Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3D nanostructured WO3/BiVO4 heterojunction derived from Papilio paris for efficient water splitting†
Chao Yin ,
Shenmin Zhu
,
Shenmin Zhu * and
Di Zhang*
* and
Di Zhang*
State Key Laboratory of Metal Matrix Composites, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai, 200240, P. R. China. E-mail: smzhu@sjtu.edu.cn; zhangdi@sjtu.edu.cn; Fax: +86-21-34202749; Tel: +86-21-34203927
First published on 23rd May 2017
Abstract
We report on a novel butterfly wing-like WO3/BiVO4 heterojunction for photocatalytic water splitting, in which BiVO4 is the primary visible light-absorber and WO3 acts as an electron conductor. The heterojunction, which is prepared by a one-step sol–gel method, achieves high light absorption and charge separation efficiencies, even without a sacrificial agent, and produces a photocatalytic O2 evolution of 20 μmol h−1 mg−1 under visible light irradiation (λ > 420 nm) and an incident photon-to-current conversion efficiency of ∼10% at 380–450 nm, both at a potential of 1.23 V versus RHE. Compared to planar WO3/BiVO4 heterojunction, the 3D nanostructured WO3/BiVO4 heterojunction shows significantly improved photocatalytic performance due to the quasi-honeycomb structure inherited from the Papilio paris and the efficient separation of the photogenerated charge at the WO3/BiVO4 interface. Synthesis details are discussed, with heterojunction morphologies and structures characterized by field emission scanning electron microscopy and X-ray diffraction.
Introduction
Photocatalytic water splitting into H2 and O2 using semiconductors is a very attractive and desirable way to meet the global energy challenge and solve environmental issues.1,2 Although several semiconductor materials show photocatalytic activity, most of them have limited utility due to the high charge carrier recombination3 and wide band gaps, which make them only active under UV light irradiation, and UV light represents merely 4–5% of the solar spectrum energy lies.4 A range of attempts have been made to improve light absorption and charge separation efficiencies, including impurity doping,5–7 loading co-catalysts,8,9 and the introduction of nanoscale porosity.10,11 Moreover, photocatalytic performance has also been improved by the formation of heterojunctions, which couple a large band gap semiconductor with a small band gap semiconductor. Heterojunctions have a more negative conduction band (CB) level, and the CB electrons can be injected from the small band gap semiconductor into the large band gap semiconductor, promising that the heterojunction can be more efficient than a single semiconductor system.12Monoclinic BiVO4 (mBiVO4) has become the top performer among all metal oxide photocatalysts under visible light owing to its relatively small band-gap energy (2.4 eV).13 BiVO4 based heterostructures with other desirable semiconductors including ZnO,14 WO3,12,15–19 nanowires of Fe2O3 (ref. 20) and others21–24 have been shown to promote the separation of electron–hole pairs and anti-photocorrosion. Among the latest state-of-the-art BiVO4-based heterojunctions, the highest efficiency was achieved by a W doped WO3/BiVO4 core/shell nanowire photoanode, synthesized on an FTO without an added catalyst, which produced a photocurrent of 3.1 mA cm−2 under simulated sunlight and an incident photon-to-current conversion efficiency of ∼60% at 300–450 nm, both at a potential of 1.23 V versus RHE.12 However, the cumbersome process and difficulty in controlling the nanomorphology of the photoanode create obstacles in its practical use.
As a matter of fact, nature offers an astonishing variety of materials with exquisite 3D hierarchical structures, which cannot be easily synthesized using available nanotechnology to date. 3D hierarchical structures from nature often display an efficient light-harvesting capacity, particularly in the region of visible light, and the abundant biogenic elements endowed from biological systems can be preserved and incorporated into the final biotemplated materials to reduce the bandgap of photocatalysts,25 leading to a new way to obtain efficient photocatalysts. Recently, we reported that a C-doped BiVO4 photocatalyst with fine 3D hierarchical structures templated from Papilio paris butterfly wings fabricated by a novel one-step sol–gel and templating method enables simultaneous control of crystal phase, nanomorphology, and element carbon doping in a single process.26 The improved photocatalytic performance was attributed to the synergetic effect of the unique morphology and doping control. We have also reported that the sequence of ceramic WO3 butterfly wings was also inherited from Papilio paris.27 We verified that the quasi-honeycomb structure adapted from Papilio paris can help WO3 harvest visible light more efficiently and boost charge transport. Nevertheless, the efficiency of these photocatalysts is not sufficient due to the still limited charge separation efficiencies. Hence heterojunction nanoarchitectures coupled with the unique structures are expected to result in the highest photocatalytic activity.
In this study, we fabricate a novel 3D nanostructured WO3/BiVO4 heterojunction derived from Papilio paris for photocatalytic water splitting, in which BiVO4 is the primary visible light-absorber and WO3 acts as an electron conductor. The heterojunction, which is prepared by a one-step sol–gel method, achieves the highest light absorption and charge separation efficiencies among BiVO4-based powder composites, even without a sacrificial agent. In the following, we will describe the synthesis and characterization of the present WO3/BiVO4 heterojunction in detail and explain the origin of the improved photocatalytic performance.
Experimental
Materials
Butterfly wing biotemplates were purchased from Shanghai Natural Wild-Insect Kingdom Co., Ltd. Analytical grade reagents such as phosphotungstic acid 44-hydrate (H3PO40W12), bismuth nitrate (Bi(NO3)3·5H2O), ammonium metavanadate (NH4VO3), ethanol, acetic acid, nitric acid, and tetramethylammonium hydroxide (TMAH) were purchased from Sinopharm Reagent Chemical Co., Ltd.Synthesis
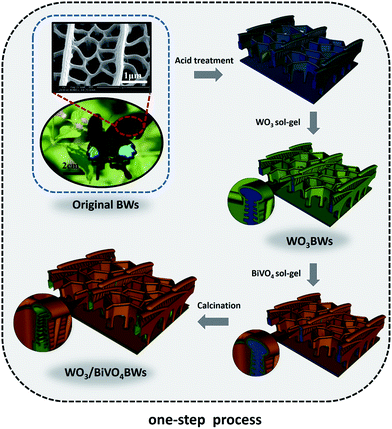
In a typical synthesis, first, Papilio paris butterfly wings, as biological templates, were air dried before use. The biological samples were pretreated by a mixture solution of acetic acid and ethanol for 6 h at room temperature and then washed with deionized water and air-dried for 24 h. The pretreated samples were carefully dipped into 20 wt% of phosphotungstic acid 44-hydrate (H3PO40W12) in ethanol solution and kept at 36 °C for 6 h, denoted as WO3BWs.27 Then, WO3/BiVO4 heterojunction was achieved by a one-step sol–gel method and a two-step sol–gel method. In the one-step sol–gel process, WO3BWs were washed with ethanol and dried overnight under vacuum at 60 °C and dipped into the BiVO4 sol–gel solution26 and kept at 36 °C for 12 h. Finally, the samples were calcined in air at 500 °C with a constant heating rate of 1 °C min−1 to format the crystal and remove the organic templates (WO3/BiVO4BWs). The synthesis process is illustrated in Scheme 1. For comparison, WO3/BiVO4BWs (two-step) were prepared by WO3BWs, which were first calcined in N2 atmosphere at 800 °C to crystallize the WO3 and dipped into the BiVO4 sol–gel solution at last.Photocatalytic activity test
The photocatalytic activities of the samples were evaluated by the amount of oxygen split from water (Labsolar-H2, Perfect Light, China) under visible light. Portions of the solid samples (10 mg) were introduced into a spectroscopic quartz probe cell, and the measurements were taken at room temperature under visible light irradiation from a 300 W Xe lamp (PLS-SXE300/300UV, Perfect Light, China) equipped with a cutoff filter L42. The visible light intensity is about 10 mA. The evolved gases were analyzed by gas chromatography with a thermal conductivity detector (GC7890II, Tianmei, Shanghai; N2 carrier gas).The electrodes were prepared according to the method from Kudo. Photoelectrochemical properties were evaluated with a three-electrode cell consisting of the prepared electrodes, a Pt electrode, and a saturated Ag/AgCl electrode as the working, counter, and reference electrodes, respectively. The working electrode was irradiated from the FTO side with visible-light through a cutoff filter (L42). The incident photon-to-photocurrent efficiency (IPCE) was calculated as follows:
| [IPCE/%] = 1240 × [photocurrent density/μA cm−2]/([wavelength/nm] × [photo flux/Wm−2]) |
Characterizations
The synthesized samples were examined by X-ray diffraction (XRD) on a D-max/2550 (Rigaku) X-ray diffractometer system operating at 1600 W power (40 kV, 40 mA) with Cu Kα radiation (λ = 0.15406 nm), at a scan rate of 4° min−1 and a step size of 0.050° in 2θ. The morphologies and microstructures of the samples were characterized using a FEI QUANTA TEG 250 scanning electron microscope (SEM) and a JEOL2011 transmission electron microscope (TEM). TEM specimens were prepared by dispersing the synthesized samples in pure ethanol, and the powder was picked up by copper grids. UV-Visible (UV-Vis) diffuse reflectance spectra of all the samples were recorded using a PerkinElmer UV-Vis-NIR spectrophotometer (750S) in the spectral range of 200–800 nm, and Raman scattering measurements were obtained in back scattering geometry on a Via+ Reflex from 150–1000 cm−1.Results and discussion
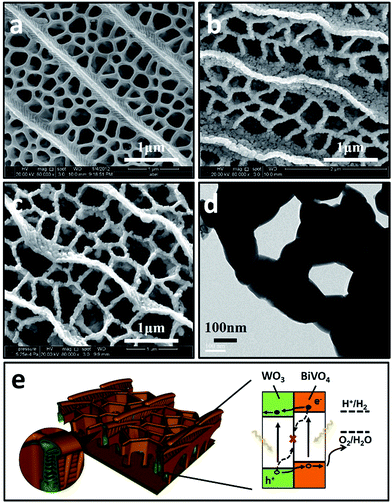
BiVO4 has a narrow band gap (2.4 eV), and its conduction band is more negative than that of WO3. Therefore coupling with photocorrosion resistant WO3, WO3/BiVO4 heterojunctions15–19 could be suitable for efficient photocatalytic water splitting under visible irradiation; see Fig. 1e. The quasi-honeycomb structure of Papilio paris butterfly wings is shown in Fig. S1,† which can absorb visible light to an utmost degree with over 95% absorption rate.28 The morphologies of WO3BWs, WO3/BiVO4BWs (two-step) and WO3/BiVO4BWs are shown in Fig. 1a–c. The quasi-honeycomb and three-layer structures of the scales can clearly be seen in the three samples. Compared with the original butterfly wing, the replicas show a 20% shrinkage for both WO3/BiVO4BWs (two-step) and WO3/BiVO4BWs after calcination (Fig. 1b and c). The scales, neatly arranged on the surface of the structure of the Papilio paris butterfly wings, were completely retained by WO3 (Fig. 1a). The parallel ridges of the scales and nanoscale ribs were retained in the WO3/BiVO4BWs, indicating an extremely fine duplication of the original scale structures using the one-step sol–gel method (Fig. 1c), and the high-resolution TEM (HRTEM) images provide the 150 nm average pore diameter (Fig. 1d). However, the further finer and elaborate architectures in the ribs of the WO3/BiVO4BWs (two-step) disappear (Fig. 1b) because of the significant grain growth of WO3 particles when the calcination temperature reaches 800 °C, suggesting a poor photocatalytic performance.Fig. 2a shows X-ray diffraction patterns of WO3BWs, BiVO4BWs, and WO3/BiVO4BWs. In the WO3BWs, the peaks of 23.3, 23.8, and 24.6° are observed corresponding to (002), (020), and (200) planes for the monoclinic phase of WO3 (JCPDS 43-1035), which is known to show the highest photocatalytic effect compared to other crystal phases.29,30 Compared with monoclinic WO3, several new peaks appeared on the XRD spectra, and they could be indexed as other WxOy phases, such as WO2.9 and W18O49. This means that the bio-template resulted in multiple tungsten oxide phases in the replicas. For bare BiVO4BWs, the main peaks can be indexed as (110), (011), (121), (040), (200) and (002) planes, which can represent the monoclinic BiVO4 (JCPDS 14-0688) without any impurity phase, illustrating that the bio-template has been removed completely by calcination at 500 °C. In the case of WO3/BiVO4BWs, monoclinic WO3 and BiVO4 are the only phases detected in XRD measurements (Fig. 2a), demonstrating that a novel 3D nanostructured WO3/BiVO4 heterojunction derived from Papilio paris with pure and high crystallinity can be achieved by the one-step sol–gel method mentioned before. As a result, the average grain size of the samples, calculated via the Scherrer formula, are 15.3, 27.2 and 30.5 nm, corresponding to WO3BWs, BiVO4BWs, and WO3/BiVO4BWs, respectively, while WO3/BiVO4BWs (two-step) shows 48.5 nm.
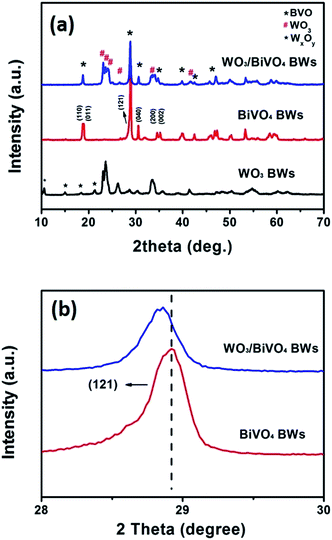 | ||
| Fig. 2 XRD patterns of (a) WO3BWs, BiVO4BWs, and WO3/BiVO4BWs. (b) Magnified peaks of (120) planes in the range of 2θ from 28° to 30°. | ||
Fig. 2b exhibits a magnified view of (121) peaks, which show 0.318° shifts to lower angles in the WO3/BiVO4BWs, compared with the BiVO4BWs. Pratap M. Rao et al. in 2014 have reported that W6+ dopes into BiVO4 by substituting for V5+ and acts as an electron donor in the WO3/BiVO4 core/shell nanowire photoanode.12 This shifting may be ascribed to lattice expansion due to the slight portion of W atoms incorporating from WO3. These XRD results indicate that little W atoms have been well inserted into V sites of the host BiVO4 lattice, induces lattice expansion without forming any segregated impurity phase.12 It has been known that impurity doping may form new bonds to promote the electron–hole separation efficiency.6 Therefore, the WO3/BiVO4BWs are expected to show enhanced photocatalytic performance.
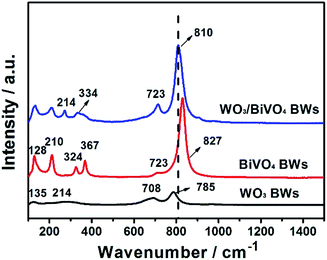
Moreover, Fig. 3 presents the Raman spectra of the WO3BWs, BiVO4BWs, and WO3/BiVO4BWs in the 150–1000 cm−1 region. The bands at 827, 723, 367, 324, 210 and 128 cm−1 in the BiVO4BWs correspond to the typical vibrations of monoclinic BiVO4. The band at 827 is assigned to the typical symmetric V–O stretching mode,31 and the one with a weak shoulder at about 712 cm−1 assigned to the antisymmetric V–O stretching mode, whereas those at 367 and 324 cm−1 are attributed to the typical symmetric and antisymmetric bending modes of the vanadate anion.32,33 Raman vibrations centered at 135, 214, 708 and 785 cm−1, characteristic of pure WO3, are also detected in the WO3BWs. The bands at 708 and 785 cm−1 are identified as the O–W–O bending and stretching mode, respectively.32
AS seen from Fig. 3, in the case of WO3/BiVO4BWs, it is clear that a shift of the Raman band to the lower wavenumber, from 827 to 810 cm−1 assigned to symmetric V–O bond stretching mode, reveals that the average lone-range symmetry of the VO4 tetrahedral becomes less regular. The blue-shift is due to the W-doping effect where W has been inserted into the V sites of the BiVO4 lattice to form the W–O bond, which has the bond length shorter than that of the V–O bond. Furthermore, the band at 785 cm−1 attributed to O–W–O bonds is broad and shifted to 810 cm−1 in WO3/BiVO4BWs, probably due to the formation of the WO3/BiVO4 heterojunction structures, which generate a new W–O–V bond,33 consistent with XRD results. It has been reported that formation of new bonds can promote electron–hole separation efficiency of photocatalysts. It would be expected that WO3/BiVO4BWs should perform the best in the photocatalytic O2 production experiments.
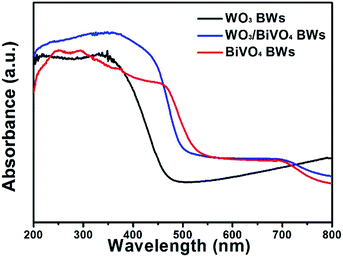
To investigate the optical absorption properties of the samples, UV/Vis spectroscopy was conducted, and the results are shown in Fig. 4. Light absorption of the bare WO3BWs starts at around 475 nm in correspondence with its band gap energy. For the bare BiVO4BWs, the onset of light absorption is around 520 nm, again corresponding to its band gap energy. In the WO3/BiVO4BWs, the main absorption edge has a red-shift of more than 20 nm compared with pure BiVO4BWs. It indicates that the band-gap energy of the samples is affected by the quasi-honeycomb structure of Papilio paris butterfly wing, and the quasi-honeycomb structure can enhance visible light absorption, which has been reported by us before.26 Moreover, compared with BiVO4BWs and WO3BWs, WO3/BiVO4BWs exhibits an increased photoabsorption at wavelengths between 200 nm and 480 nm, which is probably due to the formation of the heterojunction structure. The optical band-gap energies of the three samples are estimated from the absorption spectra using the following relationship:
| αhν = A(hν − Eg)n |
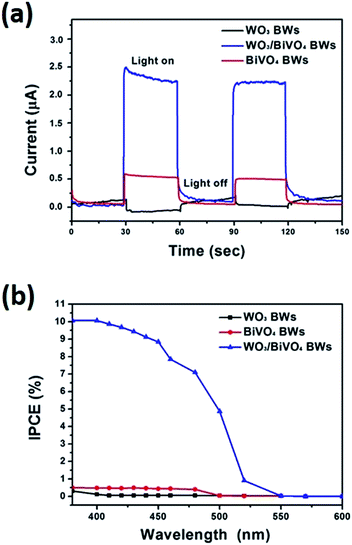
We further measure photocurrents of WO3BWs, BiVO4BWs, and WO3/BiVO4BWs by depositing each material on ITO electrodes. A fast and strong photocurrent response is observed for each switch-on/off event in both WO3/BiVO4BWs and BiVO4BWs-deposited electrodes under visible light (Fig. 5a). Under visible light irradiation (λ > 420 nm), the photocurrent of WO3/BiVO4BWs electrode (2.5 μA) is approximately 5 times higher than that of the BiVO4BWs electrode (0.5 μA), which indicates that the separation efficiency of photo-induced electrons and holes is improved significantly through the electronic interaction between BiVO4 and WO3, as expected from the results in Fig. 5a. In contrast, no photocurrent is recorded for WO3BWs under visible light irradiation, which implies no photocatalytic O2 evolution from water splitting under visible light gap.
The incident photon-to-photocurrent efficiency (IPCE) spectra of WO3BWs, BiVO4BWs, and WO3/BiVO4BWs are shown in Fig. 5b, in which the electrodes are measured at 1.23 V (vs. Ag/AgCl) in 0.5 M Na2SO4 using monochromatic light controlled with band pass filters. The IPCE is defined by the following equation:
| [IPCE/%] = 1240 × [photocurrent density/μA cm−2]/([wavelength/nm] × [photo flux/Wm−2]) |
The WO3BWs shows rising IPCE from 480 nm and the BiVO4BWs from 540 nm, in agreement with their band gap energies. The WO3/BiVO4BWs also shows the onset of IPCE at 540 nm similar to BiVO4BWs. Moreover, the WO3/BiVO4BWs electrode performs at an excellent anodic photocurrent with 10.8% of an IPCE at 400 nm at 1.23 V vs. Ag/AgCl, whereas the trivial IPCE of WO3BWs at 0.3% (at 380 nm) and BiVO4BWs at 0.7% (at 400 nm) is observed. Despite the range of light absorption of BiVO4BWs being larger than WO3/BiVO4BWs, the photoactivity was much less because of poor charge transfer characteristics as evidenced by the photocurrents study discussed above. Because WO3 cannot absorb light with wavelength between 500 and 540 nm, the IPCE in this range for WO3/BiVO4BWs originates from the absorption by the BiVO4 layers. However, unlike bare BiVO4, the WO3/BiVO4 heterojunction can utilize light at 500–540 nm for water oxidation because the good charge transfer characteristic at the interface induces rapid transfer of photoelectrons formed in BiVO4 to WO3. One order of magnitude enhancement in photoconversion efficiency indicates that WO3/BiVO4BWs may result in the best photocatalytic activity.
On the basis of the above results, the photocatalytic activities of the samples were tested for O2 evolution from water splitting under visible light range. Fig. 6 shows the amount of evolved O2 by solar light from boiled water. The amount of evolved O2 from aqueous solution after 1 h from the WO3/BiVO4BWs is ca. 200 μmol, which is 5 times as much as that of the BiVO4BWs (ca. 42 μmol), the highest photocatalytic O2 evolution among all the tested samples. The total evolved O2 after 5 h from the WO3/BiVO4BWs is ca. 950 μmol, which is more than 7.6 times that of the BiVO4BWs (ca. 125 μmol). On the contrary, WO3/BiVO4BWs (two-step) shows ca. 35 μmol (Fig. S2, ESI†) and WO3BWs produces no oxygen under visible light irradiation. The results suggest that the WO3/BiVO4BWs performs the best photocatalytic activities, compared with other hierarchical porous photocatalysts from bio-templates that we have reported before.26,27 This result shows that the WO3/BiVO4BWs are stable and indeed oxidize water into O2.
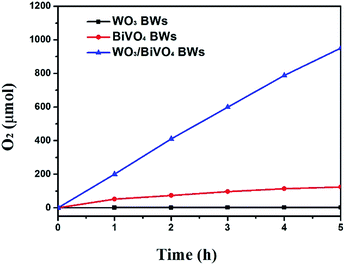 | ||
| Fig. 6 Photocatalytic O2 evolution of WO3BWs, BiVO4BWs, and WO3/BiVO4BWs under visible light irradiation (λ > 420 nm). The amount is kept the same for all the samples. | ||
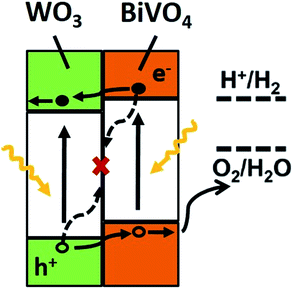
From all the results presented above, a possible mechanism for photocatalytic O2 evolution from water splitting by the WO3/BiVO4BWs has been proposed and illustrated in Fig. 7. We attribute the significantly enhanced photocatalytic performance of WO3/BiVO4BWs under visible light to more absorption of visible light as well as more efficient transfer of photogenerated electrons. The unique quasi-honeycomb structure allows more visible light waves to penetrate deep inside the photocatalyst, thus improving the light absorption capability and the formation of heterojunctions which enhances the separation of photogenerated electron–hole pairs and thus reduces charge recombination rate as evidenced by the photocurrent responses analysis.
According to the literature, for a WO3 film in Na2SO4, a flat-band potential of Vfb = 0.15 V (vs. SCE) was reported by Patil,34 while the Vfb of BiVO4 was reported as −0.62 V (vs. SCE) by Sayama35 and Li36. It is generally known that the bottoms of the conduction bands in several n-type semiconductors are more negative by 0.1 V than the flat band potential.37 In the photocatalytic oxygen evolution process (Fig. 7), illumination photons create electron–hole pairs in the WO3/BiVO4BWs at the solid/solution interface. These electron–hole pairs separate and reach the photocatalyst surface by diffusion. Owing to the positive conduction band position (>0 eV vs. NHE at pH = 7), only the reaction of holes with water to produce O2 can occur spontaneously (eqn (2)).
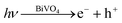 | (1) |
| 2H2O + 4h+ → 4H+ + O2 | (2) |
With the bottom of the BiVO4 conduction band more positive than that of WO3, it is favorable for the electrons to travel from the BiVO4 conduction band to WO3. Hence, electrons generated in the BiVO4 will first travel radially inward to the WO3 in the WO3/BiVO4BWs, and then travel to the current collector through WO3, in turn suppressing electron–hole recombination. Although BiVO4 absorbs a larger fraction of solar light than WO3 due to its narrower band gap as shown in Fig. 4, electrons and holes formed in BiVO4 are not fully utilized for water decomposition reaction because its poor transfer characteristics lead to recombination. However, the holes can migrate to the semiconductor/electrolyte interface either directly or after transfer from WO3 to BiVO4. The reduced recombination naturally induces photoactivity enhancement.
Conclusions
In summary, a novel 3D nanostructured WO3/BiVO4 heterojunction derived from Papilio paris for photocatalytic water splitting is fabricated by a one-step sol–gel method. The quasi-honeycomb structure of the original butterfly wings is kept perfectly in the heterojunction. The photocurrents of WO3/BiVO4BWs (2.5 μA) is approximately 5 times higher than that of a BiVO4BWs (0.5 μA), and an incident photon-to-current conversion efficiency of ∼10% at 380–450 nm (1.23 V versus RHE), indicating a higher product of light absorption and charge separation efficiencies. The WO3/BiVO4BWs, in which BiVO4 is the primary visible light-absorber and WO3 acts as an electron conductor, achieved an excellent photocatalytic O2 evolution of 20 μmol h−1 mg−1 under visible light irradiation, which is 5 times higher than BiVO4BWs, even without a sacrificial agent. The enhanced photocatalytic performance is attributed to not only the unique quasi-honeycomb structure with an efficient visible light-harvesting capacity, but also to improved separation efficiency of photo-induced electrons and holes through the electronic interaction in the WO3/BiVO4 heterojunction. These promising results open new possibilities in the design of highly photoactive heterojunction photocatalysts inherited from bio-templates for water-splitting systems.Acknowledgements
The authors gratefully acknowledge financial support for this research from the Morgan Crucible Company, the National Science Foundation of China (No. 51072117, 51171110), the National Basic Research Program of China (973 Program, 2012CB619600), the Shanghai Science and Technology Committee (0JC1407600). We also thank the Shanghai Jiao Tong University (SJTU) Instrument Analysis Center.Notes and references
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446 CrossRef CAS PubMed.
- H. P. Maruska and A. K. Ghosh, Sol. Energy Mater., 1979, 1, 411–429 CrossRef CAS.
- P. V. Kamat, Chem. Rev., 1993, 93, 267–300 CrossRef CAS.
- W. J. Jo, J. W. Jang, K. J. Kong, H. J. Kang, J. Y. Kim, H. Jun, K. P. S. Parmar and J. S. Lee, Angew. Chem., Int. Ed., 2012, 51, 1–6 CrossRef PubMed.
- W. J. Luo, Z. S. Yang, Z. Li, J. Zhang, J. Liu, Z. Zhao, Z. Wang, S. Yan, T. Yu and Z. Zou, Energy Environ. Sci., 2011, 4, 4046–4051 CAS.
- S. K. Pilli, T. E. Furtak, L. D. Brown, T. G. Deutsch, J. A. Turner and A. M. Herring, Energy Environ. Sci., 2011, 4, 5028–5034 CAS.
- F. Lin, D. G. Wang, Z. X. Jiang, Y. Ma, J. Li, R. Li and C. Li, Energy Environ. Sci., 2012, 5, 6400–6406 CAS.
- J. A. Seabold and K. S. Choi, J. Am. Chem. Soc., 2012, 134, 2186–2192 CrossRef CAS PubMed.
- R. G. Li, F. X. Zhang, D. G. Wang, J. X. Yang, M. R. Li, J. Zhu, X. Zhou, H. X. Han and C. Li, Nat. Commun., 2013, 4, 1–7 CrossRef.
- L. Chen, Q. Zhang, R. Huang, S. F. Yin, S. L. Luo and C. T. Au, Dalton Trans., 2012, 41, 9513–9518 RSC.
- P. M. Rao, L. Cai, C. Liu, I. S. Cho, C. H. Lee, J. M. Weisse, P. Yang and X. Zheng, Nano Lett., 2014, 14, 1099–1105 CrossRef CAS PubMed.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- T. W. Kim and K. S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- W. J. Luo, Z. S. Yang, Z. Li, J. Zhang, J. Liu, Z. Zhao, Z. Wang, S. Yan, T. Yu and Z. Zou, Energy Environ. Sci., 2011, 4, 4046–4051 CAS.
- S. J. A. Moniz, J. Zhu and J. W. Tang, Adv. Energy Mater., 2014, 4, 1301590 CrossRef.
- R. Saito, Y. Miseki and K. Sayama, Chem. Commun., 2012, 48, 3833–3835 RSC.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 CAS.
- K. Zhang, X. J. Shi, J. K. Kim and J. H. Park, Phys. Chem. Chem. Phys., 2012, 14, 11119–11124 RSC.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928–1933 CrossRef CAS PubMed.
- Y. Hou, F. Zuo, A. Dagg and P. Feng, Nano Lett., 2012, 12, 6464–6473 CrossRef CAS PubMed.
- A. Iwase, Y. H. Ng, Y. Ishiguro, A. Kudo and R. Amal, J. Am. Chem. Soc., 2011, 133, 11054–11057 CrossRef CAS PubMed.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. vande Krol, Nat. Commun., 2013, 4, 2195 Search PubMed.
- X. Lin, Y. S. Wang, J. Zheng, C. Liu, Y. Yang and G. B. Che, Dalton Trans., 2015, 44, 19185–19193 RSC.
- C. J. Li, S. P. Wang, T. Wang, Y. J. Wei, P. Zhang and J. L. Gong, Small, 2014, 10, 2783–2790 CrossRef CAS PubMed.
- C. Yin, S. M. Zhu, Z. X. Chen, W. Zhang, J. J. Gua and D. Zhang, J. Mater. Chem. A, 2013, 1, 8367–8378 CAS.
- C. Yin, S. M. Zhu, F. Yao, J. J. Gu, W. Zhang, Z. X. Chen and D. Zhang, J. Nanopart. Res., 2013, 15, 1812–1823 CrossRef.
- P. Vukusic, J. R. Sambles and C. R. Lawrence, Proc. R. Soc. B, 2004, 271, 237–239 CrossRef PubMed.
- U. M. García-Pérez, S. Sepúlveda-Guzmán and A. Martínez-de la Cruz, Solid State Sci., 2012, 14, 293–298 CrossRef.
- J. Kenneth, M. Donald and K. S. Choi, Energy Environ. Sci., 2012, 5, 8553–8557 Search PubMed.
- H. Liu, R. Nakamur and N. Yoshihiro, J. Electrochem. Soc., 2005, 152, 856–861 CrossRef.
- G. S. Li, D. Q. Zhang and J. C. Yu, Chem. Mater., 2008, 20, 3983–3992 CrossRef CAS.
- D. Wang, H. Jiang, X. Zong, Q. Xu, Y. Ma, G. Li and C. Li, Chem.–Eur. J., 2011, 17, 1275–1282 CrossRef CAS PubMed.
- P. S. Patil and P. R. Patil, Bull. Mater. Sci., 1996, 19, 651–656 CrossRef CAS.
- K. Sayama, A. Nomura, T. Arai, T. Sugita, R. Abe, M. Yanagida, T. Oi, Y. Iwasaki, Y. Abe and H. J. Sugihara, J. Phys. Chem. B, 2006, 110, 11352–11360 CrossRef CAS PubMed.
- M. Li, Z. L. Hao and L. Guo, Int. J. Hydrogen Energy, 2010, 35, 7127–7133 CrossRef CAS.
- Y. Matsumoto, K. Omae, I. Watanabe and E. J. Sato, J. Electrochem. Soc., 1986, 133, 711–716 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03491a |
| This journal is © The Royal Society of Chemistry 2017 |