 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Potassium fulvate-functionalized graft copolymer of polyacrylic acid from cellulose as a promising selective chelating sorbent
Hisham A. Essawy *b,
Magdy F. Mohameda,
Nabila S. Ammarc and
Hanan S. Ibrahimc
*b,
Magdy F. Mohameda,
Nabila S. Ammarc and
Hanan S. Ibrahimc
aDepartment of Chemistry, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
bDepartment of Polymers and Pigments, National Research Centre, Dokki 12622, Cairo, Egypt. E-mail: hishamessawy@yahoo.com
cDepartment of Water Pollution, National Research Centre, Dokki 12622, Cairo, Egypt
First published on 6th April 2017
Abstract
Further to our previous study on the grafting polymerization of acrylic acid (AA) from cellulose (Cell) that was accomplished in presence of potassium fulvate (KF) and methylenebisacrylamide (MBA), we report that the produced bio-based graft copolymer show improved chelating activity and promising selectivity. Preliminary investigations revealed that Cu(II) uptake was much higher than that of Co(II) and Ni(II) during competitive removal from their mixture. Selective removal of Cu(II) could proceed very efficiently from the mixture during the first 5 minutes of contact with the chelating graft at pH 5.5, which is attributed to the deprotonation of the –COOH groups above their pKa. Kinetic modeling studies revealed that the second-order model was best satisfactorily approximated, which signifies the domination of the chemisorption on the uptake process. The intraparticle diffusion model confirmed this conclusion and further proved that the uptake process was mainly governed by boundary layer effect as a result of electrostatic attraction while the diffusion-controlled step was insignificant. A thermodynamic study on the competitive adsorption revealed the exothermic nature of the process with adverse effect on the adsorption at elevated temperatures with respect to Ni(II) and Co(II) but to a much lesser extent in case of Cu(II), which signifies its greater affinity.
1. Introduction
Removal of heavy metal ions from water is necessary to eliminate the environmental difficulties resulting from the survival in water. Accumulation of these elements in living organisms, even in low concentrations, causes serious problems to health. This imposes an extreme need for finding proficient techniques to get rid of these pollutants.1–3The complexity of removing heavy metal ions effectively from water has made it necessary for the integration of efforts to develop efficient sorbents.4–8 Efficient sorbents are the ones that reveal outstanding removal capacity and high selectivity to specific metal ions.9,10 The removal efficiency and/or capacity is determined by the availability of suitable function groups in the sorbent.11 Polymeric sorbents are chelating materials exhibiting the ability to construct bonds with heavy metal ions through coordination.12 The bonding between them involves electrons donation by Lewis basic atoms such as N, O, P, S, from the sorbent to the metal ions as Lewis acids.13 In general, the accessibility to these donating centers specifies to a great extent the selectivity of a sorbent to specific metal ions.
Polyacrylic acid (AA) is a frequently employed conventional sorbent among many others proposed in the near past years.14,15 More functionalization of polymeric microspheres or graft copolymers was reported to enlarge the efficiency of removal of heavy metal ions.16,17 In a recent report, we carried out grafting polymerization of acrylic acid from cellulose and used the potassium salt of fulvic acid as multifunctional interpenetrating agent.18
Potassium fulvate provides higher number of oxygen containing groups which is expected to widen the chemical activity of the resulting network structure.19 The acquired interpenetration and multi-functionality leads consequently to greater hydrophilicity, which is thought to improve the chelation and selectivity. This eases also the mass transfer and as a result the removal process can terminate in a short time. Further, the improvement in the mechanical strength is for the sake of the handling whereas a lower cost is ensured considering that a major part of the material is a bio-based waste material. Metal ions such as Cu2+ are involved in numerous industrial activities because of its interesting properties. Excess above a critical limit can cause harmful effect on health and environment.20 We introduce in the current work potassium fulvate (KF)-modified graft copolymer of acrylic acid onto cellulose (Cell-g-PAA)/KF considering that the modification with KF and the way of its interpenetration in the structure was found interestingly to be a key step for achieving remarkable selective elimination during competitive adsorption of metal ions, when comparing with similar materials reported in the literature.
2. Experimental
2.1. Materials and methods
Acrylic acid (AA) was purchased from Sigma-Aldrich, USA. Potassium persulfate (KPS) was obtained from Loba Chemie Co. India. N,N′-methylenebisacrylamide (MBA) was ordered from Merck, Germany. Cellulose (Cell) and potassium hydroxide were supplied from Sd. fine Chem., India. Fulvic acid (FA) was obtained from Changsha Xian Shan Yuan Agriculture, Technology Co., Ltd. China (used as potassium salt of fulvic acid with 75% organic matter and 8% K2O). Stock solutions of Cu(II), Co(II) and Ni(II) were prepared in deionized water from copper(II) sulfate pentahydrate (CuSO4·5H2O) (Merck), cobalt chloride hexahydrate (CoCl2·6H2O) and nickel nitrate (Ni(NO3)2), respectively.2.2. Preparation of (Cell-g-PAA)/KF as polymeric sorbent
In a three-necked flask equipped with stirrer, reflux condenser, nitrogen line and a thermometer, 5 g of Cell was dispersed in 25 ml distilled water and the temperature was elevated to 70 °C for half an hour while the system was flushed with nitrogen to remove dissolved oxygen. A predetermined amount of KPS was dissolved in least amount of distilled water and added to the Cell suspension to allow radicals production. A mixture composed of the required amounts of AA (60% neutralized with KOH), MBA and KF was added after 15 min. The temperature was kept at 70 °C and the polymerization was continued for 180 min. The product was then washed excessively with methanol followed by drying at 70 °C until a constant weight. The product was ground into a fine powder and dispersed in distilled water at 40 °C for one day to dispose any dissolved PAA or unreacted species. The graft copolymer was separated by filtration, washed again repeatedly with distilled water and methanol and finally was dried at 65 °C for one day. For comparison, a control sample was prepared following the same route but in absence of KF.2.3. Removal of the metal ions using the polymeric sorbent
A batch sorption method was used to study the efficiency of the chelating graft polymer in the removal of metal ions. The effect of pH, contact time and temperature on the sorption for a mixture of copper, cobalt and nickel ions was studied. A mechanical shaker operated at 120 rpm was used for ensuring homogeneity of the metal ions salts in solutions. The metal ions were estimated in the samples after filtration with filter papers Whatman® (No. 44). Control samples without adsorbent were used for comparison between sorption and precipitation of metal ions. All experiments were repeated three times, the percentage of metal ions removal by the polymers was specified for all samples using inductive coupled plasma optical emission spectrometry (Agilent ICP-OES 5100, Australia) according to standard methods for the examination of water and wastewater.212.4. Characterizations
FTIR spectra were collected for the sorbent before and after metal ions adsorption using Jasco 6100 FTIR spectrophotometer (Japan) in the range 500–4000 cm−1. Dry samples were mixed with potassium bromide and pressed into discs before data acquisition. Scanning electron microscopy, SEM-Quanta FEG-250 (Holland), attached with AMETEX Energy Dispersive X-ray Analysis unit (EDAX), USA, was operated at 20 kV on gold plated adsorbent after being loaded with the metal ions for examining the morphology as well as detecting the adsorbed metal ions.3. Results and discussion
3.1. Grafting of acrylic acid onto cellulose in presence of potassium fulvate
The preparation of a selective sorbent was undergone via graft copolymerization of acrylic into cellulose while employing potassium fulvate as a multi-functional branching agent. For simplicity, these components have liability to react together considering that potassium fulvate and cellulose are multi-functional components that can react with their hydroxyl groups with the –COOH of acrylic acid. This should produce a branched macromonomer in situ, with cellulose–potassium fulvate hybrid as a backbone while vinyl groups derived from the anchored acrylic acid are protruded from the surface. The attack of potassium persulphate (KPS) as initiator induces the graft copolymerization. The high content of hydrophilic groups in the resulting interpenetrating network (IPN) may cause dissolution, which makes it necessary to ensure a regulated cross linking with methylene bisacrylamide (MBA). Methylene bisacrylamide enhances the mechanical strength while keeping good level of water absorption. The displayed scenario in Fig. 1 was approved after intensive investigations in our prior study.18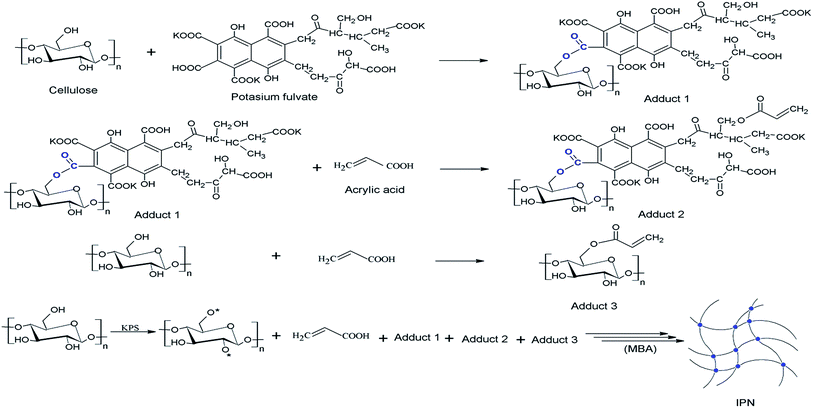 | ||
| Fig. 1 Synthesis of (Cell-g-PAA)/KF via graft copolymerization of acrylic acid onto cellulose in presence of potassium fulvate. | ||
Deficiency of selectivity is a major drawback that limits the use of many materials in the removal of heavy metal ions.22 Selectivity of a polymer ligand to specific metal ions is undertaken on the basis of charge, size and geometry of coordination.23 The chemical structure shown in Fig. 1, which was proven in our previous studies with various techniques16,18 may reveal that such complicated network with a high content of crowded functional groups may provide different chemical environments in the sense of coordination geometries and/or size of the metal ions thus expected to display selectivity to the network during competitive removal of heavy metal ions from their mixtures.
In a previous work,16 we evaluated the use of (Cell-g-PAA)/KF as chelating polymer for the removal of Cu(II) ions from aqueous solutions with initial concentration range 45–300 mg L−1. The attained efficiency of removal reached about 98.8% for the low concentration (45 mg L−1) and decreased systematically with the rise in Cu(II) concentration in the solution. As far as the current study focus on competitive removal of 3 metal ions at the same time, upon this we will fix the initial concentration from each metal ion at 50 mg L−1 for two reasons; it is the concentration at which the chelating polymer shows maximum removal efficiency for single ion removal while the total initial concentration of the ions in the solution becomes 150 mg L−1.
3.2. Effect of contact time on the capacity of removal and selectivity
The contact time between the selective chelating sorbent and mixtures of metal ions solution was studied using 50 mg L−1 from each metal ion in the mixture in order to determine the time at which an equilibrium state is reached (Fig. 2). The results reveal removal of Cu(II) proceeded very quickly especially in the first 5 min and the removal efficiency accomplished by that time was 82%. However, the achieved removal efficiency was lesser in case of Ni(II) (25%) and Co(II) (20.4%). The process levelled off within 30 min leaving behind a residual concentrations of 5.0, 40.2, and 43 mg L−1 for Cu(II), Ni(II) and Co(II), respectively, which is compiled into removal efficiency of 90, 19.6 and 14%, in that order. Therefore, the order of selectivity to the elements is Cu(II) >> Ni(II) > Co(II).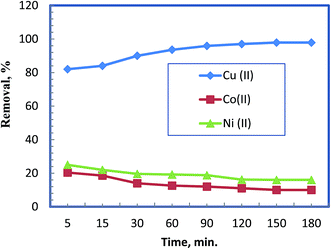 | ||
| Fig. 2 Effect of time on competitive removal of different metal ions from their mixture at pH 5.5 using 0.5 g L−1 of the chelating polymer. | ||
Also, in order to verify the selectivity of the polymeric sorbent towards Cu(II), the removal efficiency was investigated by employing the maximum attained capacity of Cu(II) (270.2 mg g−1) from our previous study 16 in presence of equivalent concentrations of the other metal ions. Therefore, the experiment was conducted using 135 mg L−1 from each metal ion using 0.5 g L−1 as sorbent dose at pH 5.5 for 30 min. The results show that Cu(II) displayed inherent remarkable activity as compared to other elements whereas no removal for Co(II) and Ni(II) was observed. Nevertheless, the removal efficiency for Cu(II) declined to 60% in the mixture. The removal of Cu(II) declined from 90% to 60% most likely due to the saturation of the sorption sites on the adsorbent, as the concentration of the metal ions increased. Evidence is the significant higher efficiency attained at lower concentrations. This trend validates the selectivity of the polymeric sorbent toward Cu(II).
3.3. Effect of pH on the capacity of removal and selectivity
Fig. 3 displays the removal selectivity of the chelating polymer as a function of the pH during competitive adsorption of Cu(II), Co(II) and Ni(II). It is apparent that a very effective selective removal of Cu(II) can be guaranteed on going with the pH from 2 onward. The selectivity increases remarkably toward Cu(II), in comparison with the other elements, and achieve the maximum at pH 5.5. No removal is noticed below pH 2 as the carboxyl groups are in protonated form, which hampers their chelation potential. This confirms that the carboxyl groups are the main chelating centers from the chelating polymer under investigation.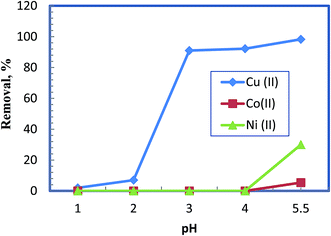 | ||
| Fig. 3 Effect of pH on competitive removal of 50 mg L−1 of different metal ions from their mixture at 0.5 g L−1 as chelating polymer dose. | ||
For more evidence of the metal ions adsorption into the polymeric structure, it was necessary to check the resulting polymer–metal ions chelate using additional tool such as FTIR as well as SEM-EDAX as displayed in Fig. 4 and 5.
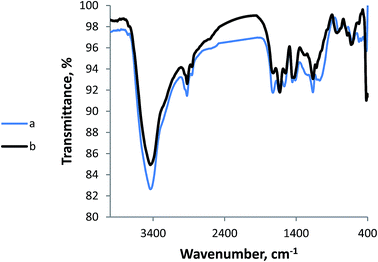 | ||
| Fig. 4 FTIR spectra of the polymeric adsorbent before and after removal of metal ions from their mixture. | ||
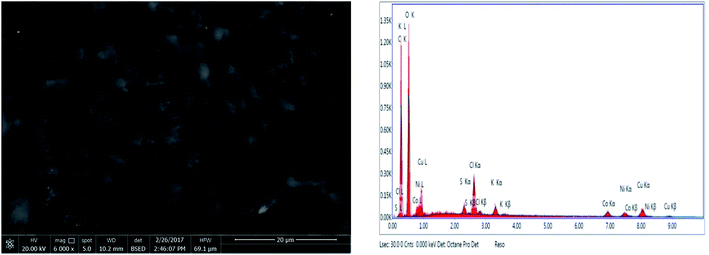 | ||
| Fig. 5 SEM and EDAX spot on the surface of the polymeric adsorbent after competitive removal of 50 mg L−1 of each metal ion from their mixture using 0.5 g L−1 of the polymer. | ||
Fig. 4a shows the FTIR spectra of the graft copolymer before the adsorption of metal ions. A band is appearing at 2858 cm−1, signifying CH2 groups of polyacrylic acid grafted chains while a peak at 1638 cm−1 is related to carbonyl groups of the grafted chains, overlapped with another carbonyl from the fulvate part (1725 cm−1). The peak in the range 3100–3751 cm−1 is featuring the OH groups emerging from both cellulose and polyacrylic acid. However, it is sharper than usual which indicates that polyacrylic acid is rendered mostly as ester after reacting with –OH groups. The phenyl parts of the potassium fulvate appear at 1561 cm−1 and 800 cm−1.
After adsorption of the metal ions, the peaks shifted toward lower wavenumbers (Fig. 4b). Namely, the bands at 1638 and 1725 moved downward to 1629 and 1719 cm−1, indicating more single bond character and linkage formation with metal ions via coordinate bonds. However, the bands of the hydroxyl groups remained almost unshifted signifying that these centers were not favoured for chelation to the metal ions. In addition, the emergence of a new band at 630 cm−1 is most likely related to metallic bonds formation.
Fig. 5 reveals that the previously reported well-defined macroporous structure of the polymeric adsorbent16,18 is maintained after the removal process. Furthermore, the elemental analysis on a surface spot using EDAX confirmed the presence of the metal ions under investigation in a relative population that lies in conformity with the measured levels after competitive removal: 4.3% for Cu(II), 2% for Ni(II) and 1.88% for Co(II).
Based on this basis in the sense of removal levels and selectivity, it is clear that the introduced chelating polymer in the current study is promising and superior in its performance as compared to other applied materials for the same purpose.3,4,9
3.4. Kinetic modeling
The uptake kinetics of Cu(II), Co(II) and Ni(II) by the graft copolymer was investigated with pseudo-first order, pseudo-second order and intra-particle diffusion models. Kinetic modeling for sorption of pollutants helps to predict the conditions at which the process can take place optimally for a given system.16 Pseudo-first order and pseudo-second order expressions are among the commonly used models for studying sorption of pollutants.24 However, the significance of particle diffusion in metal ions sorption was not considered for chelating materials.25 Accordingly, it was necessary to involve a diffusion-based kinetic model such as intra-particle diffusion to provide more understanding of the diffusion process. This model assumes the availability of active sites for simple access of metal ions, which influences the sorption kinetics for chelating system. Based on this, the sorption mechanism depends on the physical and chemical properties of the chelating material and the mass transfer as well.26 The system that gives closer conformity between the theoretical and experimental data as well as higher correlation coefficient will be considered the best applicable model.Lagregen's first order model postulates that the removal rate of metal ions is proportional to the number of free active sites in a sorbent.27 This model was employed to express the obtained kinetic data as a function of sorption rate and capacity according to eqn (1):
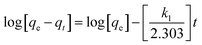 | (1) |
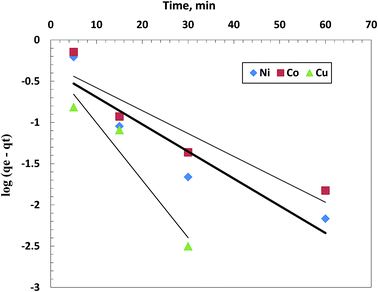 | ||
| Fig. 6 A kinetic plot for pseudo-first order model for individual removal of 50 mg L−1 of Cu(II), Co(II) and Ni(II) using 0.5 g L−1 of the chelating polymer at pH 5.5. | ||
| Pseudo-first order kinetics | Pseudo-second order kinetics | |||||
|---|---|---|---|---|---|---|
| qe Exp. (meq g−1) | qe (meq g−1) | k1 (min−1) | R2 | qe (meq g−1) | k2 (g mg−1 min−1) | R2 |
| Cu(II) 3.083 | 0.49 | 0.162 | 0.9365 | 3.09 | 1.21 | 0.999 |
| Co(II) 3.29 | 0.499 | 0.064 | 0.868 | 3.33 | 0.316 | 0.999 |
| Ni(II) 3.33 | 0.433 | 0.076 | 0.876 | 3.35 | 0.418 | 0.999 |
The pseudo-second order model assumes proportionality for the rate of removal and the square of the unoccupied sites number.24 Eqn (2) expresses the pseudo-second-order model:
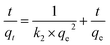 | (2) |
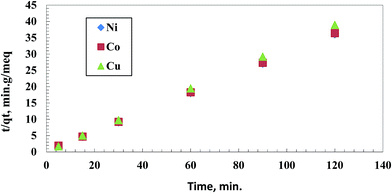 | ||
| Fig. 7 A kinetic plot for pseudo-second order model for individual removal of 50 mg L−1 of Cu(II), Co(II) and Ni(II) using 0.5 g L−1 of the chelating polymer at pH 5.5. | ||
Diffusion mechanism during metal ions uptake cannot be explored with pseudo-first order and/or pseudo-second order models. The intraparticle diffusion model was suggested to account for diffusion rate-controlled uptake of the metal ions by a chelating agent and is described by eqn (3).28
| qt = kit0.5 + Ci | (3) |
qt is the adsorbed amount of metal ions at time t, ki is the intraparticle diffusion constant which signifies the uptake rate for a diffusion-controlled process, whereas Ci is the intercept and contributes to the boundary layer effect.29 The boundary layer effect signifies that the removal is accomplished mainly by electrostatic attraction. A plot of qt and t1/2 according to eqn (4) (Fig. 8) shows a higher Ci for Cu(II) with respect to other elements, indicating greater affinity and instantaneous external surface adsorption for this element during the adsorption process. The following step illustrates the diffusion-controlled removal which encounters a gradual adsorption. The last step of the removal process exhibits a plateau as a result of saturation of the chelating polymer. This may help to predict that excellent selectivity can be undertaken within 3 min whereas afterward the polymeric sorbent cannot discriminate between the metal ions due to the high mass transfer. However, this prediction should be validated as the adsorption behavior might be something different during competitive adsorption.
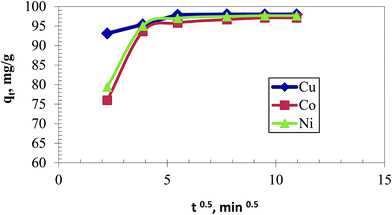 | ||
| Fig. 8 Intraparticle diffusion model for individual removal of 50 mg L−1 from each metal ion using 0.5 g L−1 of the chelating polymer at pH 5.5. | ||
The extent of Ci values in all cases implies that the uptake is principally governed by boundary layer effect (electrostatic attraction) whereas the intraparticle diffusion-controlled portion constituted a lesser extent.30,31 This explains the selective attitude of Cu(II) among the other metals ions during competitive adsorption. In general, this result provides a proof to the conclusion derived from the second-order model that chemisorption dominates the removal process.
3.5. Adsorption thermodynamics
A thermodynamic investigation for competitive removal process of the metal ions was carried out for understanding some characteristics of the process.32 It is based on the parameters qe (adsorbed amount of metal ion at equilibrium in mg L−1) and Ce (concentration of the metal ion at equilibrium in mg L−1) collected at different temperatures. A ratio of qe to Ce gives Kc. Thus, Fig. 9 is a depiction of the relation between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kc and 1/T according to eqn (4):
Kc and 1/T according to eqn (4):
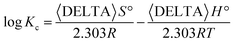 | (4) |
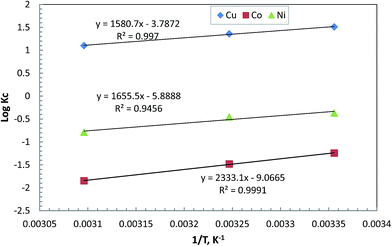 | ||
| Fig. 9 Effect of temperature on competitive removal efficiency of metal ions from their mixture (50 mg L−1 for each metal ion) at pH 5.5 using 0.5 g L−1 of sorbent. | ||
| Parameter | Temp. (K) | Cu(II) | Ni(II) | Co(II) |
|---|---|---|---|---|
| a 50 mg L−1 for each metal ion [Cu(II), Ni(II) and Co(II)] and 0.5 g L−1 of sorbent at pH 5.5. | ||||
| 〈DELTA〉G° (kJ mol−1) | 298 | −8.5 | 2.099 | 7.094 |
| 308 | −8.01 | 2.68 | 8.73 | |
| 323 | −6.81 | 4.88 | 11.43 | |
| −〈DELTA〉H° (kJ mol−1) | 30.2 | 31.689 | 44.67 | |
| −〈DELTA〉S° (J mol−1) | 72.5 | 112.74 | 173.588 | |
The negative value of 〈DELTA〉G° in case of Cu2+ (−8.5 kJ mol−1) indicates that Cu(II) adsorption process on the chelating polymer is feasible and spontaneous. Interestingly, the negativity of 〈DELTA〉G° is lessened with the temperature rise from 298 K to 323 K which confirms that the adsorption of this element becomes less spontaneous but maintained its high adsorption tendency onto by the polymeric sorbent at the expense of the other competing elements. Thereby, the positive values of 〈DELTA〉G° in case of Ni(II) and Co(II) seem not logic especially with the exothermic nature of the adsorption in all cases as signified by the negative values of 〈DELTA〉H°; −30.3, −31.689, −44.67 kJ mol−1 for Cu, Co, Ni, ions, respectively. This further indicates that the adsorption and desorption of Co and Ni ions are not in mutual thermodynamic equilibrium. That is to say that the qe cannot exceed Ce for these elements in presence of Cu(II). This reveals the much higher selectivity of the sorbent toward Cu(II) as compared to them.
The acquired negative values of 〈DELTA〉S° (−72.5, −112.74, −173.588 J mol−1 K−1 for Cu, Co, Ni ions) show a decrease in randomness at the solution–sorbent interface following the competitive removal process. However, this decrease was much greater in case of Ni(II) and Co(II) as compared to Cu(II) indicating the possibility to achieve optimal selective removal between the ions from their mixture especially at higher temperatures. Furthermore, the selectivity issue in the current study may be justified upon this basis by the geometry of coordination rather than the size of the ions.
4. Conclusions
The copolymer resulting from graft polymerization of acrylic acid onto cellulose can acquire excellent chelating potential towards heavy metal ions when modified with potassium fulvate as permanent part of the produced network structure. In addition, outstanding selectivity was noted in case of competitive removal of a mixture of metal ions. For the metal ions under investigation, they could be arranged in the following order according to the selectivity preference: Cu(II) >> Ni(II) and Co(II), when exist together. Efficient selectivity of Cu(II) can be warranted during the first few minutes of contact with the chelating graft at pH 5.5 due to the deprotonation of –COOH at this pH. Kinetic modeling studies proved the control of the chemisorption on the uptake process whereas the intraparticle diffusion model validated that the uptake was principally pursued by boundary layer effect, as a result of electrostatic attraction. Cu(II) adsorption proceeds more spontaneously at all temperatures during competitive removal on the contrary to Ni(II) and Co(II).References
- S. Shukla, R. Pai and A. Shendarkar, Adsorption of Ni(II), Zn(II) and Fe(II) on modified coir fibres, Sep. Purif. Technol., 2006, 47, 141–147 CrossRef CAS.
- H. Essawy and H. Ibrahim, Synthesis and characterization of poly(vinylpyrrolidone-co-methylacrylate) hydrogel for removal and recovery of heavy metal ions from wastewater, React. Funct. Polym., 2004, 61, 421–432 CrossRef CAS.
- A. Denizil, G. Ozkan and M. Arica, Preparation and characterization of magnetic polymethylmethacrylate microbeads carrying ethylene diamine for removal of Cu(II), Cd(II), Pb(II), and Hg(II) from aqueous solutions, J. Appl. Polym. Sci., 2000, 78, 81–89 CrossRef.
- C. Futalan, W. Tsai, S. Lin, K. Hsien, M. Dalida and M. Wan, Copper, nickel and lead adsorption from aqueous solution using chitosan-immobilized on bentonite in a ternary system, Sustainable Environ. Res., 2012, 22, 345–355 CAS.
- A. Baraka and P. J. Heslop, Preparation and characterization of melamine-formaldehyde DTPA chelating resin and its use as an adsorbent for heavy metals removal from wastewater, React. Funct. Polym., 2007, 67, 585–600 CrossRef CAS.
- U. Yildiz and O. Kemik, , The removal of heavy metal ions from aqueous solutions by novel pH-sensitive hydrogels, J. Hazard. Mater., 2010, 183, 521–532 CrossRef CAS PubMed.
- S. Kirupha, A. Murugesan, T. Vidhyadevi, P. Baskaralingam, S. Sivanesan and D. L. Ravikumar, Novel polymeric adsorbents bearing amide, pyridyl, azomethine and thiourea binding sites for the removal of Cu(II) and Pd(II) ions from aqueous solution, Sep. Sci. Technol., 2013, 48, 254–262 CrossRef.
- R. Saravanan and L. Ravikumar, The use of new chemically modified cellulose for heavy metal ion adsorption and antimicrobial activities, J. Water Resour. Prot., 2015, 7, 530–545 CrossRef CAS.
- C. Kavakl, A. Tuncel and B. Salih, Selectivity of cyclam modified poly(p-chloromethyl styrene-ethyleneglycol dimethacrylate) microbeads for Cu(II), Ni(II), Co(II) and Zn(II), Sep. Purif. Technol., 2005, 45, 32–40 CrossRef.
- Q. Saima, S. Hasany, M. Bhanger and M. Khuhawar, Enrichment of Pb(II) ions using phthalic acid functionalized XAD-16 resin as a sorbent, J. Colloid Interface Sci., 2005, 291, 84–91 CrossRef PubMed.
- C. Y. Chen, C. Chiang and C. R. Chen, Removal of heavy metal ions by a chelating resin containing glycine as chelating groups, Sep. Purif. Technol., 2007, 54, 396–403 CrossRef CAS.
- N. Pekel, H. Savas and O. Guven, Complex formation and adsorption of V3+, Cr3+ and Fe3+ ions with poly(N-vinylimidazole), Colloid Polym. Sci., 2002, 280, 46–51 CAS.
- S. Kalaivani, T. Vidhyadevi, A. Murugesan, K. Thiruvengadaravi, C. Anduradha, S. Sivanesan and L. Ravikumar, The use of new modified pol(acrylamide) chelating resin with pendent benzothiazole groups containing donor atoms in the removal of heavy metal ions from aqueous solutions, Water Resources and Industry, 2014, 5, 21–35 CrossRef.
- E. Orozco-Guareno, F. Santiago-Gutierrez, J. L. Moran-Quiroz, S. L. Hernandez-Olmos, V. Soto, W. L. Cruz, R. Manriquez and S. Gomez-Salazar, Removal of Cu(II) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels, J. Colloid Interface Sci., 2010, 349, 583–593 CrossRef CAS PubMed.
- Q. Lu, J. Yu, J. Gao, W. Jang and Y. Li, Glow-discharge electrolysis plasma induced synthesis of polyvinylpyrrolidone/acrylic acid hydrogel and its adsorption properties for heavy-metal ions, Plasma Processes Polym., 2011, 8, 803–814 CrossRef CAS.
- M. Fawzy, H. Essawy, N. Ammar and H. Ibrahim, Potassium Fulvate-Modified Graft Copolymer of Acrylic Acid Onto Cellulose as Efficient Chelating Polymeric Sorbent, Int. J. Biol. Macromol., 2017, 94, 771–780 CrossRef PubMed.
- N. A. Abdelwahab, N. S. Ammar and H. S. Ibrahim, Graft copolymerization of cellulose acetate for removal and recovery of lead ions from wastewater, Int. J. Biol. Macromol., 2015, 79, 913–922 CrossRef CAS PubMed.
- M. B. Ghazy, F. Abd El-Hai, M. F. Mohamed and H. Essawy, Potassium fulvate as co-interpenetrating agent during graft polymerization of acrylic acid from cellulose, Int. J. Biol. Macromol., 2016, 91, 1206–1214 CrossRef CAS PubMed.
- H. Essawy, M. B. Ghazy, F. Abd El-Hai and M. F. Mohamed, Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients, Int. J. Biol. Macromol., 2016, 89, 144–151 CrossRef CAS PubMed.
- Z. Liu, H. Wang, C. Liu, Y. Jiang, G. Yu, X. Mu and X. Wang, Magnetic cellulose-chitosan hydrogels prepared from ionic liquids as reusable adsorbent for removal of heavy metal ions, Chem. Commun., 2012, 48, 7350–7352 RSC.
- American Public Health Association, Standard methods for the examination of water and wastewater, Washington, DC, 2012 Search PubMed.
- S. A. Idris, S. R. Harvey and L. T. Gibson, Selective extraction of mercury(II) from water samples using mercapto functionalized-MCM-41 and regeneration of the sorbent using microwave digestion, J. Hazard. Mater., 2011, 193, 171–176 CrossRef CAS PubMed.
- L. D. Mafu, T. A. M. Msagati and B. B. Mamba, Ion-imprinted polymers for environmental monitoring of inorganic pollutants: synthesis, characterization, and applications, Environ. Sci. Pollut. Res. Int., 2013, 20, 790–802 CrossRef CAS PubMed.
- W. Plazinski, W. Rudzinski and A. Plazinska, Theoretical models of sorption kinetics including a surface reaction mechanism: a review, Adv. Colloid Interface Sci., 2009, 152, 2–13 CrossRef CAS PubMed.
- Y. S. Ho, J. C. Y. Ng and G. McKay, Kinetics of pollutant sorption by biosorbents: review, Sep. Purif. Methods, 2000, 29, 189–232 CrossRef CAS.
- C. Gerente, V. K. C. Lee, P. Le Cloirec and G. McKay, Application of chitosan for the removal of metals from wastewater by adsorption – mechanisms and models review, Crit. Rev. Environ. Sci. Technol., 2007, 37, 41–127 CrossRef CAS.
- S. Lagergren, Zurtheorie der sogenannten adsorption gelosterstoffe, Kungliga Sevenska Vetenskapas akademiens, Handlingar, 1898, 24, pp. 1–39 Search PubMed.
- J. C. Morris and W. J. Weber, Advances in Water Pollution Research, Pergamon Press, New York, 1962 Search PubMed.
- Y. Li, Q. Du, T. Liu, J. Sun, Y. Jiao, Y. Xia and D. Wu, Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto graphene, Mater. Res. Bull., 2012, 47, 1898–1904 CrossRef CAS.
- V. Fierro, V. Torne-Fernandez, D. Montane and A. Celzard, Adsorption of phenol onto activated carbons having different textural and surface properties, Microporous Mesoporous Mater., 2008, 111, 276–284 CrossRef CAS.
- B. I. Olu-Owolabi, P. N. Diagboya and K. O. Adebowale, Evaluation of pyrene sorption–desorption on tropical soils, J. Environ. Manage., 2014, 137, 1–9 CrossRef CAS PubMed.
- Y. Nuhoglu and E. Malkoc, Thermodynamic and kinetics studies for environmentally friendly Ni(II) biosorption using waste pomace of olive factory, Bioresour. Technol., 2009, 100, 2375–2380 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |
