbon chains can be largely consumed.
The methyl group at the end of the FMEE molecule may delay the degradation process.28 This result demonstrated that FMEE can be utilized by bacteria in soil. Furthermore, the ester group in the compound is not stable at all, and it can be mineralized at last.29
| Number | Compounds |
|---|---|
| 1 | Hexadecanoic acid, trimethylsilyl ester |
| 2 | Octadecanoic acid, trimethylsilyl ester |
| 3 | Octadecanoic acid, trimethylsilyl ester |
| 4 | Propanedioic acid, bis(trimethylsilyl) ester |
| 5 | Eicosenoic acid, trimethylsilyl ester |
| 6 | Eicosenoic acid, trimethylsilyl ester |
| 7 | Hexadecanoic acid, 2,3-bis[(trimethylsilyl)oxy]propyl ester |
| 8 | Docosenoic acid, trimethylsilyl ester |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). So the structure of Tween-80 was changed by this reaction and the detected composition were products from derivatization. The octadecenoic and hexadecanoic acid propyl ester had the same aliphatic chain structure compared with Tween-80. It can be inferred that these compounds may be the derived products. It has been reported that octadecenoic acid can be degraded by bacteria which have been isolated from soil.32 As Fig. 7 shows, after the first 25 days, most of the aliphatic acid ester and alcohol had decreased, which means that a large proportion of Tween-80 was reduced during this period. But some aliphatic acids, such as hexadecanoic acid and octadecenoic acid, still remained. During the degradation period, (Z)-9-tricosene was produced in the soil. This can be explained by the fact that the polyoxyethylene sorbitan was consumed firstly in the Tween-80 structure. At the same, eicosanoic acid and oleic acid can be formed because of the bacterial metabolism.
1, v/v). So the structure of Tween-80 was changed by this reaction and the detected composition were products from derivatization. The octadecenoic and hexadecanoic acid propyl ester had the same aliphatic chain structure compared with Tween-80. It can be inferred that these compounds may be the derived products. It has been reported that octadecenoic acid can be degraded by bacteria which have been isolated from soil.32 As Fig. 7 shows, after the first 25 days, most of the aliphatic acid ester and alcohol had decreased, which means that a large proportion of Tween-80 was reduced during this period. But some aliphatic acids, such as hexadecanoic acid and octadecenoic acid, still remained. During the degradation period, (Z)-9-tricosene was produced in the soil. This can be explained by the fact that the polyoxyethylene sorbitan was consumed firstly in the Tween-80 structure. At the same, eicosanoic acid and oleic acid can be formed because of the bacterial metabolism.
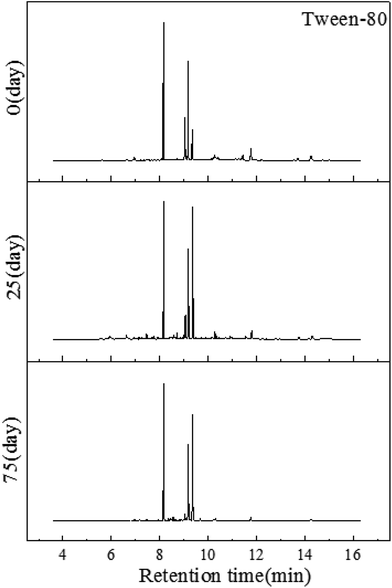 | ||
| Fig. 7 GC-MS profiles of the Tween-80 extracted on the first day, the 25th day and the 75th day, respectively. | ||
| Number | Compounds |
|---|---|
| 1 | Hexadecanoic acid, trimethylsilyl ester |
| 2 | Octadecanoic acid, trimethylsilyl ester |
| 3 | Octadecanoic acid, trimethylsilyl ester |
| 4 | Eicosenoic acid, trimethylsilyl ester |
| 5 | Eicosenoic acid, trimethylsilyl ester |
| 6 | 2-Monopalmitin trimethylsilyl ester |
| 7 | Hexadecanoic acid, propyl ester |
However, after the degradation process, the monostearin in the soil did not show any dramatic decrease. And fatty acids and fatty acid methyl esters can be detected in the soil after 75 days. This demonstrated that the degradation of hexadecanoic acid and octadecenoic acid were much slower. The substances remaining in the soil after 75 days were similar to those of the control, from which we can infer that the degradation process of Tween-80 does not do any harm to the soil environment. This is coincident with the research carried by Lee in 2013.33
Toxicity of surfactants
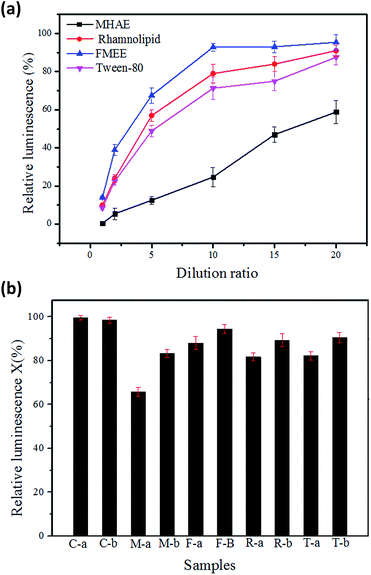
Surfactants are organic polymers which are more or less toxic to microorganisms. Their toxicity depends on their structures, as Rebello reported.34 The biotoxicity of the surfactants was assayed by using luminescent bacteria. Fig. 8(a) shows the acute toxicity of the surfactants at different dilution ratios. The surfactants stock solution had a relative luminescence lower than 20%, and the acute toxicity of the surfactants was observed when the concentration reached 50 mg L−1. The relative luminosities of MHAE, FMEE, Tween-80 and rhamnolipid were 50.8%, 80.6%, 70.2% and 78.6%, respectively, when 50 mg L−1 surfactants solutions were applied in the determination. Compared with other surfactants, FMEE had the lowest toxicity, followed by rhamnolipid, Tween-80 and MHAE. This toxicity result well explained why FMEE has the shortest lay phase and the highest degradation rate, as shown in Fig. 2 and 3. Fig. 8(b) shows the acute toxicity of soil suspensions containing different surfactants at the beginning and the end of the experiment. It can be seen that the control groups are not toxic to luminescent bacteria. However, the addition of surfactants into the soil decreased the shining of the luminescent bacteria to some extent, but when the degradation was complete, the relative luminescence of the samples was raised. This means that the acute toxicity declined as the degradation proceeded. On the other hand, the addition of MHAE decreased the relative luminescence dramatically, whereas it increased with the degradation process. We can infer that the biodegradation process of MHAE may produce somewhat less harm to microorganisms or the soil, but the higher residual MHAE inhibits the luminescence more.Conclusions
In this work, the biodegradation processes of MHAE, FMEE, Tween-80 and rhamnolipid in a soil environment were investigated. The biodegradation process in soil lasted for 75 days under natural conditions varying from 15 °C to 22 °C in the autumn in southwest of China. Finally, all four surfactants degraded to different extents. FMEE showed the most effective process, and was degraded to 2.4% of the initial concentration. And rhamnolipid, Tween-80 and MHAE followed in that order. Furthermore, most of the reduction of surfactant was due to mineralization according to the GC-MS characterization of each surfactant. Meanwhile, with the increase in the number of bacteria, most of the surfactants were degraded. The biodegradation processes depended on the consumption of surfactant by bacteria. For all of the surfactants studied can be used as carbon sources for the growth of microorganisms. Finally, all the results indicated that FMEE had the fastest rate of degradation among all the surfactants in soil. For this reason, FMEE is superior to the other three surfactants. Less harm will occur because of its lower toxicity to the environment and the lower possibility of it being scoured into water by rain. Through this investigation, it can be inferred that the biosurfactant rhamnolipid is not superior in terms of acute toxicity or in its ability to biodegrade. Factors that affect the utilization of surfactants should not only take the ability to biodegrade into consideration because it is the same or less compared with synthetic surfactants.Acknowledgements
This work was financially supported by the Open Projects of Key Laboratory of Oil & Gas Applied Chemistry of Sichuan Province, Southwest Petroleum University, PR China (YQKF201406), Open Projects of Key Laboratory of Development and Application of Rural Renewable Energy, Ministry of Agriculture, PR China. China National Petroleum Corporation Key S & T Special Projects of the development of large oil-gas fields and coalbed methane (Grant No. 2016ZX05040-003) and Xinjiang Oilfield Company No. 1 Gas Production Plant.References
- N. S. Neta, J. A. Teixeira and L. R. Rodrigues, Crit. Rev. Food Sci. Nutr., 2015, 55, 595–610 CrossRef CAS PubMed.
- K. C. Taylor and H. A. Nasr-EL-Din, Colloids Surf., A, 1996, 108(1), 49–72 CrossRef CAS.
- K. Urum, S. Grigson, T. Pekdemir and S. McMenamy, Chemosphere, 2006, 62, 1403–1410 CrossRef CAS PubMed.
- T. M. Bo Gejlsbjerg and T. T. Andersen, Chemosphere, 2003, 50, 321–331 CrossRef.
- W. Zhang, D. C. Tsang and I. M. Lo, Chemosphere, 2007, 66, 2025–2034 CrossRef CAS PubMed.
- M. T. Ammami, F. Portet-Koltalo, A. Benamar, C. Duclairoir-Poc, H. Wang and F. Le Derf, Chemosphere, 2015, 125, 1–8 CrossRef CAS PubMed.
- P. Guo, W. Chen, Y. Li, T. Chen, L. Li and G. Wang, Environ. Sci. Pollut. Res. Int., 2014, 21, 1370–1379 CrossRef CAS PubMed.
- C. Yuan and C. H. Weng, Chemosphere, 2004, 57, 225–232 CrossRef CAS PubMed.
- M. Lechuga, M. Fernandez-Serrano, E. Jurado, J. Nunez-Olea and F. Rios, Ecotoxicol. Environ. Saf., 2016, 125, 1–8 CrossRef CAS PubMed.
- B. L. Tenside, J. Surfactants Deterg., 1989, 26, 101–107 Search PubMed.
- C. J. Tang, P. Zheng, T. T. Chen, J. Q. Zhang, Q. Mahmood, S. Ding, X. G. Chen, J. W. Chen and D. T. Wu, Water Res., 2011, 45, 201–210 CrossRef CAS PubMed.
- S. Jiao, S. Zheng, D. Yin, L. Wang and L. Chen, Chemosphere, 2008, 73, 377–382 CrossRef CAS PubMed.
- A. Karci, I. Arslan-Alaton and M. Bekbolet, J. Hazard. Mater., 2013, 263(2), 275–282 CrossRef CAS PubMed.
- M. I. Bautista-Toledo, J. Rivera-Utrilla, J. D. Mendez-Diaz, M. Sanchez-Polo and F. Carrasco-Marin, J. Colloid Interface Sci., 2014, 418, 113–119 CrossRef CAS PubMed.
- J. Jensen, Sci. Total Environ., 1999, 226, 93–111 CrossRef CAS PubMed.
- B. Brycki, M. Waligorska and A. Szulc, J. Hazard. Mater., 2014, 280, 797–815 CrossRef CAS PubMed.
- M. D. Coello, C. A. Aragon and R. Rodriguez-Barroso, et al., Environ. Technol., 2009, 30, 1391–1396 CrossRef CAS PubMed.
- G. Lan, Q. Fan, Y. Liu, C. Chen, G. Li, Y. Liu and X. Yin, Biochem. Eng. J., 2015, 101, 44–54 CrossRef CAS.
- R. W. Jackson, K. Osborne, G. Barnes, C. Jolliff, D. Zamani, B. Roll, A. Stillings, D. Herzog, S. Cannon and S. Loveland, Appl. Environ. Microbiol., 2000, 66, 453–454 CrossRef CAS PubMed.
- M. M. Gonzalez, J. Martin, D. Camacho-Munoz, J. L. Santos, I. Aparicio and E. Alonso, Waste Manage., 2012, 32, 1324–1331 CrossRef CAS PubMed.
- K. A. G. Michel Dubois, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 250–256 CrossRef.
- D. G. Brown and P. R. Jaffeä, Environ. Sci. Technol., 2001, 35, 2022–2025 CrossRef CAS PubMed.
- A. Leyva, A. Quintana, M. Sanchez, E. N. Rodriguez, J. Cremata and J. C. Sanchez, Biologicals, 2008, 36, 134–141 CrossRef CAS PubMed.
- M. Castillo, E. Martínez, D. Barceló, A. Ginebreda and L. Tirapu, Analyst, 2000, 125, 1733–1739 RSC.
- Y. Yang, H. Li, J. Zhang, N. Sun and H. Sun, Food Anal. Methods, 2013, 7, 798–805 CrossRef.
- M. N. J. Matthew and J. Scott, Biochim. Biophys. Acta, 2000, 1508, 235–251 Search PubMed.
- C. C. Ang and A. S. Abdul, J. Hydrol., 1992, 138, 191–209 CrossRef CAS.
- H. S. I. Hama, T. Tamura, T. Nakamura and K. Miura, J. Surfactants Deterg., 1998, 1, 93–97 CrossRef.
- K. H. Kim and W. H. Jo, Macromol. Res., 2008, 16, 749–752 CrossRef CAS.
- J. Wen, S. P. Stacey, M. J. McLaughlin and J. K. Kirby, Soil Biol. Biochem., 2009, 41, 2214–2221 CrossRef CAS.
- G. Zeng, H. Fu, H. Zhong, X. Yuan, M. Fu, W. Wang and G. Huang, Biodegradation, 2007, 18, 303–310 CrossRef CAS PubMed.
- N. Frank, A. Lissner, M. Winkelmann, R. Huttl, F. O. Mertens, S. R. Kaschabek and M. Schlomann, Biodegradation, 2010, 21, 179–191 CrossRef CAS PubMed.
- S. Lee, J. H. Kweon and H. S. Kim, Int. Biodeterior. Biodegrad., 2013, 85, 652–660 CrossRef CAS.
- S. Rebello, A. K. Asok, S. Mundayoor and M. S. Jisha, Environ. Chem. Lett., 2014, 12, 275–287 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |