Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
One-pot synthesis of cubic PtPdCu nanocages with enhanced electrocatalytic activity for reduction of H2O2
Liangliang Tian *ab,
Yanling Chenab,
Shenping Wuab,
Yanhua Caiab,
Hongdong Liuab,
Jin Zhangab,
Cong Yangab,
Gege Heab,
Wei Xiao
*ab,
Yanling Chenab,
Shenping Wuab,
Yanhua Caiab,
Hongdong Liuab,
Jin Zhangab,
Cong Yangab,
Gege Heab,
Wei Xiao *ab,
Lu Liab,
Li Linab and
Yue Chengab
*ab,
Lu Liab,
Li Linab and
Yue Chengab
aResearch Institute for New Materials Technology, Chongqing University of Arts and Sciences, Chongqing, PR China. E-mail: tianll07@163.com; showame@aliyun.com
bCo-innovation Center for Micro/Nano Optoelectronic Materials and Devices, PR China
First published on 6th July 2017
Abstract
Despite the high electrocatalytic activity of Pt, pure Pt electrocatalysts always suffer from high cost and poor poison resistance. In this work, a cubic PtPdCu nanocage (NC) trimetallic electrocatalyst was synthesized using cuprous oxide as a sacrificial template. Being employed as a H2O2 detection electrode, PtPdCu NCs exhibit higher sensitivity (562.83 μA mM−1 cm−2) than that of PdCu NCs (210.19 μA mM−1 cm−2), PtCu NCs (411.34 μA mM−1 cm−2) and commercial Pt black (16.94 μA mM−1 cm−2). Furthermore, a PtPdCu NCs electrode presents a working potential as low as 0.05 V. The excellent electrocatalytic activity can be attributed to the suitable hollow porous structure and synergistic electrocatalysis effect between Pt, Pd and Cu. It is believed that the trimetallic PtPdCu NCs electrocatalyst has potential applications in the design of H2O2 detection electrodes.
1. Introduction
Nowadays, it is still of great importance to develop H2O2 sensors due to their crucial applications in clinical medicine, biochemistry, pharmaceuticals, food safety and environmental monitoring.1,2 As a classical electrocatalyst, Pt nanoparticles can significantly decrease the overpotential of H2O2 during electrocatalytic processes.3,4 However, the rare reserves, high cost and poor poison resistance of single Pt dramatically limit its practical applications.Design of Pt-based bi- or trimetallic nanostructures is an effective route to reduce the consumption of Pt and improve its activity for multistep catalysis.5,6 Recent reports indicate that Pd alloying can promote the poison resistance and electrocatalytic activity of Pt.7,8 In the case of H2O2, Pd atoms close to Pt could take OHad on Pd sites and highly active Pt mainly functions as actives for H2O2, leading to a bifunctional electrocatalytic mechanism.9 On the other hand, alloying of noble metals with transition metals (Fe, Co, Ni, Sn, Cu, etc.) is another approach to enhance the electrocatalytic performance. Transition metals alloying not only saves noble metals but also causes a negative shift of d-band center position and rearrangement of the surface atoms, resulting in easy desorption of intermediate products and a high active surface.10 Thus, synthesis of transition metal alloyed PtPd is an efficient way to design high-efficiency electrocatalyst for H2O2.
In this paper, Cu alloyed cubic PtPd NCs were synthesized using Cu2O as sacrificial templates and employed to construct amperometric H2O2 sensor. The hollow porous architecture provides large surface area and abundant diffusion paths for analyte and electrolyte, which is benefit for electrocatalytic kinetics. As a detection electrode for H2O2, the PtPdCu NCs electrode presents excellent electrocatalytic activity in terms of high sensitivity and eminent anti-poisoning performance due to the synergistic electrocatalysis effect.
2. Materials and methods
2.1 Synthesis of cubic PtPdCu NCs
Cubic Cu2O was synthesized according to the previous report.11 10.0 mL NaOH solution (2 M) was dropped into the stirred CuCl2·2H2O (100 mL, 0.01 M) at 55 °C. 10.0 mL ascorbic acid (0.6 M) was added after 30 min. The brick-red products were centrifugated after 3 h, followed by drying in vacuum at 40 °C overnight.For the preparation of PtPdCu NCs, 10 mg as-prepared Cu2O was dispersed into 10 mL distilled water by ultrasonic for 15 min. Then a mixed solution containing 1 mL chloroplatinic acid (20 mM) and 0.6 mL disodium tetrachloropalladate (33 mM) was added (the mole ratio of Pt and Pd is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After reacting for 30 min, 1 mL NH3·H2O (1
1). After reacting for 30 min, 1 mL NH3·H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was injected to etch the residual Cu2O cores. The ultimate products were separated by mild centrifugation after 12 h and dried at 40 °C for 24 h. PtCu NCs and PdCu NCs samples were also synthesized under the same condition using chloroplatinic acid or disodium tetrachloropalladate only.
1) was injected to etch the residual Cu2O cores. The ultimate products were separated by mild centrifugation after 12 h and dried at 40 °C for 24 h. PtCu NCs and PdCu NCs samples were also synthesized under the same condition using chloroplatinic acid or disodium tetrachloropalladate only.
2.2 Electrochemical measurements
All electrochemical measurements were performed in 0.1 M phosphate buffer solution (PBS, pH = 7.0). A three-electrode configuration was applied with Ag/AgCl (saturated with KCl) and platinum disk as the reference and counter electrode, respectively. PtPdCu NCs, PtCu NCs, PdCu NCs and Pt black modified glassy carbon electrodes (GCE, Φ = 3 mm) were employed as the working electrodes. Typically, GCE was polished with 3 μm, 0.5 μm and 0.05 μm alumina powders, respectively. Then, 5 μL of suspension (1 mg mL−1 in 0.1 wt% Nafion solution) was cast onto the polished GCE and finally dried by infrared.2.3 Materials characterizations
The phase and structure of PtPdCu NCs were characterized by X-ray diffraction (XRD) performed on a Rigaku D/Max-2400 X-ray diffractometer using Cu Kα radiation (40 kV, 60 mA). The chemical state was determined by X-ray photoelectron spectroscopy ESCALAB 250Xi (XPS, USA) using 500 μm X-ray spot and all the XPS spectra were calibrated by C 1s line at 284.8 eV. The morphologies and microstructures of PtPdCu NCs were analyzed on Hitachi S-4800 field emission scanning electron microscope (FESEM) and FEI F20 high-resolution transmission electron microscope (HRTEM). Specific surface area and porosity measurements were conducted on a Belsort-max instrument using N2 adsorption–desorption isotherms.3. Results and discussions
3.1 Characterizations
The surface state and composition of the products were confirmed by XPS. As shown in Fig. 1a, Pt 4f and Cu 3p are overlapped between 65.5 eV and 83.5 eV. The peaks located at 70.8 eV and 73.9 eV correspond to Pt 4f7/2 and Pt 4f5/2, and the peaks at 75.2 eV and 77.3 eV can be related to Cu 3p3/2 and Cu 3p1/2, respectively, demonstrating the coexist of Pt and Cu in the products. The observed peaks at 335.1 eV and 340.5 eV in Fig. 1b correspond to 3d5/2 and 3d3/2 of Pd, respectively.12 In Fig. 1c, the peaks located at binding energy of 932.4 eV and 952.3 eV refer to Cu 2p3/2 and Cu 2p1/2, respectively, which is well consistent with pure Cu. According to XPS results, the atomic ratio of Pt, Pd and Cu is 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21. Although the same amounts of PtCl62− and Pd2+ were added, the atom content of Pd is slightly higher than Pt. The reactivity between Pd2+ and Cu2O is much higher than that between PtCl62− and Cu2O. Hence, a Pd-rich layer would preferentially form around Cu2O and hinder the diffusion of fresh reagent, resulting in higher Pd content in the products. In XRD pattern (Fig. 1d), all the reflection peaks can be indexed to (111), (200), and (220) diffractions of face-centered-cubic structure. No refection peaks of Cu2O are observed in all the XRD patterns, confirming the purity of products. The reflection peaks of PtPdCu display a slight positive shift compared to PtCu, however, a negative shift compared to PdCu, indicating the successful preparation of PtPdCu alloys.13 The analysis of XPS and XRD confirm the successful preparation of PtPdCu NCs.
21. Although the same amounts of PtCl62− and Pd2+ were added, the atom content of Pd is slightly higher than Pt. The reactivity between Pd2+ and Cu2O is much higher than that between PtCl62− and Cu2O. Hence, a Pd-rich layer would preferentially form around Cu2O and hinder the diffusion of fresh reagent, resulting in higher Pd content in the products. In XRD pattern (Fig. 1d), all the reflection peaks can be indexed to (111), (200), and (220) diffractions of face-centered-cubic structure. No refection peaks of Cu2O are observed in all the XRD patterns, confirming the purity of products. The reflection peaks of PtPdCu display a slight positive shift compared to PtCu, however, a negative shift compared to PdCu, indicating the successful preparation of PtPdCu alloys.13 The analysis of XPS and XRD confirm the successful preparation of PtPdCu NCs.
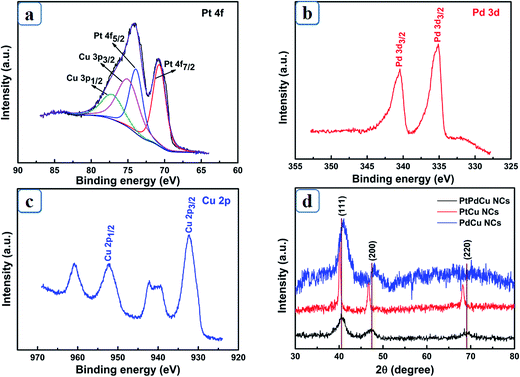 | ||
| Fig. 1 XPS spectra for the products. (a) Pt 4f; (b) Pd 3d; (c) Cu 2p; (d) XRD patterns of prepared PtPdCu, PtCu and PdCu. | ||
The difference between electrode potentials is considered to be the driving force for the reactions. The redox pair value of Cu2O/Cu (0.36 V) is much lower than that of PtCl62−/Pt (0.735 V) and Pd/Pd2+ (0.987 V). Thus, Cu2O crystals can be employed as reducing agent for PtCl62− and Pd2+. The formation mechanism of Pt and Pd can be explained as follows:13
| 2Cu2O + 4H+ + PtCl62− → Pt + 4Cu2+ + 6Cl− + 2H2O | (1) |
| Cu2O + 2H+ + Pd2+ → Pd + 2Cu2+ + H2O | (2) |
Cu atoms in the products can be ascribed to the disproportionation of Cu(I) in the presence of H+:
| Cu2O + 2H+ → Cu + Cu2+ + H2O | (3) |
Above reactions can simultaneously produce Pt, Pd and Cu atoms, resulting in the formation of PtPdCu alloys around Cu2O templates. Finally, Cu2O cores were removed by etching and PtPdCu NCs were successfully constructed.
Morphologies of the products were observed by SEM and TEM and the images were displayed in Fig. 2. As shown in Fig. 2a, the prepared Cu2O presents smooth cubic characteristics with an edge length about 500 nm. After reaction, cubic feature of Cu2O is exactly replicated by PtPdCu alloy (Fig. 2b) and the surface turns into rough and porous. The partly broken cube in Fig. 2b reveals the hollow cage-like structure of PtPdCu. The porous architecture affords amounts of paths for diffusion of analyte and desorption of intermediate products, which favours the electrocatalytic kinetics. Further insight into the details, PtPdCu NCs are constructed by aggregations of fine PtPdCu nanograins (Fig. 2c). TEM image of PtPdCu NCs in Fig. 2d provides convincing evidence for the cubic hollow structure of PtPdCu. A well-defined cubic PtPdCu NC was investigated in Fig. 2e and the shell of the cube is around 80 nm. As depicted in Fig. 2f, the grain size of PtPdCu NCs is about 4 nm. The spacing for marked adjacent lattice fringes is about 0.2218 nm, which is smaller than that of pure Pt (111) (0.2245 nm) and Pd (111) (0.2265 nm) but larger than pure Cu (111) (0.2088 nm), implying the formation of ternary PtPdCu alloy.
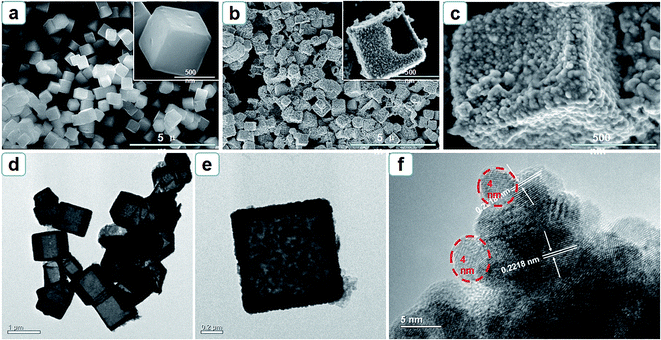 | ||
| Fig. 2 SEM images of (a) cubic Cu2O and (b, c) PtPdCu NCs; (d, e) TEM and (f) HRTEM images of PtPdCu NCs. | ||
For the characterizations of specific surface area and porosity, N2 adsorption–desorption isotherms and Barrett–Joyner–Halenda pore size distributions were measured. As shown in Fig. 3a, PtPdCu NCs possess a large specific surface area of 98.8 m2 g−1, which is much larger than that of PtCu NCs (61.9 m2 g−1) (Fig. 3b) and PdCu NCs (58.7 m2 g−1) (Fig. 3c). In addition, a larger pore volume of 0.16 cm3 g−1 is observed for PtPdCu NCs compared to PtCu NCs (0.092 cm3 g−1) and PdCu NCs (0.088 cm3 g−1). PtPdCu NCs, PtCu NCs and PdCu NCs present similar pore size distributions with mean pore diameters of 5.2 nm, 5.7 nm and 6.3 nm, respectively. On the basis of above discussions, PtPdCu NCs could offer more contact area and diffusion channels for electrolyte, which is beneficial for electrocatalysis.
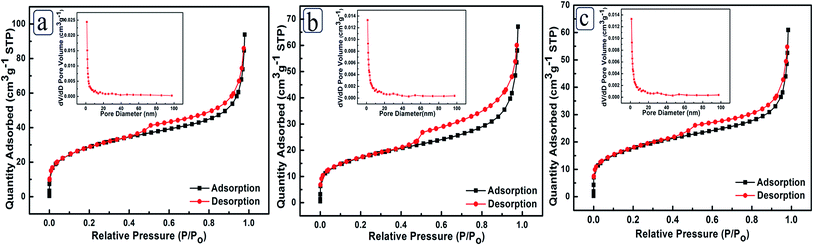 | ||
| Fig. 3 N2 adsorption–desorption isotherms of (a) PtPdCu NCs, (b) PtCu NCs and (c) PdCu NCs. Insets are the corresponding pore size distributions, respectively. | ||
3.2 Electrochemical performance
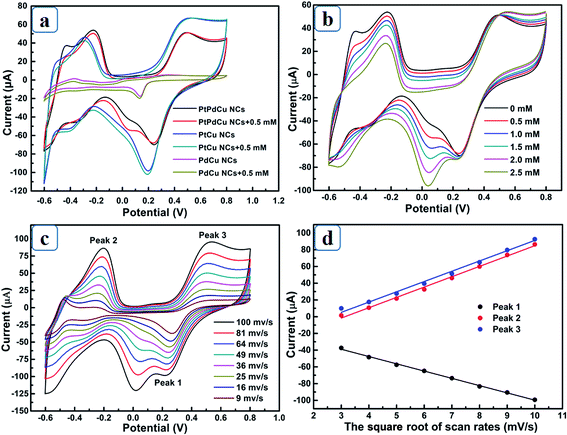
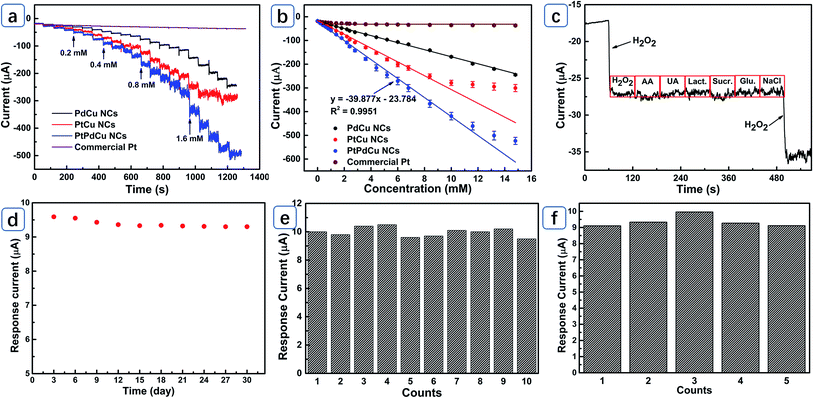
PtPdCu NCs were employed to detect H2O2 to evaluate its electrocatalytic activity. Fig. 4a displays the cyclic voltammetry curves (CVs) of different electrodes, largest current response is observed for PtPdCu NCs electrode at 0.05 V compared to PtCu NCs and PdCu NCs, demonstrating higher electrocatalytic activity towards electroreduction of H2O2. As can be seen from Fig. 4b, the peak current at 0.05 V linearly scales with the increase of H2O2 concentration, confirming potential applications for detect H2O2. CVs of PtPdCu NCs with different scan rates were recorded in Fig. 4c to estimate its kinetics process. Both the anodic peak current (peak 2 and 3) and cathodic peak current (peak 1) are proportional to the square root of scan rates (Fig. 4d), indicating a typical diffusion-controlled electrocatalytic process.14Typical amperometric responses of PtCu, PdCu, PtPdCu NCs and commercial Pt black electrodes at 0.05 V were recorded in Fig. 5a. PtCu NCs electrode presents higher current response than commercial Pt. The improved electrocatalytic activity is related to the hollow porous feature and the alloying of Cu atoms. The introduced Cu atoms not only result in a high-active Pt-rich surface in the outermost layer but also reduce the Pt–OHad bonding strength, accelerating the regeneration of Pt actives.8,10 Moreover, the PtCu NCs electrode displays broader linear range than commercial Pt, implying better anti-poisoning performance towards intermediate products. Obviously, PtCu NCs electrode shows higher current response than PdCu NCs electrode, indicating that Pt sites are the main electroreduction active sites for H2O2. The alloyed Pd atoms modulates the surface electronic structure of PtCu NCs and acts as promoting centers for the generation of OHad species, guaranteeing the activity of Pt active.15 PtPdCu NCs electrode displays larger response current compared to PtCu NCs, PdCu NCs and commercial Pt black electrodes, confirming the advantages of designed microstructure and synergistic electrocatalytic effect.16 From Fig. 5b, the response current of PtPdCu NCs electrode linearly increases with the H2O2 concentration between 1.5 μM (limit of detection) and 11.6 mM with a sensitivity of 562.83 μA mM−1 cm−2, which is higher than that of PdCu NCs (210.19 μA mM−1 cm−2), PtCu NCs (411.34 μA mM−1 cm−2) and commercial Pt black (16.94 μA mM−1 cm−2). The sensing performance of PtPdCu NCs electrode was compared with other reported Pt-based electrodes in Table 1. It is found that PtPdCu NCs electrode presents low overpotential, high sensitivity and wide linear range towards detection of H2O2. The excellent electrocatalytic activity can be essentially attributed to large surface area and highly porous architecture of PtPdCu NCs electrode, which is benefit for diffusion of analyte and desorption of intermediate products.
| Electrode | Potential (V) | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | Reference |
|---|---|---|---|---|
| a Nanoparticles.b MWCNTs = multi-wall carbon nanotubes; NDs = nanohybrids.c PPy = polypyrrole; GCE = glassy carbon electrode.d Reduced graphene oxide.e Poly(diallydimethylammonium chloride). | ||||
| PtPdCu NCs | 0.05 (Ag/AgCl) | 562.8 | 0.0015–11.6 | This work |
| 3,4-Ethylenedioxythiophene-Pt NPsa | −0.55 (Ag/AgCl) | 19.3 | 0.0016–6 | 17 |
| Pt–MnOx | 0.35(Ag/AgCl) | 123 | 0.002–4 | 18 |
| Pt–SnO2@C | 0.5 (—) | 241 | 0.001–0.17 | 19 |
| MWCNTs/Pt NHsb | 0 (Ag/AgCl) | 205.8 | 0.01–2 | 20 |
| Pt/PPy/GCEc | −0.1 ((Ag/AgCl)) | 80.4 | 1–8 | 21 |
| Pt0.5Au0.5@C | 0.3 (Ag/AgCl) | 210.3 | 0.007–6.5 | 22 |
| Nafion/Pt NPsa/RGOd | −0.1 (Ag/AgCl) | 132.8 | 0.005–3 | 23 |
| Pt flowers/gold slice | −0.2 (Ag/AgCl) | 314 | 0.1–0.9 | 24 |
| PDDAe/Pt | 0.6 (Ag/AgCl) | 500 | 0.000042–0.16 | 25 |
Selectivity of PtPdCu NCs electrode was estimated by adding one-tenth ascorbic acid (AA), uric acid (UA), lactose (Lact.), sucrose (Sucr.), glucose (Glu.) and NaCl species during H2O2 detection at 0.05 V (Fig. 5c). PtPdCu NCs electrode only present mild interference towards AA (4.5%) and sucrose (5.4%). In addition, the second addition of 0.2 mM H2O2 retains about 93% of its original response, demonstrating excellent selectivity and anti-poisoning performance. Long term stability of the PtPdCu NCs electrode was tested by recording the current responses for 0.2 mM H2O2 for 30 days (Fig. 5d). PtPdCu NCs electrode still retains 97% of its original current response, revealing excellent stability. The reproducibility of PtPdCu NCs electrode was tested by measuring its response current towards 0.2 mM H2O2 for ten times and the relative standard deviation (RSD) for current response is about 3.34% (Fig. 5e). In addition, the current responses towards 0.2 mM H2O2 of five different electrodes were recorded and the RSD for current responses is about 3.63% (Fig. 5f). PtPdCu NCs electrode possesses low applied potential, high sensitivity, reliable stability and excellent reproducibility, indicating potential practicability in nonenzymatic H2O2 electrochemical sensor.
4. Conclusions
In conclusions, cubic PtPdCu NCs synthesized by sacrificial template method were applied to detect H2O2. The electrode exhibits excellent electrocatalytic activity towards H2O2 in terms of low applied potential, high sensitivity, reliable stability and excellent reproductivity. It is believed that the hollow porous PtPdCu NCs have potential applications in design of electrochemical sensors.Acknowledgements
This study is supported by National Natural Science Foundation of China (21403020, 51503022), Basic and Frontier Research Program of Chongqing Municipality (cstc2016jcyjAX0014, cstc2015jcyjA50036, CSTC2015JCYJBX0126, CSTC2016SHMSZX20001, cstc2014jcyjA50033), Scientific and Technological Research Program of Chongqing Municipal Education Commission (KJ1601133, KJ1601104, KJ1711285).References
- W. Lian, L. Wang, Y. Song, H. Yuan, S. Zhao, P. Li and L. Chen, Electrochim. Acta, 2009, 54, 4334–4339 CrossRef CAS.
- M. Q. Wang, Y. Zhang and S. J. Bao, Electrochim. Acta, 2016, 190, 365–370 CrossRef CAS.
- K. Huang, Y. Li and Y. Xing, Electrochim. Acta, 2013, 103, 44–49 CrossRef CAS.
- L. L. Tian, X. H. Zhong, W. P. Hu, B. T. Liu and Y. Li, Nanoscale Res. Lett., 2014, 9, 68–72 CrossRef PubMed.
- D. S. Wang and Y. D. Li, Adv. Mater., 2011, 23, 1044–1060 CrossRef CAS PubMed.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 13934–13937 CrossRef CAS PubMed.
- X. Q. Huang, Y. J. Li, Y. J. Li, H. L. Zhou, X. F. Duan and Y. Huang, Nano Lett., 2012, 12, 4265–4270 CrossRef CAS PubMed.
- X. H. Niu, C. Chen, H. L. Zhao, Y. Chai and M. B. Lan, Biosens. Bioelectron., 2012, 36, 262–266 CrossRef CAS PubMed.
- Y. B. Zhou, G. Yu, F. F. Chang, B. N. Hu and C. J. Zhong, Anal. Chim. Acta, 2012, 757, 56–62 CrossRef CAS PubMed.
- V. R. Stamenkovic, B. Fowler, B. S. Mun, G. F. Wang, P. N. Ross, C. A. Lucas and N. M. Markovic, Science, 2007, 315, 493–497 CrossRef CAS PubMed.
- D. F. Zhang, H. Zhang, L. Guo, K. Zheng, X. D. Han and Z. Zhang, J. Mater. Chem., 2009, 19, 5220–5225 RSC.
- H. M. Duan and C. X. Xu, Electrochim. Acta, 2015, 152, 417–424 CrossRef CAS.
- F. Hong, S. D. Sun, H. J. You, S. C. Yang, J. X. Fang, S. W. Guo, Z. M. Yang, B. J. Ding and X. P. Song, Cryst. Growth Des., 2011, 11, 3694–3697 CAS.
- M. R. Guascito, E. Filippo, C. Malitesta, D. Manno, A. Serra and A. Turco, Biosens. Bioelectron., 2008, 24, 1057–1063 CrossRef CAS PubMed.
- A. X. Yin, X. Q. Min, W. Zhu, W. C. Liu, Y. W. Zhang and C. H. Yan, Chem.–Eur. J., 2012, 18, 777–782 CrossRef CAS PubMed.
- J. Lan, K. Wang, Q. Yuan and X. Wang, Mater. Chem. Front., 2017, 1, 1217–1222 RSC.
- L. C. Chang, H. N. Wu, C. Y. Lin, Y. H. Lai, C. W. Hu and K. C. Ho, Nanoscale Res. Lett., 2012, 7, 319–326 CrossRef PubMed.
- H. Kivrak, O. Alal and D. Atbas, Electrochim. Acta, 2015, 176, 497–503 CrossRef CAS.
- H. Lu, S. Yu and F. Yang, Colloids Surf., B, 2012, 101, 106–110 CrossRef PubMed.
- Z. Miao, D. Zhang and Q. Chen, Materials, 2014, 7, 2945–2955 CrossRef CAS.
- X. J. Bian, X. F. Lu, E. Jin, L. R. Kong, W. J. Zhang and C. Wang, Talanta, 2010, 81, 813–818 CrossRef CAS PubMed.
- O. G. Sahin, Electrochim. Acta, 2015, 180, 873–878 CrossRef CAS.
- C. Zhang, H. Jiang, R. Ma, Y. Y. Zhang and Q. Chen, Ionics, 2017, 1–9, DOI:10.1007/s11581-016-1944-2.
- J. Wan, W. Wang and G. Yin, J. Cluster Sci., 2012, 23, 1061–1068 CrossRef CAS.
- P. Karam and L. I. Halaoui, Anal. Chem., 2008, 80, 5441–5448 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |