Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An octupolar bis(porphyrinato) terbium(III) complex with the highest off-resonant hyperpolarizability†
Junshan Sun and
Yinfeng Han*
College of Chemistry and Chemical Engineering, Taishan University, Taian 271000, Shandong, China. E-mail: sunjunshan79@tsu.edu.cn
First published on 25th April 2017
Abstract
A novel bis(porphyrinato) terbium double-decker complex HTb(DADAPor)2 with a twisted cuboid octupolar molecular symmetry exhibits the largest off-resonant first hyperpolarizability, as evaluated by harmonic light scattering measurements with 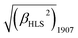 = 1700 × 10−30 esu.
= 1700 × 10−30 esu.
Octupolar molecules with highly delocalized π-electron systems have paved new ways in the development of new nonlinear optical (NLO) molecular materials due to their broader range of nonlinear tensorial coefficients in comparison with the more common dipolar one-dimensional D–π–A schemes.1,2 The most general 3D octupolar architecture is made of a cube bearing eight alternatively positive and negative charges at its corners (that are induced by the presence of electron-donors and electron-acceptors) and strong electronic interactions between donor and acceptor groups along the cube edges.3 In addition, this “perfect” octupolar cubic geometry displays an overall tetrahedral symmetry (Td). However, practical octupolar molecular NLO materials developed up to date usually take reduced dimensionality.3c For example, projecting the charges of the octupolar cube on a plane perpendicular to the three-fold symmetry axis of the cube and containing its center result in a 2D alternate distribution of three negative and three positive charges.1a In fact, 3D octupolar molecules with a cube conformation and/or a tetrahedral symmetry are still remaining extremely rare4 since strong NLO responses require highly conjugated electronic distributions corresponding to a planar molecular geometries. However, new species of octupolar NLO rare earth complexes composed of a ABAB-type phthalocyanine ligand associating two kinds of alternative substituents with slightly different electron-donating properties at the peripheral positions were recently developed.5 These compounds take advantage of an intense intramolecular π–π interaction between the two phthalocyanines in a bis(phthalocyaninato) rare earth(III) sandwich complex.
Porphyrins, as another important class of tetrapyrrole system with a large polarizable π-conjugated system, have also been extensively involved in the design and synthesis of typical NLO molecular materials because of their large oscillator strengths and substantial polarizabilities.6 In particular, the porphyrin-based D–π–A systems mediated by an ethynyl unit between porphyrin and other strong oscillators usually create unusually polarizable and hyperpolarizable structures, and therefore enable the development of a variety of materials that possess unusual NLO properties.7 However, all porphyrin-based 3D molecules developed so far are in fact assemblies of a few (3 or 4) porphyrin units organized in a tetrahedral geometry by binding them together to a single sp3-hybridized atom (carbon, silicium) that prevents from any significant electronic interactions between porphyrin moieties.8 Truly porphyrin-based octupolar molecules employing the cubic molecular structure with eight alternating charges at the cube corners with noticeable electronic interactions between electron donor and electron acceptor groups has not yet been reported.
In the present paper, a metal free porphyrin featuring a crosswise DADA arrangement for the electron-donating and electron-withdrawing groups at the four meso-positions, namely 21H,23H-[5,15-bis(4-carboxyethyl-ethynyl)-10,20-bis(4-diphenyl-amino)phenyl]porphyrin, was designed and synthesized. The bis(porphyrinato) terbium double-decker complex HTb(DADAPor)2 fabricated from this novel porphyrin ligand affords a typical generic template of an octupolar molecule with a relatively lower symmetry, D2, than the Td symmetry displayed by a truely octupolar cube, Scheme 1. This symmetry, in combination with an intense intramolecular π–π interaction and the lack of any absorption beyond 800 nm due to the reduced protonated nature of the double-decker molecule, is expected to result in a high off-resonant first hyperpolarizability for this double-decker compound.3–5
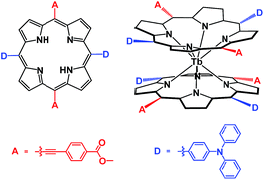 | ||
| Scheme 1 Schematic molecular structures of the DADA-type metal free porphyrin H2(DADAPor) and double-decker HTb(DADAPor)2. | ||
Special attention will be paid here to the contribution of resonance effects to the first hyperpolarizability β. Oudar and co-worker have proposed a two-level dispersion model to account for the resonance effects in the destination of β values.2 If the fundamental laser frequency is close to the resonance frequency (ω0)] or its first harmonic (2ω0), in which 0 corresponds to one of the absorption bands of the molecule, the NLO property9 can be strongly increased due to the significant resonance contribution. According to the very recent theoretical study, the resonant hyperpolarizability for bis(phthalocyaninato) yttrium skeleton becomes 10 to 103 times larger than the off-resonant value.10 As a consequence, a off-resonant hyperpolarizability β(0) should be taken as the evaluating criterion to compare the intrinsic NLO activities among various molecular materials.
It has been revealed that the electronic polarizability and the nature of the transitions between electronic states for molecular materials are responsible for the NLO response. As a consequence, for the purpose of engineering NLO chromophores, careful consideration over the molecular symmetry must be taken into account. Noncentrosymmetry being a necessary prerequisite for all even-order nonlinear effects, the donor–bridge–acceptor (D–Br–A) moiety has been developed as a typical classic molecular architecture for the dipolar moment molecule. Associated with their large π-conjugated systems, a number of porphyrin-bridged dipolar D–π–A chromophores featuring large dynamic hyperpolarizabilities have been developed.11 However, the ground-state electrostatic interaction for dipolar molecules usually favors a detrimental centrosymmetric aggregation in the condensed phase, which in turn minimizes the second-order NLO properties. Aiming to overcome this drawback in the case of dipolar D–porphyrin–A chromophores, porphyrin-based octupolar compounds were proposed as alternative molecules. With the robust and benchmark dipolar chromophore, ruthenium(II)[5-(4′-ethynyl-(2,2′;6′,2′′-terpyridinyl))-10,20-bis(2′,6′-bis(3,3-dimethyl-1-butyloxy)phenyl)porphinato]zinc(II)-(2,2′;6′,2′′-terpyridine)2+ bis-hexafluorophosphate(RuPZn) as starting material, a porphyrin-containing supramolecular system displaying an octupolar NLO response was created.12 Nevertheless, taking advantage of the intense intramolecular π–π interaction in bis(phthalocyaninato) rare earth(III) complexes, new species of octupolar NLO molecular materials composed of the most important artificial porphyrin analogue, phthalocyanine, with ABAB-type arrangement bringing two kinds of alternative substituents with slightly different electron-donating ability at the peripheral positions, were also developed.5 However, the absorption band between 800–1100 nm for bis(phthalocyaninato) lutetium compound [Lu(Pc*)2] associated with the presence of one unpaired electron in the double-decker molecule is responsible for a strong absorption of the scattered light at the second harmonic wavelength (953 nm) by the chromophore,13 indicating a significant contribution of multilevel resonance enhancements to the second-order NLO response of Lu(Pc*)2.
In the present case, in order to construct the target octupolar bis(porphyrinato) terbium double-decker complex, a novel DADA-type metal free porphyrin has been synthesized. In order to strengthen the electron-donor and electron acceptor character of D and A, respectively, two carboxymethyl-phenylethynyl substituents as typical electron-accepting groups were introduced onto the opposite meso-positions of the porphyrin ligand while two di(phenylamino)phenyl moieties as typical electron-donating groups were grafted onto the remaining two opposite meso-positions. As a result, the metal free [5,15-bis(4-carboxymethyl-phenylethynyl)-10,20-bis(4-diphenylamino)phenyl] porphyrin H2(DADAPor) was designed and synthesized for the first time, (Scheme 1). Its reaction with Tb(acac)3·nH2O (acac = acetylacetonate) in refluxing 1,2,4-trichlorobenzene (TCB) led to the first bis(porphyrinato) terbium(III) double-decker complex HTb(DADAPor)2 exhibiting a typical generic template of a 3D octupolar molecule with a D2 symmetry, Scheme 1. In particular, the reduced protonated nature of this double-decker species excludes any absorption in the near-IR range beyond 800 nm as detailed below,14 then strongly reducing resonance contributions to the second-order NLO response of this compound as evaluated by harmonic light scattering measurements at 1907 nm. Satisfactory elemental analysis result was obtained for the target bis(porphyrinato) terbium double-decker complex after repeated column chromatography followed by recrystallization. Its MALDI-TOF mass spectrum clearly shows intense signal for the corresponding protonated molecular ion [M + H]+. The isotopic pattern closely resembles the simulated one, Fig. S1 and Table S1 (ESI†). Nevertheless, this compound was further characterized with a range of spectroscopic techniques including IR and electronic absorption spectroscopy.
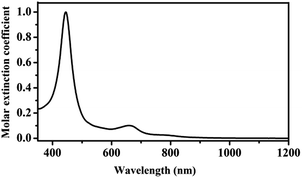
The electronic absorption spectrum of HTb(DADAPor)2 was recorded in CHCl3 and shown in Fig. 1, with corresponding data summarized in Table S2 (ESI†). As can be seen, the electronic absorption spectrum of HTb(DADAPor)2 shows a typical Soret band at 445 nm and a weak, broad Q band at 660 nm. No near-IR absorption band was observed for this homoleptic double-decker, indicating the protonated nature of this double-decker in the form of HTb(DADAPor)2 and, consequently, the off-resonant nature for the first hyperpolarizability of this material.
In the IR spectrum of HTb(DADAPor)2, Fig. S2 (ESI†), the absorption due to the symmetric stretching vibration of the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C– groups is observed at about 2348 cm−1. This is close to the band observed at 2351 cm−1 for metal free porphyrin. However, due to the removing of seven of the eight inner pyrrole hydrogen atoms in the bis(porphyrinato) terbium double-decker compound and the significantly decreased interaction of the remaining hydrogen atom (which tautomerises around the four pyrrole nitrogen atoms of one of the two porphyrins in the double-decker molecule15) with the pyrrole nitrogen atoms, only a very weak absorption for the characteristic N–H stretching vibration of pyrrole moiety was observed around 3310 cm−1, instead of the relatively intense band usually observed for standard porphyrins.
C– groups is observed at about 2348 cm−1. This is close to the band observed at 2351 cm−1 for metal free porphyrin. However, due to the removing of seven of the eight inner pyrrole hydrogen atoms in the bis(porphyrinato) terbium double-decker compound and the significantly decreased interaction of the remaining hydrogen atom (which tautomerises around the four pyrrole nitrogen atoms of one of the two porphyrins in the double-decker molecule15) with the pyrrole nitrogen atoms, only a very weak absorption for the characteristic N–H stretching vibration of pyrrole moiety was observed around 3310 cm−1, instead of the relatively intense band usually observed for standard porphyrins.
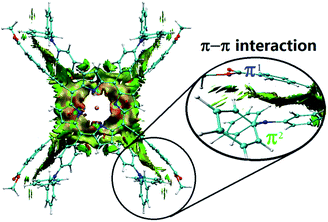
In spite of several attempts, it was not possible to obtain HTb(DADAPor)2 single crystals suitable for X-ray diffraction analysis. As a consequence, to provide information about the molecular configuration of this double-decker compound, theoretical calculations have been performed to optimize the molecular geometry. As shown in Fig. 2, topology analysis for the molecular skeleton was carried out on the basis of DFT calculation results. Instead of a standard cubic structure, the present bis(porphyrinato) terbium double-decker molecule actually displays a twisted cuboid structure with along x/y edge of 15–16 Å and a short z edge of 4–5 Å, with a twisting angle of 17°, leading to a decrease in the molecular symmetry from the Td point group for a model cubic octupolar structure to the D2 point group for the present twisted cuboid octupolar one.
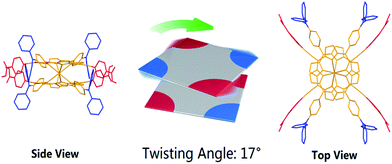 | ||
| Fig. 2 The simulated molecular structure of HTb(DADAPor)2 with a twisted octupolar cuboid skeleton and the group point of D2. | ||
In order to clarify the effect of the widespread π–π interactions within HTb(DADAPor)2 on the molecular structure, reduced density gradient (RDG) analysis was carried out. As clearly exhibited in Fig. 3, π–π interactions in this double-decker molecule exist not only between the two tetrapyrrole chromophores but also between the peripheral donor and acceptor substituents of different porphyrin ligands. Careful inspection over the π–π interactions among the peripheral substituents gives further detailed information about the interaction between π1[–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–(C6H4)–(C
C–(C6H4)–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–CH3] of one porphyrin and π2[–(C6H4)–N–(C6H5)2] of the other porphyrin in the double-decker molecule, including a π1(C
O)–O–CH3] of one porphyrin and π2[–(C6H4)–N–(C6H5)2] of the other porphyrin in the double-decker molecule, including a π1(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C)⋯π2(C6H4) pair, a π1(C6H4)⋯π2(NC3) pair, and a π1(C
C)⋯π2(C6H4) pair, a π1(C6H4)⋯π2(NC3) pair, and a π1(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)⋯π2(C6H5) pair. In addition, σ(–CH3) at the end of the π1 system is also attracted by π2(C6H5) via the n–π interaction. As a result, these two peripheral aromatic substituents get close to each other with a distance between 3.5 and 4.0 Å. This in turn results in a twisted cuboid octupolar molecular structure with a decreased molecular symmetry of D2 employed by HTb(DADAPor)2 as shown in Fig. 2.
O)⋯π2(C6H5) pair. In addition, σ(–CH3) at the end of the π1 system is also attracted by π2(C6H5) via the n–π interaction. As a result, these two peripheral aromatic substituents get close to each other with a distance between 3.5 and 4.0 Å. This in turn results in a twisted cuboid octupolar molecular structure with a decreased molecular symmetry of D2 employed by HTb(DADAPor)2 as shown in Fig. 2.
For this sandwich-type octupolar chromophore of HTb(DADAPor)2, we determine its molecular first hyperpolarizability β (for 1907) by harmonic light scattering16a (HLS, also named hyper-Rayleigh scattering16b) experiments in chloroform solution. The incident and harmonic wavelengths at 1907 and 953 nm, respectively, are quite remote from the main absorption band of the molecule, then minimizing resonance contributions to the β tensor. Considering these conditions, we report here a very large off-resonant first hyperpolarizability with a value of 1700 × 10−30 esu. If we neglect the contribution of the weak Q band around 660 nm, we may apply the two-level dispersion model16b to infer a “static β(0) value of 1300 × 10−30 esu.
It is worth noting that the sandwich-type bis(phthalocyaninato) lutetium double-decker complex Lu(Pc*)2 shows a higher second-order NLO response at 1907 nm, βHLS = 5750 × 10−30 esu.5 However, direct comparison is definitely not appropriate because of the presence of several absorption bands in the visible-near IR range for Lu(Pc*)2 associated with the presence of one unpaired electron in the double-decker molecule.13 This reveals the significant contribution of a multilevel resonance enhancement to the high β value of Lu(Pc*)2, contrary to our present case where HTb(DADAPor)2 does not show any strong absorption in visible or near-IR region beyond the Soret band due to its reduced protonated nature.14 This results in a resonance-free first hyperpolarizability for HTb(DADAPor)2 as measured at 1907 nm. On the basis of other off-resonant βHRS values reported thus far for most octupolar NLO materials, which are smaller than 103 × 10−30 esu,3–5 the present result therefore represents the largest resonance-free first hyperpolarizability for octupolar molecular materials.
It must be pointed out that according to the geometry inferred from molecular modelling, the twist angle between the two porphyrin derivatives of this double-decker molecule is only 17°, which is much smaller than the value reported in ref. 5 for phthalocyanine derivatives. If this trend could be confirmed by experimental structure determination, the present porphyrine-based strategy opens the ways to the synthesis of Td – symmetry “perfect octupoles with a real cubic geometry. Further effort towards designing and synthesizing novel bis(tetrapyrrole) rare earth compounds with further enhanced resonance-free second-order NLO response is in progress.
Briefly summarizing above, a novel metal free porphyrin featuring a crosswise DADA arrangement for the electron-donating and electron-withdrawing groups at the four meso-positions was designed, synthesized, and employed to construct a bis(porphyrinato) terbium double-decker complex with octupolar molecular symmetry. This, in combination with the intense intramolecular π–π interaction and the lack of any absorption beyond 800 nm in the double-decker molecule, results in the largest off-resonant first hyperpolarizability as evaluated by harmonic light scattering measurements with 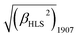 = 1700 × 10−30 esu.
= 1700 × 10−30 esu.
Experimental section
General remarks
4-(Diphenylamino)benzaldehyde and methyl 4-bromobenzoate were purchased from Aladdin Inc. CuI, bis(triphenylphosphine)palladium(II) dichloride, and trimethylsilylacetylene were bought from Energy Chemical Inc. Column chromatography was carried out on silica gel (Merck, Kieselgel 60, 200–300 mesh) with the indicated eluents. All other reagents and solvents were used as received. The compounds of methyl 4-ethynylbenzoate17 and metal free [5,15-dibromo-10,20-(4-diphenylamino)phenyl]porphyrin18 were prepared according to the literature procedures.The 1H NMR spectra were recorded on a 400 MHz spectrometer in CDCl3 with shifts referenced to SiMe4 (0.00 ppm). MALDI-TOF mass spectra were taken on a Bruker BIFLEX III ultra-high resolution Fourier transform ion cyclotron resonance (FT-IR) mass spectrometer with alpha-cyano-4-hydroxy cinnamic acid as the matrix. Elemental analyses were performed on an Elementar Vavio El III. Electronic absorption spectra were recorded on a Hitachi U-4100 spectrophotometer in CHCl3 solution at ambient temperature.
Synthesis of metal free [5,15-bis(4-carboxymethyl-phenylethynyl)-10,20-bis(4-diphenylamino)phenyl]porphyrin H2(DADAPor)
The mixture of metal free [5,15-dibromo-10,20-(4-diphenylamino)phenyl]porphyrin (238 mg, 0.025 mmol), Pd(PPh3)2Cl2 (0.165 mg, 0.24 mmol), and CuI (0.58 mg, 0.31 mmol) in 20 mL dry THF was stirred at 50 °C under nitrogen for 2 h. The precipitate was removed by filtration and the volatiles were removed under reduced pressure. The residue was chromatographed on a silica gel column using CHCl3/n-haxane ether (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent. After evaporating the solvent under reduced pressure, the target compound was obtained as a greenish brown solid, 186 mg (67%). 1H NMR (400 MHz, CDCl3): δ 0.652–0.670 (d, 6H, CH3O−); 7.05–7.46 (d, 20H, Ph); 8.05–8.46 (d, 8H, Ph), 9.058 (d, 2H, pyrolle-H), 9.65 (d, 2H, pyrolle-H). MALDI-TOF MS: an isotopic cluster peaking at m/z 1116.63 [M + H]+; calculated for C76H52O4N6, 1115.54. Anal. calcd (%) for H2(DADAPor)·(H2O)2·(CH3OH): C, 81.99; H, 4.71; N, 7.55; found: C, 81.58; H, 4.92; N, 7.91.
1) as eluent. After evaporating the solvent under reduced pressure, the target compound was obtained as a greenish brown solid, 186 mg (67%). 1H NMR (400 MHz, CDCl3): δ 0.652–0.670 (d, 6H, CH3O−); 7.05–7.46 (d, 20H, Ph); 8.05–8.46 (d, 8H, Ph), 9.058 (d, 2H, pyrolle-H), 9.65 (d, 2H, pyrolle-H). MALDI-TOF MS: an isotopic cluster peaking at m/z 1116.63 [M + H]+; calculated for C76H52O4N6, 1115.54. Anal. calcd (%) for H2(DADAPor)·(H2O)2·(CH3OH): C, 81.99; H, 4.71; N, 7.55; found: C, 81.58; H, 4.92; N, 7.91.
Synthesis of HTb(DADAPor)2
A mixture of metal free porphyrin (127.6 mg, 0.11 mmol) and Tb(acac)3·nH2O (25.0 mg, 0.021 mmol) in TCB (2.0 mL) was heated to reflux under a nitrogen atmosphere for about 8 h. The solvent was removed under reduced pressure and the residue was chromatographed on a silica gel column with CHCl3 and methanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the eluent. After the first green band containing unreacted porphyrin, a brown band containing mainly the target double-decker compounds was collected. Repeated chromatography followed by recrystallization from CHCl3 and CH3OH gave a purple powder with the yield of 25%. MALDI-TOF MS: an isotopic cluster peaking at m/z 2397.55 [M + H]+; calculated for C152H100O8N12TbH, 2396.41. Anal. calcd (%) for HTb(DADAPor)2: C, 76.66; H, 4.23; N, 7.06; found: C, 76.84; H, 4.62; N, 7.25.
1 v/v) as the eluent. After the first green band containing unreacted porphyrin, a brown band containing mainly the target double-decker compounds was collected. Repeated chromatography followed by recrystallization from CHCl3 and CH3OH gave a purple powder with the yield of 25%. MALDI-TOF MS: an isotopic cluster peaking at m/z 2397.55 [M + H]+; calculated for C152H100O8N12TbH, 2396.41. Anal. calcd (%) for HTb(DADAPor)2: C, 76.66; H, 4.23; N, 7.06; found: C, 76.84; H, 4.62; N, 7.25.
Calculation summary
Long-range corrected density functional method ωB97XD is employed for geometric calculation.19 The basis sets of 6-311+G(d) and SDD are employed for C/N/H/O and Tb, respectively.20 The π–π interactions are plotted using the reduced density gradient (RDG) at the same level.21 All the calculations were carried out using Multiwfn 3.2 (ref. 22) and Gaussian 09 D.01.23 Computational details are given in ESI.†HLS measurements
Hyper-Rayleigh Scattering (HRS) experiment16 is based on a two-photon scattering process, in which the intensity of the second harmonic scattered light, generated by focusing an intense laser beam onto an isotropic solution, is measured as a function of the incoming laser intensity. Measurements were performed at an IR incident wavelength (1907 nm) chosen to minimize the frequency dispersion of molecular hyperpolarizabilities. A 10−5 M solution of chromophore HTb(DADAPor)2 in CHCl3 was prepared and passed through 0.2 μm filters to remove any particles or dust. The experiments was performed at a low chromophore concentration where the linearity of the HRS signal for HTb(DADAPor)2 as a function of chromophore concentration confirmed that no significant aggregation of NLO molecules occurred in this experiment.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21571137), and Talent Foundation of Taishan University (No. Y2015-1-002) is gratefully acknowledged.Notes and references
- (a) J. Zyss, Nonlinear Opt., 1991, 1, 3 CAS; (b) J. Zyss, J. Chem. Phys., 1993, 98, 6583 CrossRef CAS; (c) V. L. Floch, S. Brasselet, J. Zyss, B. R. Cho, S. H. Lee, S. J. Jeon, M. Cho, K. S. Min and M. P. Suh, Adv. Mater., 2005, 17, 196 CrossRef.
- J. L. Oudar and D. S. J. Chemla, Chem. Phys., 1977, 66, 2664 CAS.
- (a) M. Hanack, T. Schneider, M. Barthel, J. S. Shirk, S. R. Flom and R. G. S. Pong, Coord. Chem. Rev., 2011, 235, 219 Search PubMed; (b) O. Maury and B. Le, Acc. Chem. Res., 2005, 38, 691 CrossRef CAS PubMed; (c) J. Zyss and I. Ledoux, Chem. Rev., 1994, 94, 77 CrossRef CAS; (d) T. Baumgartner and R. Réau, Chem. Rev., 2006, 106, 4681 CrossRef CAS PubMed; (e) C. G. Claessens, D. González-Rodríguez, D. M. Rodríguez-Morgade, A. Medina and T. Torres, Chem. Rev., 2014, 114, 2192 CrossRef CAS PubMed; (f) P. C. Ray, Chem. Rev., 2010, 110, 5332 CrossRef CAS PubMed.
- (a) S. Roke and G. Gonella, Annu. Rev. Phys. Chem., 2012, 63, 353 CrossRef CAS PubMed; (b) M. S. Shchepinov and V. A. Korshun, Chem. Soc. Rev., 2003, 32, 170 RSC; (c) A. L. Kanibolotsky, I. F. Perepichkaz and P. G. Skabara, Chem. Soc. Rev., 2010, 39, 2695 RSC; (d) J. Lacour and V. Hebbe-Viton, Chem. Soc. Rev., 2003, 32, 373 RSC.
- (a) M. M. Ayhan, A. Singh, C. Hirel, A. G. Gürek, V. Ahsen, E. Jeanneau, I. Ledoux-Rak, J. Zyss, C. Andraud and Y. Bretonniere, J. Am. Chem. Soc., 2012, 134, 3655 CrossRef CAS PubMed; (b) M. M. Ayhan, A. Singh, E. Jeanneau, V. Ahsen, J. Zyss, I. Ledox-Rak, A. G. Gürek, C. Hirel, Y. Bretonniere and C. Andraud, Inorg. Chem., 2014, 53, 4359 CrossRef CAS PubMed; (c) W. Cao, K. Wang, I. Ledoux-Rak and J. Z. Jiang, Inorg. Chem. Front., 2016, 3, 1146 RSC.
- (a) C. G. Liu, W. Guan, P. Song, L. K. Yan and Z. M. Su, Inorg. Chem., 2009, 48, 6548 CrossRef CAS PubMed; (b) H. T. Uyeda, Y. Z. Zhao, K. Wostyn, I. Asselberghs, K. Clays, A. Persoons and M. J. Therien, J. Am. Chem. Soc., 2002, 124, 13806 CrossRef CAS PubMed; (c) T. G. Zhang, Y. X. Zhao, I. Asselberghs, A. Persoons, K. Clays and M. J. Therien, J. Am. Chem. Soc., 2005, 127, 9710 CrossRef CAS PubMed; (d) T. Ishizuka, L. E. Sinks, K. Song, S. T. Hung, A. Nayak, K. Clays and M. J. Therien, J. Am. Chem. Soc., 2011, 133, 2884 CrossRef CAS PubMed; (e) L. Karki, F. W. Vance, J. T. Hupp, S. M. Lecours and M. J. Therien, J. Am. Chem. Soc., 1998, 120, 2606 CrossRef CAS; (f) N. Jiang, G. Zuber, S. Keinan, A. Nayak, W. T. Yang and M. J. Therien, J. Phys. Chem. C, 2012, 116, 9724 CrossRef CAS; (g) K. D. Mey, J. Perez-Moreno, J. E. Reeve, I. Lopez-Duarte, I. Boczarow, H. L. Aanderson and K. Clays, J. Phys. Chem. C, 2012, 116, 13781 CrossRef CAS.
- (a) M. Yeung, A. C. H. Ng, M. G. B. Drew, E. Vorpagel, E. M. Breitung, R. J. Mcmahon and D. K. P. Ng, J. Org. Chem., 1998, 63, 7143 CrossRef CAS PubMed; (b) M. Pizzotti, F. Tessore, A. O. Biroli and R. Ugo, J. Phys. Chem. C, 2009, 113, 11131 CrossRef CAS; (c) P. C. Ray, P. Bonifassi and J. Leszczynski, J. Phys. Chem. A, 2008, 112, 2870 CrossRef CAS PubMed; (d) P. C. Ray and Z. Saumudeen, J. Phys. Chem. A, 2006, 110, 12342 CrossRef CAS PubMed.
- M. Quintiliani, J. Pérez-Moreno, I. Asselberghs, P. Vázquez, K. Clays and T. Torres, J. Phys. Chem. B, 2010, 114, 6309 CrossRef CAS PubMed.
- (a) T. Verbiest, K. Clays and V. Rodriguez, Second Order Nonlinear Optical Characterization Techniques: An Introduction, CRC Press, Taylor & Francis Group, Boca Raton, USA, 2009, ch. 1–2, pp. 1–61 Search PubMed; (b) R. P. Feynman, R. B. Leighton and M. Sands, Resonance, in Feynman Lectures on Physics, World Publishing Company, Beijing, China, 2011, vol. 1, ch. 23 Search PubMed; (c) R. W. Boyd, Nonlinear Optics, Elsevier Press, San Diego, USA, 3rd edn, 2008 Search PubMed.
- D. Qi and J. Jiang, ChemPhysChem, 2015, 16, 1889 CrossRef CAS PubMed.
- (a) X. Hu, D. Xiao, S. Keinan, L. Asselberghs, M. J. Therien, K. Clays, W. Yang and D. N. Beratan, J. Phys. Chem. C, 2010, 114, 2349 CrossRef CAS; (b) J. E. Reeve, H. A. Collins, K. De Mey, M. M. Kohl, K. J. Thorley, O. Paulsen, K. Clays and H. L. Anderson, J. Am. Chem. Soc., 2009, 131, 2758 CrossRef CAS PubMed; (c) M. J. Therien, Nature, 2009, 458, 716 CrossRef CAS PubMed.
- T. Ishizuka, L. E. Sinks, K. Song, S. T. Hung, A. Nayak, K. Clays and M. J. Therien, J. Am. Chem. Soc., 2011, 133, 2884 CrossRef CAS PubMed.
- (a) Y. Bian, Y. Zhang, Z. Ou and J. Jiang, Chemistry of Sandwich Tetrapyrrol Rare Earth Complex, in Handbook of Porphyrin Science, World Scientific Press, Singapore, 2011, vol. 14, ch. 64, pp. 249–460 Search PubMed; (b) J. Jiang and D. K. P. Ng, Acc. Chem. Res., 2009, 42, 79 CrossRef CAS PubMed; (c) W. Cao, Y. Zhang, H. Wang, K. Wang and J. Jiang, RSC Adv., 2015, 5, 17732 RSC; (d) H. Zhang, R. Wang, P. Zhu, Z. Lai, J. Han, C. F. Choi, D. K. P. Ng, X. Cui, C. Ma and J. Jiang, Inorg. Chem., 2004, 43, 4740 CrossRef CAS PubMed; (e) R. Wang, R. Li, Y. Bian, C. F. Choi, D. K. P. Ng, J. Dou, D. Wang, P. Zhu, C. Ma, R. D. Hartnell, D. P. Arnold and J. Jiang, Chem.–Eur. J., 2005, 11, 7351 CrossRef CAS PubMed; (f) R. Wang, Y. Li, R. Li, D. Y. Y. Cheng, P. Zhu, D. K. P. Ng, M. Bao, X. Cui, N. Kobayashi and J. Jiang, Inorg. Chem., 2005, 44, 2114 CrossRef CAS PubMed; (g) Y. Bian, R. Wang, J. Jiang, C. H. Lee, J. Wang and D. K. P. Ng, Chem. Commun., 2003, 1194 RSC; (h) J. Jiang, K. Kasuga and D. P. Arnold, Sandwich-type phthalocyaninato and porphyrinato metal complexes, in Supramolecular Photosensitive and Electroactive Materials, ed. H. S. Nalwa, Academic Press, New York, USA, 2001, ch. 2, pp. 113–210 Search PubMed; (i) D. K. P. Ng and J. Jiang, Chem. Soc. Rev., 1997, 26, 433 RSC.
- (a) J. P. Collman, J. L. Kendall, J. L. Chen, K. A. Collins and J. C. Marchon, Inorg. Chem., 2000, 39, 1661 CrossRef CAS PubMed; (b) N. Pan, Y. Bian, M. Yokoyama, R. Li, T. Fukuda, S. Neya, J. Jiang and N. Kobayashi, Eur. J. Inorg. Chem., 2008, 35, 5519 CrossRef.
- I. Bertini, A. Coutsolelos, A. Dikiy, C. Luchinat, G. A. Spyroulias and A. Troganis, Inorg. Chem., 1996, 35, 6308 CrossRef CAS.
- (a) P. D. Maker, Phys. Rev. A, 1970, 1, 923 CrossRef CAS; (b) K. Clays and A. Persoons, Phys. Rev. Lett., 1991, 66, 2980 CrossRef CAS PubMed.
- B. Liu, W. Zhu, Y. Wang, W. Wu, X. Li, B. Chen, Y. Long and Y. Xie, J. Mater. Chem., 2012, 22, 7434 RSC.
- M. G. Vivas, D. L. Silva, L. D. Boni, Y. Bretonniere, C. Andraud, F. Laibe-Darbour, J. C. Mulatier, R. Zalesny, W. Bartkowiak, S. Canuto and C. R. Mendonca, J. Phys. Chem. B, 2012, 116, 14677 CrossRef CAS PubMed.
- J. D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615 RSC.
- (a) R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS; (b) T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, J. Comput. Chem., 1983, 4, 294 CrossRef CAS; (c) M. Dolg, U. Wedig, H. Stoll and H. Preu, J. Chem. Phys., 1987, 86, 866 CrossRef CAS.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498 CrossRef CAS PubMed.
- (a) T. Lu and F. J. Chen, Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed; (b) T. Lu, Multiwfn 3.2, Uinversity of Science and Technology Beijing, Beijing, 2014 Search PubMed.
- M. J. Frisch, et al., Gaussian 09, revision D.01, Gaussian, Inc., Pittsburgh PA, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Computational details. Validity of the present calculating level of ωB97XD/6-311+G(d)/MWB28. Mass spectrum of HTb(DADAPor)2. The 1H NMR spectrum of HTb(DADAPor)2. The IR spectra of H2DADAPor and HTb(DADAPor)2. Mass spectroscopic and elemental analysis data for H2DADAPor and HTb(DADAPor)2. Electronic absorption and fluorescence spectra data for H2DADAPor and HTb(DADAPor)2 at the concentration of 2.0 × 10−6 M in CHCl3. See DOI: 10.1039/c7ra03033a |
| This journal is © The Royal Society of Chemistry 2017 |