Open Access Article
Open Access ArticleMethod for electroless nickel plating on the surface of CaCO3 powders
Junjun Huang acde and
Zhenming Chen*ab
acde and
Zhenming Chen*ab
aDepartment of Chemical and Materials Engineering, Hefei University, Hefei City, 230601, P. R. China. E-mail: xinya-gui@163.com; Fax: +86 774 5271906; Tel: +86 774 5271906
bCollege of Chemistry and Bioengineering, Hezhou University, Hezhou City, 542899, P. R. China
cKey Laboratory of Materials for Energy Conversion, Chinese Academy of Sciences, Hefei City, 230601, P. R. China
dHefei Lucky & Technology Industry Co. Ltd, Hefei City, 230041, P. R. China
eDepartment of Polymer Science and Engineering, Hefei University of Technology, Hefei City, 230009, P. R. China
First published on 12th May 2017
Abstract
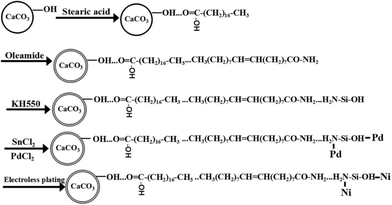
In this work, electroless nickel plating on the surface of CaCO3 powders successively modified with stearic acid, oleamide and 3-amino-propyltriethoxysilane in that order was developed. Hydrophobic and hydrophilic coatings were deposited onto the surface of the CaCO3 powders in that order. On the one hand, the hydrophobic chains coated onto the surface of the modified powders could effectively protect the powders from chemical reaction with acidic solutions (the sensitizing solution and the activating solution). On the other hand, active groups formed on the surface of the hydrophilic coating could adsorb a palladium catalyst for electroless nickel plating. It was observed that Ni–P particles were deposited on the surface of the modified CaCO3 powders after electroless plating. The size of the particles was less than 100 nm. The mechanical bonds between the modified powders and the plated particles were strong, leading to good adhesion. The saturation magnetization of the plated powders was around 1.5 emu g−1. By changing the type of inorganic powder and plating bath, the established method can potentially be adopted for the plating of metals such as copper, cobalt, and nickel onto different types of powders.
1. Introduction
Metallized (such as Ni, Cu and Co) inorganic powders have attracted attention in recent years due to their numerous potential applications in fields such as conductive paints, electromagnetic shielding paints, and thermal conductive fillers.1–7 Electroless deposition is the preferred method for the metallization of powders.3–7 Electroless plating onto the surface of Al2O3,3 WC,4 Cr3C2,5 B4C (ref. 6) and ZrO2 (ref. 7) powders has been reported. In these studies, pretreatment, sensitization, activation, and then plating were the most commonly used steps in the electroless plating process.3–7 Pretreatment can enhance the interfacial adhesion between the metal nanoparticle catalysts and the substrates.8–10 Activated particles (such as Au, Ag and Pd) are adsorbed by the active groups when the pretreated powders are successively immersed in a sensitizing solution and then in an activating solution.8–10 The activated particles serve as seeds to catalyze for electroless plating onto the surface of the powders.8–10It is well known that the sensitizing solution and activating solution are usually acidic SnCl2 solution and acidic PdCl2 solution, respectively, where the pH is adjusted using HCl so as to avoid the formation of Sn(OH)Cl and Pd(OH)2,11–13 which would reduce the catalytic performance. This will facilitate the formation of Sn2+ and [PdCl4]2− in the acidic solutions. Only the Sn2+ and [PdCl4]2− ions in the sensitizing solution and activating solution are useful for electroless reactions.11–13 However, many carbonate powders (such as CaCO3 and MgCO3) easily react with acids, so these powders cannot be activated in acidic PdCl2 solution.
Calcifying organisms incorporate carbon directly from seawater into their skeletons in the form of inorganic minerals such as CaCO3, and many species of invertebrate have shells or skeletons made of CaCO3. Calcite or marble is crushed and graded to produce many kinds of technical natural CaCO3 with different fineness.14 CaCO3 powders are characterized by low cost, high whiteness and natural abundance.14 They are widely used in rubber, plastic, paint, and paper. Nickel-plated CaCO3 powders may help to expand the applications of conductive fillers and electromagnetic shields. Polymer-based composites will have good anti-static and electromagnetic shielding properties if a resin is added into the plated CaCO3 to reduce the resistivity. In addition, plated CaCO3 has wide applications in electronic slurries, conductive paints and other fields because of its good oxidation resistance, thermal stability and low cost. However, activation of CaCO3 in acidic PdCl2 solution and then electroless nickel plating onto the surface of CaCO3 is still not well studied. In this work, electroless nickel plating onto the surface of CaCO3 powders successively modified with stearic acid, oleamide and 3-amino-propyltriethoxysilane (KH550) in that order was developed. The structural properties and magnetic performance of the plated powders were investigated systematically using an LCR bridge, scanning electron microscopy (SEM) and X-ray diffraction (XRD).
2. Experimental
Commercial CaCO3 powders were purchased from Kelong Micro-powder Co. Ltd (Hezhou, China). The size of the particles was 800 mesh. The powders were dropped into a high-speed mill at 453 K for 30 min. Then, the high-speed mill was cooled to 373 K. Stearic acid (0.2 wt%) and oleamide (0.8 wt%) were added twice at intervals of 30 min in order, and stirring of the mixture was continued for another 20 min. After that, the covered powders were immersed in KH550 solution (40 wt%) at 60 °C for 5 min, and then rinsed again and dried in an oven at 80 °C for 60 min. The modified CaCO3 powders were sensitized with SnCl2 solution (adjusted to pH 1 using HCl) and activated with PdCl2 solution (adjusted to pH 2 using HCl). Then the powders were cleaned with deionized water. After that, the powders were immersed in an electroless nickel plating bath (Na3C6H5O7·2H2O 8 g L−1, NiSO4·7H2O 5 g L−1, NH4Cl 18 g L−1, NaH2PO2·H2O 15 g L−1). The bath temperature was 333 K. The pH value was 10. The plating time was 3 min. Then, the plated powders were ultrasonically treated for 20 min.The surface morphology of the powders was observed by SEM (JEOL, JSM-5600LV). The chemical structures of the samples were measured by XRD (Rigaku D/max-2550V) and X-ray photoelectron spectroscopy (XPS, Shimadzu, AXIS Ultra DLD). Information on the surface functionalities of the powders was obtained by Fourier transform infrared spectroscopy (FT-IR, Thermo, Nicolet 6700). A links systems energy dispersive spectrometer (EDS) was used for elemental analysis. The magnetic performance of the plated powders was investigated using an LCR bridge (JEOL, HP4284A).
3. Results and discussion
Fig. 1 shows the FT-IR spectra of the pristine CaCO3 powders (a), and the powders modified with stearic acid and oleamide (b), and then KH550 (c) in that order. The peaks at about 876 cm−1, 1040 cm−1, 1430 cm−1, 1454 cm−1 and ∼3000 cm−1 are due to the (C–O3)2−, C–N, C–O, N–H2 and O–H characteristic peaks, respectively.11,15,16 As shown in Fig. 1(b), the peak at ∼3000 cm−1 disappeared and a peak at 1454 cm−1 appeared.11,15,16 These results indicated that amine and hydrophobic groups were attached onto the surface of the CaCO3 powders modified with stearic acid and oleamide. The hydrophobic chains could effectively protect the CaCO3 powders from moisture and ion penetration. As shown in Fig. 1(c), the relative intensity of the peak at 1454 cm−1 increased and a peak at ∼3000 cm−1 appeared. These results indicated that more amine groups were attached onto the surface of the powders. In addition, the surface of the powders became hydrophilic, which is helpful for the dispersion of the modified powders in the sensitizing solution, activating solution and plating solution.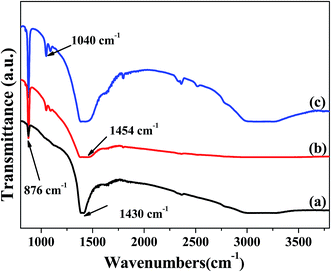 | ||
| Fig. 1 FT-IR spectra of (a) the pristine CaCO3 powders, and the powders modified with (b) stearic acid and oleamide, and then (c) KH550 in that order. | ||
Fig. 2 shows the XPS spectra of the (a) pristine CaCO3 powders, (b) modified powders, (c) sensitized powders, (d) activated powders and (e) plated powders. The peaks at about 286 eV, 336.3 eV, 398.8 eV, 439 eV, 486 eV, 534 eV, 649 eV, 728 eV and 795 eV are due to C 1s, Ca 2p3/2, Pd 3d, N 1s, Ca 2s, Sn 3d, O 1s, Ni, and Sn 3p, respectively.17,18 The peak positions of Ca 2p3/2 and Pd 3d are very close. As shown in Fig. 2(b), the characteristic peak of nitrogen was detected, which indicated that amine groups were formed on the surface of the modified CaCO3 powders. Meanwhile, an Sn 3d signal was detected in the spectrum, as shown in Fig. 2(c). This indicated that the surface structure of the modified powders facilitated the adsorption of tin ions. Fig. 3 shows the deconvoluted Pd 3d XPS spectra of the (a) modified CaCO3 powders, (b) sensitized powders and (c) activated powders. The characteristic peak of palladium was only detected in the XPS spectrum of the activated powders. As shown in Fig. 3(c), the peak at about 336.3 eV was assigned to Pd 3d, which is below the standard value of the Pd 3d peak position (338.4 eV).19,20 These results indicated that the active groups adsorbed Pd atoms via chelation, which reduces the electron density around the Pd atoms.19–21 It was indicated that the surface structure of the modified powders facilitated the chemisorption of palladium ions. The chemisorbed Pd atoms were used as the catalyst for electroless plating,8,18,19 and as shown in Fig. 2(e), an Ni signal was detected in the spectrum. The chemical reaction process can be summarized as follows:15
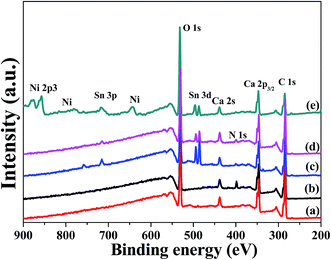 | ||
| Fig. 2 XPS spectra of the (a) pristine CaCO3 powders, (b) modified powders, (c) sensitized powders, (d) activated powders and (e) plated powders. | ||
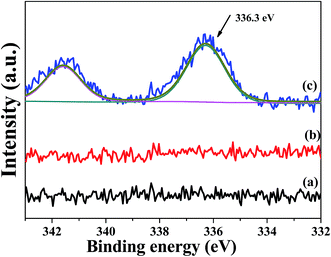 | ||
| Fig. 3 Pd XPS spectra of the (a) surface modified CaCO3 powders, (b) sensitized powders and (c) activated powders. | ||
Sodium hypophosphite cannot reduce Ni2+ directly. The Pd atoms can adsorb sodium H2PO2−, H+ and Ni2+ onto the surface of the powders. The reduced Ni can also act as the medium, which causes Ni2+ to be continuously deposited.16
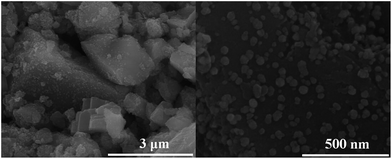
Fig. 4 shows SEM images of the (a) pristine CaCO3 powders, and (b) and (c) plated powders, and (d) EDS of the plated powders. As shown in Fig. 4(a) and (b), it was found that the surface of the pristine CaCO3 powders appeared as corrugations. The rough surface of the powders could provide a large area for mechanical bonds between the powders and the plated coating, resulting in good adhesion. It was also found that well-distributed fine particles were formed on the surface of the modified CaCO3 powders after electroless plating, which resulted from the aggregation of the deposited nickel atoms to form agglomerated nickel particles, the sizes of which are all below 100 nm. Fig. 4(d) shows the chemical compositions of the plated powders measured using EDS. The signals of nickel, phosphorous, chlorine and oxygen were found. It was suggested that Ni–P particles were deposited on the surface of the modified CaCO3 powders after electroless plating.
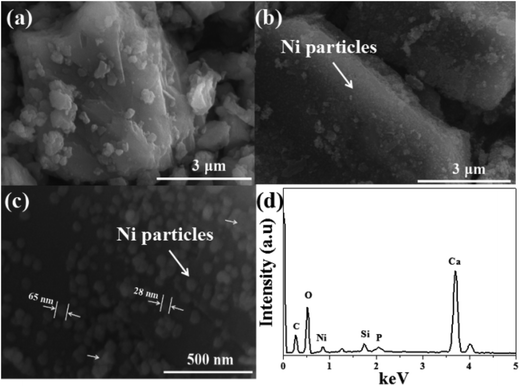 | ||
| Fig. 4 SEM images of the (a) pristine CaCO3 powders, and (b) and (c) plated powders, and (d) EDS of the plated powders. | ||
Fig. 5 shows a schematic diagram of electroless nickel plating onto the surface of the CaCO3 powders. As shown in Fig. 5, the CaCO3 powders and modifying macromolecules were bound together by hydrogen bonds and intermolecular forces. On the one hand, the surface energy and the charge of the powders can be significantly decreased by adding stearic acid. This macromolecule improves the dispersion of the powders in dry conditions. Hydrophobic and hydrophilic coatings were sequentially deposited on the surface of the CaCO3 powders in that order. On the other hand, the surface of the powders was insulated by organic layers and organic–inorganic materials with core–shell structures were formed. The hydrophobic chains could effectively protect the powders from moisture and ion penetration. The surface of the powders was hydrophobic, which could prevent acid solutions (the sensitizing solution and activating solution) from touching the powders, such that H+ does not chemically react with the powders. Then, amine groups and oxhydryl groups were grafted onto the hydrophobic coating when the powders were further modified with hydrolyzed KH550. At the same time, the surface of the powders became hydrophilic. The surface active groups were expected to be used as the scaffold for chemisorption of Pd2+ via their ion exchanging or coordinating behaviors. The adsorbed Pd atoms were used as a catalyst for electroless plating onto the surface of the CaCO3 powders. Ni–P particles were deposited on the surface of the modified CaCO3 powders after electroless plating.
Fig. 6 shows XRD patterns of the (a) pristine CaCO3 powders, (b) plated powders and (c) plated powders after ultrasonic treatment. The characteristic peaks at 22°, 29°, 35°, 39°, 42°, 47°, 48° and 57° are attributed to CaCO3.21–24 In addition, the peak at about 45° belongs to nickel [111], as shown in Fig. 6(b). It was observed that nickel crystallites were plated on the surface of the modified CaCO3 powders. As shown in Fig. 6(c), the characteristic peak of nickel crystallites was also detected in the plated sample after ultrasonic treatment. Fig. 7 shows SEM images of the plated powders after ultrasonic treatment. It was found that nickel particles were also attached to the surface of the ultrasound-treated powders. The amine groups adsorbed Pd atoms and Ni atoms. The nickel particles are connected to the substrates through Pd–N coordinate bonds. Accordingly, the fracture energy per unit area of the interface is the Pd–N bond energy, which is 2.12 J m−3.23,25 The results indicated that the mechanical bonds between the modified CaCO3 powders and nickel particles were strong, leading to good adhesion.
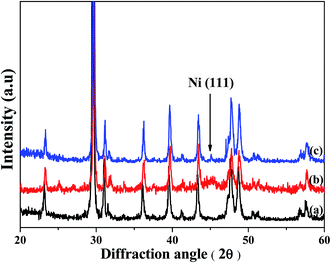 | ||
| Fig. 6 XRD patterns of the (a) pristine CaCO3 powders, (b) plated powders and (c) plated powders after ultrasonic treatment. | ||
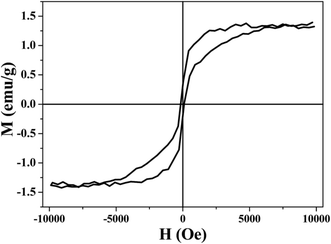
Fig. 8 shows the magnetic hysteresis loops of the plated CaCO3 powders. As shown in Fig. 8, the plated powders have a certain magnetism. The saturation magnetization of the plated CaCO3 powders was around 1.5 emu g−1. It is well known that CaCO3 powders are not magnetic, so the presence of magnetism suggested that nickel particles were deposited on the surface of the modified CaCO3 after electroless nickel plating, which will be useful for future applications.
4. Conclusions
In this work, electroless nickel plating on the surface of CaCO3 powders successively modified with stearic acid, oleamide and KH550 in that order was developed. It was found that hydrophobic chains were coated on the surface of the stearic acid and oleamide-modified powders, which could effectively protect the powders from chemical reaction with acid solutions (the sensitizing solution and activating solution). At the same time, active groups were formed on the surface of the hydrophobic coating after immersing in KH550 solution, which could adsorb palladium catalysts for electroless nickel plating. Ni–P particles were deposited on the surface of the modified CaCO3 powders after electroless plating. The size of the particles was less than 100 nm. The mechanical bonds between the modified powders and particles were strong, leading to good adhesion. The saturation magnetization of the plated powders was around 1.5 emu g−1.Acknowledgements
The authors would like to thank the General Project of Natural Science in Colleges and Universities in Anhui Province (Grant No. KJ2015B1105905), and the Opening Project of CAS Key Laboratory of Materials for Energy Conversion (Grant No. KF2016001).References
- C. Li, P. Zhang and Z. Jiang, Effect of nano Cu coating on porous Si prepared by acid etching Al–Si alloy powder, Electrochim. Acta, 2015, 161, 408–412 CrossRef CAS.
- J. Jiang, H. Chen and L. Zhu, et al., Effect of Heat Treatment on Structures and Mechanical Properties of Electroless Ni–P–GO Composite Coatings, RSC Adv., 2016, 6, 109001–109008 RSC.
- G. P. Ling and Y. Li, Influencing factors on the uniformity of copper coated nano-Al2O3 powders prepared by electroless plating, Mater. Lett., 2005, 59, 1610–1613 CrossRef CAS.
- L. Luo, Y. Wu, J. Li and Y. Zheng, Preparation of nickel-coated tungsten carbide powders by room temperature ultrasonic-assisted electroless plating, Surf. Coat. Technol., 2011, 206, 1091–1095 CrossRef CAS.
- L. Luo, J. Yu and J. Luo, et al., Preparation and characterization of Ni-coated Cr3C2 powder by room temperature ultrasonic-assisted electroless plating, Ceram. Int., 2010, 36, 1989–1992 CrossRef CAS.
- J. P. Deepa, V. G. Resmi, T. P. D. Rajan, C. Pavithran and B. C. Pai, Studies on the influence of surface pre-treatments on electroless copper coating of boron carbide particles, Appl. Surf. Sci., 2011, 257, 7466–7474 CrossRef CAS.
- G. Wen, Z. X. Guo and C. K. L. Davies, Microstructural characterisation of electroless-nickel coatings on zirconia powder, Scr. Mater., 2000, 43, 307–311 CrossRef CAS.
- A. Azarniya, F. Salatin, M. R. Eskandaripoor and A. Rasooli, A kinetic study on the mechanism of hydrogen evolution in Ni–P coated titanium hydride powder, Adv. Powder Technol., 2014, 26, 259–266 CrossRef.
- Z. J. Song, J. L. Xie, P. H. Zhou, X. H. Wang, T. H. Liu and L. J. Deng, Toughened polymer composites with flake carbonyl iron powders and their electromagnetic/absorption properties, J. Alloys Compd., 2013, 551, 677–681 CrossRef CAS.
- S. Y. Tong, M. J. Tung, W. S. Ko, Y. T. Huang, Y. P. Wang, L. C. Wang and J. M. Wu, Effect of Ni fillers on microwave absorption and effective permeability of NiCuZn ferrite/Ni/polymer functional composites, J. Alloys Compd., 2013, 550, 39 CrossRef CAS.
- Z. P. Sun, J. J. Huang, L. B. Wang, X. C. Zhang, M. L. Li and B. Tang, Method for electroless nickel plating on poly(ethylene terephthalate) substrate modified with primer and self-assembled monolayer, J. Mater. Sci.: Mater. Electron., 2015, 26, 10132–10137 CrossRef CAS.
- I. Lee, P. T. H. And and M. F. Rubner, Selective Electroless Nickel Plating of Particle Arrays on Polyelectrolyte Multilayers, Chem. Mater., 2003, 15, 4583–4589 CrossRef CAS.
- Y. Liu, Y. Xu, Y. Zhu, Y. Liu, S. Zheng, Y. Liu, A. Langrock and C. Wang, Selenium@mesoporous carbon composite with superior lithium and sodium storage capacity, ACS Nano, 2013, 7, 3627–3634 CrossRef CAS PubMed.
- C. Viravaidya, M. Li and S. Mann, Microemulsion-based synthesis of stacked calcium carbonate (calcite) superstructures, Chem. Commun., 2004, 10, 2182 RSC.
- R. Herrera, X. Erdocia, R. Llano-Ponte and J. Labidi, Characterization of hydrothermally treated wood in relation to changes on its chemical composition and physical properties, J. Anal. Appl. Pyrolysis, 2014, 107, 256–266 CrossRef CAS.
- Q. Zhou, H. Chen and Y. Wang, Region-selective electroless gold plating on polycarbonate sheets by UV-patterning in combination with silver activating, Electrochim. Acta, 2010, 55, 2542–2549 CrossRef CAS.
- J. L. Cao, Z. K. Wua, J. Yang, S. Y. Lia, H. X. Tang and G. Y. Xie, Site-selective electroless plating of copper on a poly(ethylene terephthalate) surface modified with a self-assembled monolayer, Colloids Surf., A, 2012, 415, 374–379 CrossRef CAS.
- J. Y. Choi, T. J. Kim and S. Lee, et al., Drop-on-demand printing of conductive ink by electrostatic field induced inkjet head, Appl. Phys. Lett., 2008, 93, 193508 CrossRef.
- V. Dimitrov and T. Komatsu, Classification of Simple Oxides: A Polarizability Approach, J. Solid State Chem., 2002, 163, 100–112 CrossRef CAS.
- S. L. Ma, B. Xu, G. Z. Wu, Y. F. Wang, F. Ma, D. Y. Ma, K. W. Xu and T. Bell, Microstructure and mechanical properties of SiCN hard films deposited by an arc enhanced magnetic sputtering hybrid system, Surf. Coat. Technol., 2008, 202, 5379–5382 CrossRef CAS.
- Z. P. Sun, J. J. Huang, Q. Liu, M. Gao, M. Y. Li, F. Zhao, W. Cheng and B. Tang, Effects of temperature on Ni coating on poly(ethylene terephthalate) substrate modified with primer, J. Mater. Sci.: Mater. Electron., 2016, 27, 5892–5898 CrossRef CAS.
- R. L. Blaine and H. E. Kissinger, Homer Kissinger and the Kissinger equation, Thermochim. Acta, 2012, 14, 1–6 CrossRef.
- M. L. Wang, Z. G. Yang and C. Zhang, et al., Trans. Mater. Heat Treat., 2015, 7, 183–189 Search PubMed.
- J. Georgieva and S. Armyanov, Electroless deposition and some properties of Ni–Cu–P and Ni–Sn–P coatings, J. Solid State Electrochem., 2007, 11, 869–876 CrossRef CAS.
- H. E. Evans, Stress effects in high temperature oxidation of metals, Int. Mater. Rev., 1995, 40, 1–40 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |