Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparative intestinal bacteria-associated pharmacokinetics of 16 components of Shengjiang Xiexin decoction between normal rats and rats with irinotecan hydrochloride (CPT-11)-induced gastrointestinal toxicity in vitro using salting-out sample preparation and LC-MS/MS†
Huanyu Guan ab,
Xiaoming Wanga,
Shiping Wangb,
Yang Hea,
Jiajing Yuea,
Shanggao Liaob,
Yuanda Huanga and
Yue Shi*a
ab,
Xiaoming Wanga,
Shiping Wangb,
Yang Hea,
Jiajing Yuea,
Shanggao Liaob,
Yuanda Huanga and
Yue Shi*a
aInstitute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100193, China. E-mail: shiyue1029@126.com; Fax: +86-10-57833270; Tel: +86-10-57833255
bSchool of Pharmaceutical Sciences, Guizhou Medical University, Guiyang 550004, China
First published on 11th September 2017
Abstract
Shengjiang Xiexin decoction (SXD) exerts protective effects against gastrointestinal injury induced by irinotecan hydrochloride (CPT-11). The intestinal bacteria-associated in vitro pharmacokinetics of 16 components of SXD in normal rats and those with CPT-11-induced gastrointestinal toxicity were compared in this study. A sensitive and reproducible ultra-high-performance liquid chromatography coupled to tandem mass spectrometry (UHPLC-MS/MS) method was developed for the quantification of 16 components of SXD in a rat intestinal bacteria incubation system, using naringin, naringenin and tetrahydropalmatine as internal standards (ISs). The samples were prepared via salting-out assisted liquid–liquid extraction (SALLE) with NaCl to reduce matrix effects. Chromatographic separation was performed on a sub-2 μm analytical column with acetonitrile and 0.1% aqueous formic acid as mobile phase. All of the analyzed components and ISs were detected via multiple reaction monitoring (MRM) scanning with electrospray ionization. The proposed method was successfully applied for the in vitro pharmacokinetic analysis of the multiple components of a complex mixture consisting of a traditional Chinese medicine (TCM) and an intestinal bacterial incubation system. The pharmacokinetic parameters of some flavonoid glycosides and aglycones in the rats with CPT-11-induced gastrointestinal toxicity were significantly different (p < 0.05, p < 0.01) from those in the normal rats, which suggested that consumption of CPT-11 could qualitatively and/or quantitatively alter the intestinal bacteria as well as the metabolic activities of enzymes. The in vitro pharmacokinetic analysis of these components in the intestinal bacterial incubation system provided valuable information for achieving a deeper understanding of the mechanisms involved in the alteration of intestinal bacteria induced by CPT-11 and further in vivo pharmacokinetic research on SXD. The intestinal bacteria-based pharmacokinetic method could benefit the study of interactions between TCMs and chemical drugs in clinical use.
1. Introduction
Irinotecan hydrochloride (CPT-11) is a promising antitumor derivative of camptothecin, a topoisomerase I inhibitor.1 However, at higher dosages, CPT-11 can cause severe and uncontrollable diarrhea, which is one of the main side-effects of CPT-11 and has impeded its utilization in more aggressive antitumor regimens.2,3 CPT-11 is converted by carboxylesterase enzymes mainly in the liver into its active metabolite, SN-38, which is considered to be responsible for the induction of diarrhea.4 SN-38 is then detoxified to SN-38 glucuronide (SN-38G) by UDP-glucuronosyltransferase, and is excreted via bile.5 SN-38G excreted into the intestinal lumen through bile may be deconjugated by bacterial β-glucuronidase, releasing SN-38, which leads to the accumulation of SN-38 in the intestine.6 SN-38 in the cecal and colonic contents directly damages the intestinal epithelium and induces delayed-onset diarrhea.7 Bacterial β-glucuronidase is involved in the metabolism of CPT-11 and plays a crucial role in the intestinal toxicity of CPT-11.8 Many microflora in the gastrointestinal tract can produce β-glucuronidase. Changes in the intestinal microflora occur after CPT-11 treatment, and an increase in the expression of β-glucuronidase has been observed.9,10 Moreover, the translocation of special bacteria induced by CPT-11 can cause infection.11 Therefore, antibiotics have been used to reduce the level of the microflora and decrease the bacterial β-glucuronidase activity in the gastrointestinal tract to alleviate the CPT-11-induced diarrhea.2,6,12 Nevertheless, there are some obvious drawbacks to the antidiarrheal treatment, which is detrimental to commensal bacteria. Admittedly, intestinal bacteria are involved in the metabolism of carbohydrate, the production of vitamins, and the processing of bile acids, sterols, and xenobiotics. Fortunately, potent bacterial β-glucuronidase inhibitors that do not affect commensal bacteria have been identified.13Traditional Chinese Medicine (TCM) has been used to treat or prevent cancer-related symptoms and chemotherapy-associated toxicity for thousands of years. Most of TCM are orally administered in the form of decoctions and are therefore inevitably brought into contact with bacteria and enzymes in the alimentary tract. As described in “Shang Han Lun”, Shengjiang Xiexin decoction (SXD) is a classic TCM formula to be used for the treatment of gastroenteritis, ulcerative colitis and diarrhea,14 consisting of eight herbs in the ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 on a dry weight basis: Pinellia ternata (“banxia” in Chinese, the rhizome of P. ternata (Thunb.) Breit.), Glycyrrhiza uralensis (“gancao” in Chinese, the radix of G. uralensis Fisch.), Coptis chinensis (“huanglian” in Chinese, the rhizome of C. chinensis Franch.), Ziziphus jujuba (“dazao” in Chinese, the fruit of Z. jujuba Mill.), Zingiber officinale (“ganjiang” in Chinese, the rhizome of Z. officinale Rosc.), Scutellaria baicalensis (“huangqin” in Chinese, the radix of S. baicalensis Georgi.), Codonopsis pilosula (“dangshen” in Chinese, the radix of C. pilosula (Franch.) Nannf.) and Zingiberis recens (“shengjiang” in Chinese, the rhizome of Z. recens.). The combination of these herbs is based upon the rule of “Jun-Chen-Zuo-Shi”, known as “Emperor–Minister–Assistant–Courier”. Among them, C. chinensis and S. baicalensis serve as “Jun” and “Chen” to alleviate the gastrointestinal toxicity, respectively.15
12 on a dry weight basis: Pinellia ternata (“banxia” in Chinese, the rhizome of P. ternata (Thunb.) Breit.), Glycyrrhiza uralensis (“gancao” in Chinese, the radix of G. uralensis Fisch.), Coptis chinensis (“huanglian” in Chinese, the rhizome of C. chinensis Franch.), Ziziphus jujuba (“dazao” in Chinese, the fruit of Z. jujuba Mill.), Zingiber officinale (“ganjiang” in Chinese, the rhizome of Z. officinale Rosc.), Scutellaria baicalensis (“huangqin” in Chinese, the radix of S. baicalensis Georgi.), Codonopsis pilosula (“dangshen” in Chinese, the radix of C. pilosula (Franch.) Nannf.) and Zingiberis recens (“shengjiang” in Chinese, the rhizome of Z. recens.). The combination of these herbs is based upon the rule of “Jun-Chen-Zuo-Shi”, known as “Emperor–Minister–Assistant–Courier”. Among them, C. chinensis and S. baicalensis serve as “Jun” and “Chen” to alleviate the gastrointestinal toxicity, respectively.15
Regarding modern clinical practice, when patients in several hospitals were orally administered SXD two days prior to the initiation of chemotherapy to prevent CPT-11-induced gastrointestinal toxicity, it was found that this treatment reduced the incidence of diarrhea.16 Moreover, SXD has been reported to regulate the CPT-11-induced apoptosis and necrosis of intestinal mucosal and functional cells.17 It is also noteworthy that SXD can decreased the activity of β-glucuronidase after irinotecan administration.18 Baicalin, a known flavonoid in SXD, is a β-glucuronidase inhibitor19 that inhibits the uptake of SN-38 in a concentration-dependent manner.20 Moreover, as a traditional medicines, SXD is composed of multiple components, and the flavonoids, alkaloids and triterpenoid saponins in SXD are considered the most important active components of the mixture.21–24 Some flavonoids, alkaloids and saponins can be transformed by intestinal bacteria25–28 to their metabolites, which exhibit different pharmacological activities. However, the intestinal bacteria-associated pharmacokinetics of these components in vitro are not clear. In addition, the co-existence of multiple compounds in TCMs and chemical drugs may lead to the intestinal bacteria-based metabolic and pharmacokinetic interactions. There are few available studies on such interactions involving intestinal bacteria because of the complexity of the chemical components of TCM and the intestinal bacteria system.
In a previous study, we carried out the simultaneous quantification of 14 constituents of SXD using UFLC-MS/MS.29 An analytical method has also been developed for the simultaneous quantification of flavonoids, alkaloids and triterpenoid saponins in Banxia xiexin decoction, which is analogous to SXD formula.30 Analytical conditions for the individual determination of several flavonoids,21,28,31,32 alkaloids,33,34 and triterpenoid saponins35,36 in biological matrices have been reported. However, there is little available information about the quantification and in vitro pharmacokinetics of flavonoids, alkaloids and triterpenoid saponins from TCM formulas in complex intestinal bacterial incubation systems. In addition, no data on the intestinal bacteria-associated pharmacokinetics of the major components of SXD under interaction with CPT-11 have been reported.
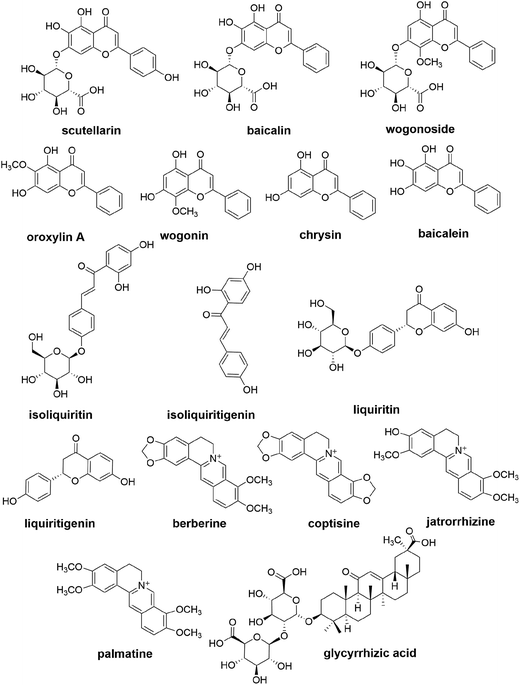
In the present study, a sensitive, specific and precise method was established for the simultaneous determination of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine, jatrorrhizine, glycyrrhizic acid, liquiritin and liquiritigenin (Fig. 1) in an in vitro rat intestinal bacterial incubation system, via one sample preparation combined with two chromatographic conditions. The method was validated and utilized to compare the intestinal bacteria-associated pharmacokinetics of 16 components of SXD in vitro between normal rats and those with CPT-11-induced gastrointestinal toxicity.
2. Experimental
2.1 Materials and reagents
All medicinal plants were purchased from Huamiao Traditional Chinese Medicine Engineering Technology Development Center (Beijing, China). The reference standards of baicalin, glycyrrhizic acid, baicalein, liquiritin, berberine, palmatine, naringin (used as an internal standard, IS1), naringenin (IS2) and tetrahydropalmatine (IS3) were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Oroxylin A, wogonin, jatrorrhizine, scutellarin, wogonoside, isoliquiritin, isoliquiritigenin, chrysin and liquiritigenin (purity ≥98%) were purchased from Shanghai Yuanye Biological Technology Co. Ltd. (Shanghai, China). Coptisine (purity ≥98%) was purchased from Aladdin Industrial Co., Ltd. (Shanghai, China), and Campo® (CPT-11) for injection was obtained from Pfizer (Bentley, WA, Australia). General anaerobic medium broth (GAM broth) was purchased from Shanghai Kayon Biological Technology Co., Ltd. (Shanghai, China). Anaerobic packs were purchased from Mitsubishi Gas Chemical Company (Tokyo, Japan).HPLC-grade methanol and acetonitrile were obtained from Honeywell Burdick & Jackson Company (Morristown, NJ, USA). Formic acid (MS grade) was purchased from Fisher Scientific (Madrid, Spain). Deionized water for HPLC analysis was prepared using a Milli-Q water purification system (Millipore, Milford, MA, USA). All other reagents were of analytical grade.
2.2 Equipment and LC-MS/MS conditions
For LC-MS/MS, a Shimadzu LC-30AD series UHPLC (Shimadzu, Kyoto, Japan) consisting of a dual solvent delivery system (two LC-30AD pumps), refrigerated auto-sampler (SIL-30AC), column oven (CTO-20AC) and degasser (DGU-20A5R) was employed, which was coupled to an AB SCIEX Qtrap 4500 system (AB SCIEX, Foster City, CA, USA) equipped with an electrospray ionization source (Turbo Ionspray) for mass spectrometric detection. Data analysis was performed using AB SCIEX Analyst 1.6 Software (AB SCIEX).The UHPLC separation was achieved on an ACQUITY UPLC® BEH C18 column (2.1 mm × 100 mm, 1.7 μm) using acetonitrile (A) and 0.1% aqueous formic acid (B) as the mobile phase at a flow rate of 0.3 mL min−1. The injection volume was set to 10 μL. The auto-sampler was conditioned at 10 °C. All analyzed components were quantified in multiple reaction monitoring (MRM) mode. The optimized conditions were as follows: curtain gas (CUR): 10.0 psi; collision gas (CAD): medium; IonSpray voltage (IS): −4500 V (in negative ionization mode) and 4500 V (in positive ionization mode); source temperature: 500 °C; GS1: 40 psi; and GS2: 40 psi. The MS/MS transitions (m/z), declustering potentials (DP), collision energies (CE), entrance potentials (EP) and collision cell exit potentials (CXP) of the analyzed components and ISs are listed in Table 1. Two gradient elution programs were employed for different compounds in different ion modes. Oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, naringin (IS1) and naringenin (IS2) were detected in negative ionization mode with elution program I: 5–5% A at 0–1 min; 5–15% A at 1–3 min; 15–15% A at 3–5 min; 15–20% A at 5–8 min; 20–20% A at 8–11 min; 20–35% A at 11–15 min; 35–45% A at 15–20 min; 45–100% A at 20–23 min; 100–100% A at 23–26 min; 100–5% A at 26–26.1 min and 5–5% A at 26.1–28 min, while isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine, jatrorrhizine, naringenin (IS2) and tetrahydropalmatine (IS3) were detected in the positive ionization mode with elution program II: 5–5% A at 0–1 min; 5–15% A at 1–3 min; 15–15% A at 3–5 min; 15–20% A at 5–9 min; 20–20% A at 9–12 min; 20–25% A at 12–16 min; 25–45% A at 16–21 min; 45–100% A at 21–23 min; 100–100% A at 23–26 min; 100–5% A at 26–26.1 min and 5–5% A at 26.1–28 min.
| Compounds | Precursor | Production | DP (V) | CE (V) | EP (V) | CXP (V) |
|---|---|---|---|---|---|---|
| Oroxylin A | 282.9 [M − H]− | 267.8 | −90 | −26 | −8 | −19 |
| Baicalin | 445.1 [M − H]− | 269.0 | −70 | −31 | −10 | −18 |
| Baicalein | 268.9 [M − H]− | 222.8 | −130 | −32 | −8 | −16 |
| Wogonoside | 459.0 [M − H]− | 267.9 | −90 | −43 | −11 | −19 |
| Wogonin | 283.0 [M − H]− | 267.8 | −82 | −25 | −11 | −18 |
| Chrysin | 252.9 [M − H]− | 142.9 | −130 | −37 | −10 | −10 |
| Scutellarin | 461.0 [M − H]− | 285.0 | −95 | −30 | −8 | −20 |
| Glycyrrhizic acid | 821.3 [M − H]− | 351.0 | −160 | −56 | −10 | −25 |
| Liquiritin | 417.2 [M − H]− | 255.0 | −110 | −28 | −11 | −18 |
| Liquiritigenin | 255.0 [M − H]− | 134.9 | −110 | −21 | −10 | −9 |
| Isoliquiritin | 419.0 [M + H]+ | 257.1 | 132 | 25 | 10 | 20 |
| Isoliquiritigenin | 257.1 [M + H]+ | 137.0 | 100 | 33 | 12 | 12 |
| Berberine | 336.0 [M]+ | 320.1 | 105 | 41 | 9 | 24 |
| Coptisine | 320.1 [M]+ | 292.0 | 95 | 40 | 8 | 20 |
| Palmatine | 352.2 [M]+ | 336.0 | 100 | 40 | 11 | 26 |
| Jatrorrhizine | 338.1 [M]+ | 322.1 | 100 | 40 | 5 | 26 |
| Naringin (IS1) | 579.1 [M − H]− | 271.0 | −165 | −44 | −11 | −19 |
| Naringenin (IS2) | 270.9 [M − H]− | 150.9 | −153 | −26 | −13 | −9 |
| 273.1 [M + H]+ | 153.0 | 110 | 32 | 9 | 12 | |
| Tetrahydropalmatine (IS3) | 356.1 [M + H]+ | 192.1 | 120 | 35 | 9 | 16 |
2.3 Preparation of standards and quality control samples
Stock solutions of the standards were prepared by individually dissolving 16 reference substances in methanol to obtain a final concentration of 1.0 mg mL−1. These stock solutions were mixed to obtain a final mixed stock solution by adding the appropriate volumes. The mixed stock solution was serially diluted with 50% methanol to obtain working standard solutions with the desired concentrations. The required IS stock solutions containing tetrahydropalmatine (0.25 μg mL−1), naringin (1.0 μg mL−1) and naringenin (1.0 μg mL−1) were prepared in 50% methanol and used at concentrations of 25, 100 and 100 ng mL−1, respectively, in each working solution and sample.Quality control (QC) samples for each compound were prepared by spiking 100 μL of the standard working solutions into 1 mL of the blank incubation solution for intestinal bacteria inactivated by acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), to obtain the following concentrations (LLOQ, low, medium and high concentrations): 10, 20, 100 and 400 ng mL−1 for oroxylin A; 20, 40, 200 and 800 ng mL−1 for baicalin, baicalein, wogonoside and wogonin; 2, 4, 40, and 160 ng mL−1 for chrysin and jatrorrhizine; 3, 6, 60 and 240 ng mL−1 for scutellarin; 12, 24, 120 and 480 ng mL−1 for glycyrrhizic acid; 8, 16, 160 and 640 ng mL−1 for liquiritin; 10, 20, 200 and 800 ng mL−1 for liquiritigenin; 3, 6, 60 and 240 ng mL−1 for isoliquiritin; 25, 50, 200 and 400 ng mL−1 for coptisine and palmatine; 50, 100, 400 and 800 ng mL−1 for berberine and 1, 2, 20 and 80 ng mL−1 for isoliquiritigenin.
1, v/v), to obtain the following concentrations (LLOQ, low, medium and high concentrations): 10, 20, 100 and 400 ng mL−1 for oroxylin A; 20, 40, 200 and 800 ng mL−1 for baicalin, baicalein, wogonoside and wogonin; 2, 4, 40, and 160 ng mL−1 for chrysin and jatrorrhizine; 3, 6, 60 and 240 ng mL−1 for scutellarin; 12, 24, 120 and 480 ng mL−1 for glycyrrhizic acid; 8, 16, 160 and 640 ng mL−1 for liquiritin; 10, 20, 200 and 800 ng mL−1 for liquiritigenin; 3, 6, 60 and 240 ng mL−1 for isoliquiritin; 25, 50, 200 and 400 ng mL−1 for coptisine and palmatine; 50, 100, 400 and 800 ng mL−1 for berberine and 1, 2, 20 and 80 ng mL−1 for isoliquiritigenin.
2.4 Preparation of SXD
The mixture of the eight crude herbal drugs was prepared according to the previously described formula, which was immersed in distilled water for 30 min. Next, a ten-fold volume of water was added, and the resulting mixture was decocted twice by boiling for 1 h. After filtration, the filtrates were combined, evaporated, and concentrated to form an extract. The obtained SXD extract (1.0 g) was extracted with 100 mL of 60% methanol and was ultrasonicated for 30 min. After centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, 10 μL of the supernatant was injected into the LC-MS/MS system to analyze the content of all 16 constituents in the SXD powder. The contents of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine and jatrorrhizine, were determined to be 202.74 ± 28.41, 17
000 rpm for 10 min, 10 μL of the supernatant was injected into the LC-MS/MS system to analyze the content of all 16 constituents in the SXD powder. The contents of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine and jatrorrhizine, were determined to be 202.74 ± 28.41, 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990.87 ± 1678.98, 215.63 ± 25.20, 13
990.87 ± 1678.98, 215.63 ± 25.20, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744.29 ± 1872.70, 242.92 ± 15.50, 12.56 ± 1.36, 578.08 ± 76.20, 2132.42 ± 316.42, 1821.92 ± 148.52, 11.32 ± 0.65, 564.84 ± 38.10, 20.05 ± 2.13, 7981.74 ± 981.55, 4036.53 ± 361.63, 3073.06 ± 471.40, and 813.70 ± 78.78 mg kg−1, respectively.
744.29 ± 1872.70, 242.92 ± 15.50, 12.56 ± 1.36, 578.08 ± 76.20, 2132.42 ± 316.42, 1821.92 ± 148.52, 11.32 ± 0.65, 564.84 ± 38.10, 20.05 ± 2.13, 7981.74 ± 981.55, 4036.53 ± 361.63, 3073.06 ± 471.40, and 813.70 ± 78.78 mg kg−1, respectively.
2.5 Animal handling
Thirty male Sprague-Dawley rats (weighing 200 ± 20 g), which were purchased from Department of Laboratory Animal Science of Peking University Health Center (Beijing, China), were maintained under a standard 12/12 h-light/dark cycle, at 20–25 °C and 40–60% humidity, with water and food available ad libitum for one week to adapt to the environment prior to the experiment. All experiments were performed according to the National Institutes of Health Guidelines for Animal Research and were approved by the Ethics Committee of the Institute of Medicinal Plant Development, CAMS & PUMC.The animals were randomly divided into group GT and C (n = 15). CPT-11 was administered intravenously (i.v.) at a dose of 60 mg per kg per day to the group GT rats via the tail vein for four consecutive days,3 while corresponding administration of saline was performed in group C rats. Body weight and diarrhea scores3 were monitored throughout the experimental period. Briefly, the severity of diarrhea was scored as follows: 0, normal; 1, soft feces or small black feces; 2, muddy feces; 3, watery feces or mucous feces. Animals were sacrificed by cervical dislocation under anesthesia. The colonic contents were collected aseptically in a sterile container 72 h after the final administration.
2.6 Incubation experiments
Samples of 2.0 g of the colonic contents were immediately mixed initially with 8 mL of aseptic physiological saline, and homogenized using a vortex-mixer. The homogenized mixture was centrifuged at 2000 rpm for 10 min, and 5 mL of the suspension was inoculated into 45 volumes of GAM broth, which was then incubated at 37 °C in an anaerobic pack for 24 h. The resulting mixture of bacteria was centrifuged at 4000 rpm for 10 min, and the residue was suspended in 5 mL of aseptic physiological saline to be used as the intestinal bacterial mixture.The intestinal bacterial mixture was inoculated into GAM broth in the presence of SXD extract (351.6 μg mL−1) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v). The cultured mixture was incubated anaerobically at 37 °C. Finally, a 1 mL aliquot of the cultured mixture was taken out, and the reaction was terminated by adding an equivalent volume of acetonitrile/water-saturated n-butanol (1
4 (v/v). The cultured mixture was incubated anaerobically at 37 °C. Finally, a 1 mL aliquot of the cultured mixture was taken out, and the reaction was terminated by adding an equivalent volume of acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36 and 48 h.
1, v/v) at 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36 and 48 h.
2.7 Sample preparation
Salting-out assisted liquid–liquid extraction (SALLE) was employed for sample preparation. Phase separation in the terminated incubation mixture was induced by the addition of NaCl until saturation. The organic phase was transferred to a clean tube and was evaporated to dryness under a steady stream of nitrogen gas. The residue was reconstituted with 1 mL of 50% methanol, followed by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The supernatant was then transferred to an auto-sampler vial. First, 10 μL of the supernatant was injected into the LC-MS/MS system for the simultaneous analysis of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, naringin (IS1) and naringenin (IS2) using elution program I. Next, another 10 μL was injected into the LC-MS/MS system for the simultaneous analysis of isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine, jatrorrhizine, naringenin (IS2) and tetrahydropalmatine (IS3) under the chromatographic condition using elution program II.
000 rpm for 10 min. The supernatant was then transferred to an auto-sampler vial. First, 10 μL of the supernatant was injected into the LC-MS/MS system for the simultaneous analysis of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, naringin (IS1) and naringenin (IS2) using elution program I. Next, another 10 μL was injected into the LC-MS/MS system for the simultaneous analysis of isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine, jatrorrhizine, naringenin (IS2) and tetrahydropalmatine (IS3) under the chromatographic condition using elution program II.
2.8 Method validation
The method was validated in terms of selectivity, calibration curve, sensitivity, precision, accuracy, recovery, matrix effect, dilution integrity and stability, in accordance with the guideline for bioanalytical method validation (2011) from the European Medicines Agency (EMA).37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to prepare the calibration curves in the following ranges: 10–500 ng mL−1 for oroxylin A; 20–1000 ng mL−1 for baicalin, baicalein, wogonoside and wogonin; 2–200 ng mL−1 for chrysin and jatrorrhizine; 3–300 ng mL−1 for scutellarin; 12–600 ng mL−1 for glycyrrhizic acid; 8–800 ng mL−1 for liquiritin; 10–1000 ng mL−1 for liquiritigenin; 3–300 ng mL−1 for isoliquiritin; 25–500 ng mL−1 for coptisine and palmatine; 50–1000 ng mL−1 for berberine; and 1–100 ng mL−1 for isoliquiritigenin.
1, v/v) to prepare the calibration curves in the following ranges: 10–500 ng mL−1 for oroxylin A; 20–1000 ng mL−1 for baicalin, baicalein, wogonoside and wogonin; 2–200 ng mL−1 for chrysin and jatrorrhizine; 3–300 ng mL−1 for scutellarin; 12–600 ng mL−1 for glycyrrhizic acid; 8–800 ng mL−1 for liquiritin; 10–1000 ng mL−1 for liquiritigenin; 3–300 ng mL−1 for isoliquiritin; 25–500 ng mL−1 for coptisine and palmatine; 50–1000 ng mL−1 for berberine; and 1–100 ng mL−1 for isoliquiritigenin.For all 16 components, each calibration curve was constructed by plotting the peak area ratio of the analyzed component to the IS versus the nominal concentration of the analyzed component using a 1/x-weighted linear least-square regression model. The LLOQ was the lowest concentration of the analyzed component on the calibration curve with an acceptable accuracy and precision. The accuracy (relative error, RE) of the LLOQ sample was within ±20% and the precision (relative standard deviation, RSD) was less than 20%.
Six batches of the blank intestinal bacterial incubation solution inactivated by acetonitrile/water-saturated n-butanol from individual rats were used to prepare QC samples at a low and a high level of concentration to evaluate the relative matrix effect. The matrix factor (MF) of each analyzed component for each batch was determined by calculating the ratio of the peak area of the analyzed component in the present of matrix to that in pure standard solutions. The IS-normalized MF was calculated by dividing the MF of the analyzed component by the MF of the IS. The RSD of the IS-normalized MF calculated from the six batches of the present matrix should not exceed 15%.
2.9 Data analysis
All calibration and quantification data were calculated using AB SCIEX Analyst 1.6 Software. Pharmacokinetic parameters, area under the concentration time curve to the respective sampling point (AUC0−t), mean resident time (MRT0−t), half-life (T1/2) and clearance rate (CL) for glycosides and alkaloids, and AUC0−t, maximum concentration (Cmax) and time to achieve maximum concentration (Tmax) for aglycones, were evaluated with Phoenix WinNonlin 6.0 (Certara, USA). An unpaired Student t-test was employed to compare the differences in pharmacokinetic parameters between group GT and C. Differences in body weight and diarrhea scores were analyzed via repeated measures analysis of variance and one-way ANOVA test.3. Results and discussion
3.1 Method development
Sample preparation and analytical technology were optimized for the determination of target components in a complex matrix. A TCM formula has been known as a complex system containing tens or even hundreds of different chemical constituents. The analysis of target components is interfered by the biological matrix and the other coexisting components in TCM formula. In the present study, it was important to take into account the interferences caused by non-target components in SXD, intestinal bacteria and incubation solution. In addition, because a large number of samples must be analyzed for pharmacokinetic studies, a rapid, simple and cost-effective sample preparation method is required.To obtain the 16 analyzed components with minimal matrix interference, PPT (acetone and acetonitrile), LLE (ethyl acetate and water-saturated n-butanol), solid-phase extraction (SPE) (Oasis HLB and Agela Cleanert PEP-SPE) and SALLE (acetonitrile with NaCl as the salting-out reagent and acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with NaCl) were evaluated. PPT using acetone or acetonitrile and LLE using water-saturated n-butanol were not considered because the ion intensities of the analyzed components were reduced due to matrix effects, although the extraction recoveries were relatively high. LLE using ethyl acetate and SALLE using acetonitrile and NaCl provided low extraction recoveries. Although the recovery range of SPE was acceptable, it was time consuming and cost prohibitive. Moreover, matrix interference were not completely eliminated for all of the analyzed components when SPE was performed.
1, v/v) with NaCl) were evaluated. PPT using acetone or acetonitrile and LLE using water-saturated n-butanol were not considered because the ion intensities of the analyzed components were reduced due to matrix effects, although the extraction recoveries were relatively high. LLE using ethyl acetate and SALLE using acetonitrile and NaCl provided low extraction recoveries. Although the recovery range of SPE was acceptable, it was time consuming and cost prohibitive. Moreover, matrix interference were not completely eliminated for all of the analyzed components when SPE was performed.
SALLE using acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with NaCl could provide relatively high extraction recoveries, low matrix interferences and good repeatability for most of analyzed components satisfying the requirements of quantification for determination of pharmacokinetic parameters. Finally, the SALLE method was performed using acetonitrile/water-saturated n-butanol (1
1, v/v) with NaCl could provide relatively high extraction recoveries, low matrix interferences and good repeatability for most of analyzed components satisfying the requirements of quantification for determination of pharmacokinetic parameters. Finally, the SALLE method was performed using acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and NaCl to prepare the samples.
1, v/v) and NaCl to prepare the samples.
However, quantification of glycyrrhetic acid, a metabolite of glycyrrhizic acid by intestinal bacteria, was compromised in this study due to its low recovery. As a saponin aglycone, the low polarity of glycyrrhetic acid makes its extraction difficult from bacterial incubation solution using acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the extract solvent. Fortunately, the recoveries of glycyrrhetic acid and other target components in further study can be improved by controlling the pH values and choosing appropriate solvents (acetone, methanol, ethanol and acetonitrile, etc.) for SALLE.
1, v/v) as the extract solvent. Fortunately, the recoveries of glycyrrhetic acid and other target components in further study can be improved by controlling the pH values and choosing appropriate solvents (acetone, methanol, ethanol and acetonitrile, etc.) for SALLE.
Compared with conventional LLE and PPT, SALLE in the present study provided a cleaner extract, effectively removing macromolecules. High extraction efficiencies were obtained for all 16 components of SXD via SALLE without the occurrence of emulsification. Compared with SPE, SALLE used much less solvent and was much faster and simpler to perform. Moreover, this was the first time that SALLE has been performed using acetonitrile/water-saturated n-butanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with NaCl for the extraction of target components in bacterial incubation solution.
1, v/v) with NaCl for the extraction of target components in bacterial incubation solution.
To achieve the maximum sensitivity and response, the precursor and product ion pairs for MRM detection, as well as their corresponding DP, CE, EP and CXP values, were optimized for the quantification of each analyzed component. The results are given in Table 1.
To improve resolution and decrease runtime, methanol, acetonitrile, ammonium acetate and formic acid were tested as potential mobile phases. Acetonitrile and 0.1% aqueous formic acid were employed as the mobile phase because the best separation of all the analyzed components from each other and the minimal influence of the matrix effect were achieved. Moreover, to improve the separation efficiency for the four alkaloids and isoliquiritin, the chromatographic condition II (in which the proportion of the organic phase between 12 and 16 min was decreased compared with that in the chromatographic condition I) was performed to obtain a better separation for berberine, coptisine, palmatine, jatrorrhizine and isoliquiritin.
3.2 Method validation
| Components | Calibration equation | r | Linear range (ng ml−1) | LLOQ (ng ml−1) |
|---|---|---|---|---|
| Oroxylin A | y = 0.0518x + 0.558 | 0.9994 | 10–500 | 10 |
| Baicalin | y = 0.0125x + 0.071 | 0.9987 | 20–1000 | 20 |
| Baicalein | y = 0.00431x + 0.0765 | 0.9989 | 20–1000 | 20 |
| Wogonoside | y = 0.0129x + 0.0155 | 0.9983 | 20–1000 | 20 |
| Wogonin | y = 0.0195x + 0.167 | 0.9988 | 20–1000 | 20 |
| Chrysin | y = 0.0173x + 0.0127 | 0.9993 | 2–200 | 2 |
| Scutellarin | y = 0.00653x + 0.00312 | 0.9990 | 3–300 | 3 |
| Glycyrrhizic acid | y = 0.00378x + 0.006 | 0.9992 | 12–600 | 12 |
| Liquiritin | y = 0.00717x + 0.00829 | 0.9994 | 8–800 | 8 |
| Liquiritigenin | y = 0.0234x + 0.0888 | 0.9993 | 10–1000 | 10 |
| Isoliquiritin | y = 0.00153x + 0.00267 | 0.9996 | 3–300 | 3 |
| Isoliquiritigenin | y = 0.0249x + 0.00376 | 0.9996 | 1–100 | 1 |
| Berberine | y = 0.0115x + 0.127 | 0.9994 | 50–1000 | 50 |
| Coptisine | y = 0.00253x + 0.00777 | 0.9993 | 25–500 | 25 |
| Palmatine | y = 0.00623x + 0.0138 | 0.9996 | 25–500 | 25 |
| Jatrorrhizine | y = 0.00585x + 0.00156 | 0.9988 | 2–200 | 2 |
| Components | Conc. (ng ml−1) | Intra-day (n = 5) | Inter-day (n = 5) | ||
|---|---|---|---|---|---|
| Precision (RSD, %) | Accuracy (RE, %) | Precision (RSD, %) | Accuracy (RE, %) | ||
| Oroxylin A | 10 | 14.70 | −3.95 | 5.02 | −2.44 |
| 20 | 8.60 | −5.02 | 4.77 | −8.95 | |
| 200 | 3.30 | 0.58 | 4.49 | −4.26 | |
| 400 | 3.27 | −2.38 | 6.15 | −7.46 | |
| Baicalin | 20 | 19.22 | −14.70 | 18.96 | −5.90 |
| 40 | 2.76 | −1.98 | 7.59 | −6.34 | |
| 200 | 4.09 | 7.00 | 6.30 | 0.07 | |
| 800 | 7.45 | −5.05 | 5.33 | −9.25 | |
| Baicalein | 20 | 11.74 | 10.20 | 7.68 | 16.50 |
| 40 | 4.59 | 4.30 | 10.17 | −6.26 | |
| 200 | 2.40 | 8.25 | 2.97 | 4.94 | |
| 800 | 3.61 | −7.58 | 5.67 | −10.21 | |
| Wogonoside | 20 | 14.31 | −7.80 | 6.68 | −4.06 |
| 40 | 1.87 | −1.53 | 7.30 | −4.10 | |
| 200 | 5.57 | 5.38 | 4.87 | 0.54 | |
| 800 | 3.62 | −2.40 | 13.31 | −8.45 | |
| Wogonin | 20 | 8.00 | −14.98 | 3.16 | −17.43 |
| 40 | 7.85 | −12.43 | 2.56 | −12.62 | |
| 200 | 2.26 | 12.00 | 5.11 | 6.70 | |
| 800 | 4.17 | 1.13 | 10.08 | −4.69 | |
| Chrysin | 2 | 8.37 | −4.83 | 6.25 | −7.78 |
| 4 | 1.87 | −1.95 | 7.93 | −3.54 | |
| 40 | 2.12 | −2.55 | 2.89 | 0.33 | |
| 160 | 4.41 | −0.95 | 7.55 | −4.45 | |
| Scutellarin | 3 | 19.34 | −0.70 | 9.40 | 10.90 |
| 6 | 2.53 | 3.75 | 7.17 | 2.88 | |
| 60 | 6.48 | −13.68 | 3.80 | −10.00 | |
| 240 | 4.69 | −9.53 | 6.26 | −4.58 | |
| Glycyrrhizic acid | 12 | 4.24 | −11.95 | 3.75 | −9.55 |
| 24 | 2.47 | −13.90 | 1.50 | −12.98 | |
| 120 | 4.04 | −14.85 | 6.33 | −10.86 | |
| 480 | 3.65 | −8.80 | 1.12 | −8.07 | |
| Liquiritin | 8 | 9.89 | 5.63 | 13.81 | −1.05 |
| 16 | 7.69 | −3.85 | 4.61 | −8.54 | |
| 160 | 7.93 | −10.23 | 14.28 | −5.81 | |
| 640 | 3.90 | 6.36 | 7.96 | −14.07 | |
| Liquiritigenin | 10 | 5.58 | −7.65 | 1.72 | −8.76 |
| 20 | 4.93 | 0.88 | 4.72 | −2.45 | |
| 200 | 2.56 | 2.88 | 2.56 | 5.96 | |
| 800 | 1.38 | 0.58 | 8.86 | 0.43 | |
| Isoliquiritin | 3 | 4.72 | 13.00 | 8.40 | 6.67 |
| 6 | 5.76 | −4.58 | 9.41 | −5.72 | |
| 60 | 5.32 | −9.90 | 14.23 | −2.91 | |
| 240 | 5.87 | −9.62 | 4.94 | −7.06 | |
| Isoliquiritigenin | 1 | 14.37 | 14.00 | 8.26 | 7.71 |
| 2 | 9.97 | −6.90 | 6.32 | −10.88 | |
| 20 | 3.00 | −8.67 | 2.48 | −7.03 | |
| 80 | 3.89 | 0.57 | 11.26 | −7.93 | |
| Berberine | 50 | 9.47 | −8.20 | 17.86 | 5.07 |
| 100 | 1.90 | 2.40 | 2.97 | 5.58 | |
| 400 | 3.24 | 3.60 | 4.70 | 1.93 | |
| 800 | 3.62 | −10.42 | 11.24 | −0.24 | |
| Coptisine | 25 | 9.87 | −10.58 | 16.68 | 1.38 |
| 50 | 7.45 | 0.60 | 2.46 | 1.51 | |
| 200 | 3.88 | 3.00 | 1.34 | 1.76 | |
| 400 | 4.41 | −9.86 | 5.86 | −4.41 | |
| Palmatine | 25 | 7.54 | 14.67 | 3.58 | 11.83 |
| 50 | 0.82 | 9.60 | 7.31 | 4.22 | |
| 200 | 3.98 | −1.18 | 3.41 | −3.51 | |
| 400 | 4.38 | −10.62 | 9.99 | −3.83 | |
| Jatrorrhizine | 2 | 7.66 | 8.25 | 4.89 | 12.13 |
| 4 | 9.16 | 12.76 | 6.20 | 5.23 | |
| 40 | 4.71 | 10.61 | 13.10 | −3.91 | |
| 160 | 3.94 | −6.12 | 5.17 | −0.16 | |
| Components | Conc. (ng ml−1) | Extract recovery | At 10 °C for 48 h | At −80 °C for 2 months | Freeze–thaw cycles | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (n = 5) | RSD (%) | Remain (%) | RSD (%) | Remain (%) | RSD (%) | Remain (%) | RSD (%) | ||
| Oroxylin A | 20 | 93.14 | 3.19 | 107.31 | 5.01 | 93.38 | 9.37 | 112.50 | 9.08 |
| 100 | 86.44 | 6.33 | 95.97 | 1.89 | 84.81 | 14.12 | 110.78 | 2.41 | |
| 400 | 84.46 | 7.23 | 102.37 | 2.53 | 92.07 | 9.97 | 90.38 | 2.49 | |
| Baicalin | 40 | 65.62 | 4.03 | 91.10 | 4.56 | 103.51 | 7.31 | 110.50 | 9.32 |
| 200 | 59.72 | 1.12 | 89.89 | 4.70 | 88.30 | 3.25 | 104.46 | 6.54 | |
| 800 | 61.22 | 2.71 | 86.68 | 3.62 | 108.15 | 5.71 | 95.20 | 1.54 | |
| Baicalein | 40 | 76.96 | 9.95 | 107.26 | 1.32 | 100.77 | 8.57 | 93.07 | 1.81 |
| 200 | 62.94 | 8.72 | 93.02 | 4.55 | 102.76 | 8.06 | 104.55 | 9.98 | |
| 800 | 65.68 | 7.06 | 85.73 | 0.14 | 96.68 | 4.09 | 97.05 | 9.13 | |
| Wogonoside | 40 | 94.71 | 3.62 | 86.53 | 5.92 | 94.77 | 6.87 | 97.71 | 8.63 |
| 200 | 83.63 | 2.01 | 87.21 | 4.19 | 102.18 | 5.78 | 103.43 | 11.55 | |
| 800 | 83.23 | 1.00 | 85.55 | 3.50 | 89.95 | 1.64 | 97.27 | 3.51 | |
| Wogonin | 40 | 62.26 | 2.97 | 102.11 | 1.97 | 86.27 | 7.90 | 109.68 | 4.47 |
| 200 | 64.90 | 4.65 | 110.14 | 1.34 | 85.77 | 0.74 | 111.11 | 5.40 | |
| 800 | 65.95 | 7.37 | 95.93 | 4.47 | 89.68 | 4.77 | 95.28 | 1.12 | |
| Chrysin | 4 | 71.84 | 2.81 | 106.90 | 3.51 | 112.85 | 2.95 | 110.45 | 4.54 |
| 40 | 62.01 | 4.65 | 108.11 | 3.03 | 113.00 | 1.33 | 101.64 | 6.68 | |
| 160 | 63.97 | 7.95 | 109.15 | 4.93 | 106.74 | 8.49 | 88.64 | 6.45 | |
| Scutellarin | 6 | 56.36 | 6.15 | 106.71 | 3.83 | 98.19 | 11.28 | 89.75 | 3.82 |
| 60 | 59.32 | 3.54 | 103.30 | 6.92 | 85.44 | 6.07 | 86.24 | 3.27 | |
| 240 | 54.50 | 2.92 | 107.98 | 5.13 | 100.46 | 6.74 | 85.83 | 2.00 | |
| Glycyrrhizic acid | 24 | 86.60 | 6.83 | 85.12 | 3.50 | 97.84 | 10.79 | 110.38 | 13.30 |
| 120 | 70.56 | 4.05 | 91.82 | 2.33 | 91.64 | 10.64 | 110.04 | 5.94 | |
| 480 | 68.85 | 4.12 | 90.37 | 8.97 | 102.97 | 7.01 | 94.57 | 2.70 | |
| Liquiritin | 16 | 79.04 | 7.04 | 113.68 | 1.85 | 112.32 | 9.67 | 93.61 | 3.89 |
| 160 | 77.91 | 7.76 | 113.54 | 4.86 | 94.57 | 6.58 | 92.43 | 7.74 | |
| 640 | 73.79 | 6.02 | 112.25 | 3.25 | 97.63 | 4.41 | 98.45 | 3.70 | |
| Liquiritigenin | 20 | 85.43 | 2.28 | 104.35 | 8.59 | 101.14 | 3.68 | 104.79 | 8.57 |
| 200 | 85.85 | 3.59 | 99.16 | 1.92 | 101.58 | 2.63 | 94.97 | 2.38 | |
| 800 | 81.55 | 4.01 | 91.31 | 6.12 | 96.58 | 7.70 | 95.87 | 1.47 | |
| Isoliquiritin | 6 | 90.79 | 2.40 | 102.33 | 1.12 | 86.34 | 2.63 | 103.13 | 5.03 |
| 60 | 79.62 | 3.54 | 94.83 | 0.72 | 97.06 | 5.84 | 106.74 | 1.09 | |
| 240 | 82.39 | 4.23 | 102.77 | 2.99 | 90.85 | 3.76 | 98.77 | 4.43 | |
| Isoliquiritigenin | 2 | 72.16 | 1.68 | 105.97 | 3.31 | 86.50 | 3.79 | 88.08 | 8.38 |
| 20 | 61.97 | 6.46 | 118.31 | 1.34 | 91.37 | 2.85 | 98.85 | 3.87 | |
| 80 | 58.02 | 7.28 | 109.04 | 0.74 | 94.77 | 9.77 | 98.27 | 1.53 | |
| Berberine | 100 | 76.63 | 5.39 | 94.48 | 1.12 | 87.05 | 7.11 | 102.40 | 6.89 |
| 400 | 89.90 | 5.77 | 90.16 | 1.52 | 87.36 | 3.09 | 96.98 | 4.52 | |
| 800 | 71.43 | 1.95 | 99.83 | 6.59 | 85.26 | 2.58 | 104.25 | 2.88 | |
| Coptisine | 50 | 64.81 | 1.65 | 104.34 | 7.64 | 86.21 | 5.33 | 97.81 | 2.69 |
| 200 | 72.16 | 5.47 | 87.75 | 5.92 | 100.69 | 3.49 | 91.23 | 7.61 | |
| 400 | 61.47 | 4.49 | 111.55 | 10.49 | 88.29 | 5.74 | 95.17 | 1.45 | |
| Palmatine | 50 | 80.02 | 4.49 | 92.19 | 3.06 | 96.76 | 2.06 | 101.13 | 5.51 |
| 200 | 93.30 | 8.91 | 94.34 | 2.86 | 103.46 | 1.43 | 99.49 | 4.39 | |
| 400 | 72.75 | 5.30 | 96.86 | 6.57 | 100.34 | 4.87 | 103.47 | 2.28 | |
| Jatrorrhizine | 4 | 78.53 | 2.96 | 107.31 | 0.66 | 111.02 | 2.67 | 104.77 | 5.49 |
| 40 | 84.20 | 3.67 | 102.46 | 4.92 | 101.85 | 2.30 | 95.85 | 1.64 | |
| 160 | 68.36 | 5.93 | 108.13 | 5.05 | 98.54 | 5.39 | 101.95 | 1.80 | |
| Components | Conc. (ng ml−1) | RSD of IS normalised MF (%) | Components | Conc. (ng ml−1) | RSD of IS normalised MF (%) |
|---|---|---|---|---|---|
| Oroxylin A | 20 | 7.72 | Liquiritin | 16 | 12.9 |
| 400 | 14.8 | 640 | 13.2 | ||
| Baicalin | 40 | 13.0 | Liquiritigenin | 20 | 6.96 |
| 800 | 13.5 | 800 | 10.4 | ||
| Baicalein | 40 | 10.4 | Isoliquiritin | 6 | 14.1 |
| 800 | 8.74 | 240 | 12.8 | ||
| Wogonoside | 40 | 13.1 | Isoliquiritigenin | 2 | 9.31 |
| 800 | 14.8 | 80 | 6.42 | ||
| Wogonin | 40 | 6.54 | Berberine | 100 | 8.53 |
| 800 | 13.8 | 800 | 10.9 | ||
| Chrysin | 4 | 10.6 | Coptisine | 50 | 10.5 |
| 160 | 11.8 | 400 | 9.23 | ||
| Scutellarin | 6 | 12.7 | Palmatine | 50 | 7.88 |
| 240 | 14.4 | 400 | 8.45 | ||
| Glycyrrhizic acid | 24 | 12.6 | Jatrorrhizine | 4 | 11.0 |
| 480 | 10.6 | 160 | 12.8 |
| Components | RE (%) | RSD (%) | Components | RE (%) | RSD (%) |
|---|---|---|---|---|---|
| Oroxylin A | 0.98 | 6.08 | Liquiritin | 8.98 | 6.28 |
| Baicalin | −2.56 | 4.71 | Liquiritigenin | −0.26 | 3.46 |
| Baicalein | −1.52 | 5.33 | Isoliquiritin | 14.50 | 2.61 |
| Wogonoside | −6.44 | 5.49 | Isoliquiritigenin | 4.15 | 3.19 |
| Wogonin | 4.92 | 4.22 | Berberine | −10.30 | 4.58 |
| Chrysin | 14.60 | 4.22 | Coptisine | −5.90 | 5.93 |
| Scutellarin | 3.60 | 5.45 | Palmatine | 7.50 | 4.15 |
| Glycyrrhizic acid | 1.52 | 5.30 | Jatrorrhizine | 3.52 | 5.07 |
3.3 Incidence of CPT-11-induced diarrhea
The animals' body weight (Fig. 3) and diarrheal symptoms were monitored after the first administration of CPT-11 throughout the experimental period. The body weight ratio in the GT group declined significantly (p < 0.05), reaching its lowest point at day 5 (Fig. 3). Delayed-onset diarrhea started at day 4 and became worst at day 5, with an average diarrhea score of 2.1.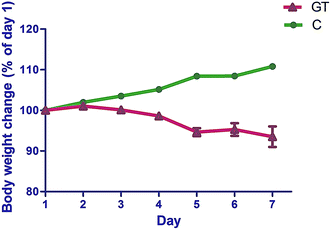 | ||
| Fig. 3 Body weight changes of normal rats (C) and rats with CPT-11 induced gastrointestinal toxicity (GT). | ||
3.4 Application of the method to pharmacokinetic study
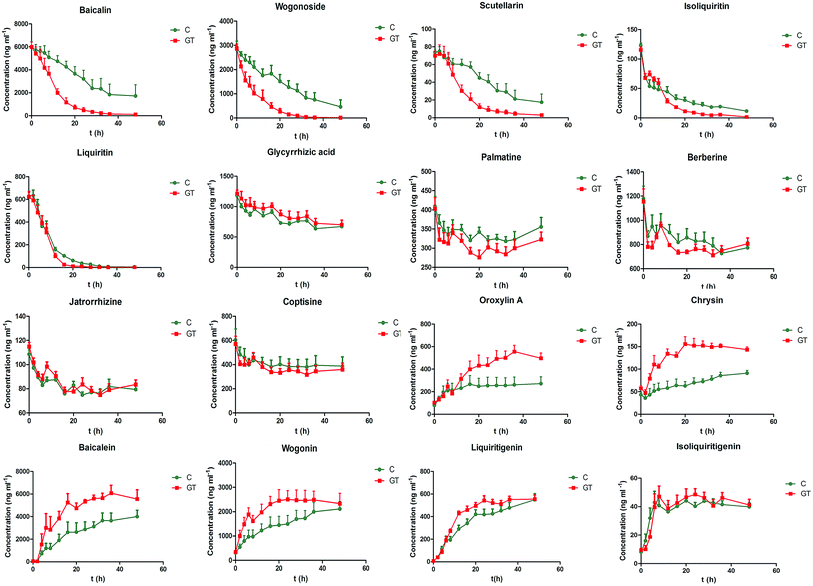
The developed ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was applied to determine the concentrations of all 16 analyzed components in the intestinal bacterial incubation system at 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36 and 48 h in groups C and GT. The mean concentration–time curves of the 16 analyzed components in the bacterial incubation solutions of normal rats (group C) and those with CPT-11-induced gastrointestinal toxicity (group GT) are shown in Fig. 4. A non-compartment model was used to calculate the pharmacokinetic parameters of the 16 analyzed components in the two groups, which are shown in Tables 7 and 8.| Compounds | Group | AUC0−t (ng h ml−1) | MRT0−t (h) | T1/2 (h) | CL (ml kg−1 h−1) |
|---|---|---|---|---|---|
| a Statistical difference between group C and GT, *p < 0.05, **p < 0.01. | |||||
| Baicalin | C | 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433.60 ± 69 433.60 ± 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 190.67 190.67 |
16.73 ± 4.49 | 15.66 ± 9.30 | 39.44 ± 20.95 |
| GT | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714.16 ± 16 714.16 ± 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666.12* 666.12* |
9.25 ± 1.85* | 8.50 ± 5.45 | 95.27 ± 24.95** | |
| Wogonoside | C | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098.78 ± 23 098.78 ± 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901.65 901.65 |
16.00 ± 4.39 | 14.50 ± 5.06 | 57.57 ± 17.88 |
| GT | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 466.17 ± 11 466.17 ± 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.68** 607.68** |
7.55 ± 1.89** | 6.47 ± 1.77 | 262.70 ± 160.04* | |
| Scutellarin | C | 2000.22 ± 755.61 | 16.67 ± 3.65 | 14.92 ± 4.75 | 100.71 ± 43.50 |
| GT | 1014.65 ± 461.50* | 10.42 ± 2.50* | 7.96 ± 2.28* | 220.64 ± 76.74* | |
| Glycyrrhizic acid | C | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695.00 ± 1605.87 695.00 ± 1605.87 |
22.05 ± 0.52 | 52.69 ± 7.89 | 8.56 ± 1.23 |
| GT | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 732.60 ± 6951.73 732.60 ± 6951.73 |
21.88 ± 1.18 | 51.35 ± 8.95 | 8.38 ± 2.27 | |
| Liquiritin | C | 6442.02 ± 895.80 | 7.78 ± 1.45 | 5.82 ± 0.92 | 100.27 ± 13.10 |
| GT | 5131.50 ± 1180.01 | 5.60 ± 0.60* | 4.73 ± 0.87 | 129.50 ± 28.79 | |
| Isoliquiritin | C | 1542.40 ± 407.50 | 16.10 ± 1.72 | 17.58 ± 5.91 | 116.41 ± 42.74 |
| GT | 1066.04 ± 226.65 | 9.79 ± 1.06** | 8.17 ± 2.55* | 189.66 ± 41.56* | |
| Berberine | C | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 818.20 ± 6052.09 818.20 ± 6052.09 |
22.73 ± 0.75 | 155.45 ± 83.45 | 16.86 ± 8.32 |
| GT | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123.20 ± 2206.68 123.20 ± 2206.68 |
22.92 ± 0.99 | 145.29 ± 79.21 | 18.61 ± 9.87 | |
| Coptisine | C | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468.60 ± 6889.76 468.60 ± 6889.76 |
22.98 ± 0.72 | 140.87 ± 66.10 | 18.73 ± 9.29 |
| GT | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548.40 ± 4558.49 548.40 ± 4558.49 |
22.85 ± 0.96 | 125.35 ± 77.86 | 23.39 ± 13.77 | |
| Palmatine | C | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.00 ± 1465.51 000.00 ± 1465.51 |
23.32 ± 0.49 | 178.43 ± 65.74 | 12.15 ± 3.68 |
| GT | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 523.80 ± 1827.48 523.80 ± 1827.48 |
23.12 ± 0.61 | 118.83 ± 57.10 | 20.31 ± 9.38 | |
| Jatrorrhizine | C | 3909.58 ± 265.03 | 23.04 ± 0.25 | 127.33 ± 46.51 | 17.27 ± 4.63 |
| GT | 4017.88 ± 369.03 | 22.80 ± 0.44 | 132.27 ± 44.07 | 17.67 ± 9.28 | |
| Compounds | Group | AUC0−t (ng h ml−1) | Cmax (ng ml−1) | Tmax (h) |
|---|---|---|---|---|
| a Statistical difference between group C and GT, *p < 0.05, **p < 0.01. | ||||
| Baicalein | C | 127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 761.98 ± 50 761.98 ± 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920.84 920.84 |
4082.50 ± 1123.58 | 36.00 ± 8.64 |
| GT | 221![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 389.61 ± 37 389.61 ± 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170.01* 170.01* |
6825.00 ± 1118.23* | 32.00 ± 13.47 | |
| Wogonin | C | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 504.30 ± 32 504.30 ± 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315.43 315.43 |
2189.00 ± 678.59 | 43.20 ± 6.57 |
| GT | 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.00 ± 31 497.00 ± 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 749.46 749.46 |
2714.00 ± 873.32 | 21.60 ± 17.52* | |
| Chrysin | C | 3324.62 ± 900.04 | 98.66 ± 17.32 | 39.20 ± 13.97 |
| GT | 6411.08 ± 530.95** | 174.00 ± 25.93** | 28.40 ± 15.52 | |
| Oroxylin A | C | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539.89 ± 6800.95 539.89 ± 6800.95 |
301.20 ± 149.08 | 34.40 ± 14.31 |
| GT | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 463.42 ± 4701.65 463.42 ± 4701.65 |
591.80 ± 129.03* | 30.40 ± 6.69 | |
| Liquiritigenin | C | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792.71 ± 3615.60 792.71 ± 3615.60 |
550.60 ± 119.37 | 42.40 ± 12.52 |
| GT | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028.03 ± 2263.81 028.03 ± 2263.81 |
577.60 ± 68.70 | 32.00 ± 10.20 | |
| Isoliquiritigenin | C | 1872.57 ± 271.96 | 51.34 ± 10.80 | 26.80 ± 15.47 |
| GT | 1957.04 ± 463.40 | 53.16 ± 11.26 | 23.20 ± 16.59 | |
A negative control experiment was carried out to demonstrate that the changes in the concentrations of the 16 analyzed components of SXD in the intestinal bacterial incubation system were caused by the bacteria. The SXD extract was anaerobically incubated in GAM broth in the absence of the intestinal bacteria for 48 h at 37 °C, then processed and analyzed via the proposed method. The peak areas obtained at 48 h were compared with those obtained at 0 h. The observed deviations in the peak area were calculated as percentages. The results revealed that the peak area percentages of the analyzed components were within the range of 92.7% to 109.3%, with RSDs of less than 3.45% (n = 5), which implied that the concentrations of the analyzed components were not affected by the anaerobic medium broth or cultured conditions.
Among the three flavonoid glycosides (scutellarin, baicalin and wogonoside) of SXD, the CL of scutellarin was greater than that of baicalin and wogonoside in the control group. The degree of metabolism was closely related to the chemical structure. Compared with the structures of baicalin and wogonoside, scutellarin exhibit one more 4′-position hydroxyl, which contributes to its excellent degree of microbial degradation.42 The lower CL of wogonoside indicated its stability to the bacteria, which resulted from the steric hindrance of methoxyl at the 8-position. In the GT group, the significantly increased CLs of baicalin, wogonoside and scutellarin implied that the bacteria from rats with CPT-11-induced gastrointestinal toxicity catalyzed the degradations of three flavonoid glycosides. CPT-11 has been reported to increase the levels of Enterococcus spp., Clostridium spp., Escherichia coli, Serratia spp., Staphylococcus spp., Peptostreptococcus spp. and Bacillus spp. in the colon.9,10 Among them, Clostridium spp., Escherichia coli and Staphylococcus spp. produced β-glucuronidase.10 The increased level of the above three species of bacteria up-regulated the expression of β-glucuronidase. The extent of the increase in the CL of wogonoside (approximately 4.6-fold) was greater than that of baicalin (approximately 2.4-fold) and scutellarin (approximately 2.2-fold), although the three flavonoid glycosides were all hydrolyzed by bacterial β-glucuronidase. The other metabolic pathway/degree of wogonoside was presumed to be altered in GT group. Moreover, the exposure levels (AUC0−t) and the MRT0−t of baicalin, wogonoside and scutellarin decreased in the GT group, which was indicative of an increase in their biotransformation rate.
In contrast to the above flavonoid glycosides, the concentration of liquiritin (a flavanone glycoside) in group C declined rapidly, reaching 10.2% of the initial level at 20 h, while the concentration in group GT declined to 4.12% at 16 h. The metabolic rate is related to the type and site of glycosidic linkage. Moreover, liquiritin undergoes deoxygenation and acetylation by bacterial enzymes besides hydrogenation, methylation and deglycosylation which are the main metabolic pathways of baicalin, wogonoside and scutellarin.43 The MRT0−t of liquiritin decreased in the group GT, while the AUC0−t of liquiritin was not significantly different from that in group C. This observation indicated that liquiritin could be completely degraded by intestinal bacteria within 48 h in both groups. However, CPT-11 could influence the bacteria-associated metabolic pathway or/and velocity of liquiritin. The increased level of Clostridium spp. by CPT-11 was speculated to accelerate the degradation rate of liquiritin.43 Similar changes were observed in the pharmacokinetic parameters of isoliquiritin, which exhibits a similar structure to that of liquiritin.
Although glycyrrhizic acid and baicalin are both glucuronide conjugates of aglycones, the CL of glycyrrhizic acid was significantly lower than that of the flavonoid glycosides. These results were supported by the report that the β-glucuronidase hydrolyzing glycyrrhizic acid might be different from the enzyme hydrolyzing baicalin, although the two compounds are both metabolized by β-glucuronidases.44 The former aimed to hydrolyze β-D-diglucuronide, while the latter might select β-D-monoglucuronide to hydrolyze. Notably, there were no significantly differences in the pharmacokinetic parameters of glycyrrhizic acid between the two groups in the present study. It can be speculated that CPT-11 alters the activity of the β-glucuronidase hydrolyzing β-D-monoglucuronide but not the enzyme hydrolyzing β-D-diglucuronide.
The concentration–time courses of the four alkaloids showed that the degradations of alkaloids by intestinal bacteria were relatively slow, and there were no significant differences in the pharmacokinetic parameters between the two groups. The slightly increased concentration of palmatine from 32 to 48 h resulted from the biotransformation of other alkaloids in Coptis chinensis.45 The increased concentration of berberine from 36 to 48 h could be attributed to the oxidization of its metabolite dihydroberberine back to berberine.27
CPT-11 may induce changes in the metabolic behavior of glycosides and aglycones due to its impact on intestinal bacteria, which accelerates the degradation rate of glycosides to improve the production of aglycones. Increased accumulation of aglycones produced by intestinal bacteria in the intestine might mean improving absorption of aglycones and increasing bioavailability of aglycones. Flavonoid aglycones resulting from the metabolism of the corresponding glycoside by intestinal bacteria, such as baicalein, chrysin, oroxylin A and wogonin, have anti-inflammatory effects,31 which may alleviate CPT-11-induced diarrhea. Moreover, chrysin has been shown to up-regulate UGT1A1 to improve the conversion of SN-38 to SN-38G in the gastrointestinal tract.47 Although CPT-11 does not alter the bacteria-associated metabolic behavior of berberine, berberine could be transformed to its intestine-absorbable form by the intestinal bacteria and enter into the blood to exert its anti-diarrheal action.27,48 Therefore, the interactions between CPT-11, intestinal bacteria and SXD are proposed, in which CPT-11 alters the intestinal bacteria qualitatively and quantitatively and thus changes metabolic behavior of SXD, resulting in protective constituents from SXD alleviating the gastrointestinal toxicity induced by CPT-11 in turn.
4. Conclusion
In the present study, a salting-out sample preparation and UHPLC-MS/MS method was developed for the determination of oroxylin A, baicalin, baicalein, wogonoside, wogonin, chrysin, scutellarin, glycyrrhizic acid, liquiritin, liquiritigenin, isoliquiritin, isoliquiritigenin, berberine, coptisine, palmatine and jatrorrhizine in a complex incubation system for rat intestinal bacteria for the first time. The method was rapid, simple and efficient, and help solving critical problems for analyses of target components in complex biological samples, such as eliminating interferences caused by biological matrix and non-target components, especially for analyses of those in a complex mixture consisting of TCM and intestinal bacteria incubation system. The proposed method was successfully applied to the intestinal bacteria-associated pharmacokinetics of the above-mentioned components in vitro, offering technical references in the field of research on the interaction between intestinal bacteria and TCM. This study was also the first to compare the pharmacokinetic parameters of the 16 components in bacterial incubation solutions from normal rats and those with CPT-11-induced gastrointestinal toxicity. Our findings will be useful for achieving a deeper understanding of the mechanisms involved in the changes in intestinal bacteria induced by CPT-11 and further pharmacokinetic comparisons of the components between normal rats and those with CPT-11-induced gastrointestinal toxicity in vivo. In summary, the developed UHPLC-MS/MS method is useful for the evaluation of SXD components in biological processes, and the intestinal bacteria-based pharmacokinetic method applied in the present investigation will likely be beneficial to the study of interactions between TCMs and chemical drugs in current clinical practice.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant no. 81274054), the Beijing Municipal Natural Science Foundation (grant no. 7142109), the Science and Technology Major Programmer for Major Drug Discovery (grant no. 2012ZX09301002-001-028) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (grant no. 2016-I2M-1-012). The authors are very thankful to the Drug Discovery Facility, which belongs to the Center of Biomedical Analysis in Tsinghua University.References
- P. Herviou, D. Richard, L. Roche, J. Pinguet, F. Libert, A. Eschalier, X. Durando and N. Authier, J. Pharm. Biomed. Anal., 2016, 118, 284–291 CrossRef CAS PubMed.
- K. Takasuna, T. Hagiwara, M. Hirohashi, M. Kato, M. Nomura, E. Nagai, T. Yokoi and T. Kamataki, Cancer Chemother. Pharmacol., 1998, 42, 280–286 CrossRef CAS PubMed.
- A. Kurita, S. Kado, N. Kaneda, M. Onoue, S. Hashimoto and T. Yokokura, Cancer Chemother. Pharmacol., 2000, 46, 211–220 CrossRef CAS PubMed.
- A. Kurita, S. Kado, N. Kaneda, M. Onoue, S. Hashimoto and T. Yokokura, Cancer Chemother. Pharmacol., 2003, 52, 349–360 CrossRef PubMed.
- M. Onoue, A. Kurita, S. Kado, T. Matsumoto, N. Kaneda, K. Uchida, I. Kato and T. Yokokura, Cancer Chemother. Pharmacol., 2008, 61, 595–605 CrossRef CAS PubMed.
- K. Takasuna, T. Hagiwara, K. Watanabe, S. Onose, S. Yoshida, E. Kumazawa, E. Nagai and T. Kamataki, Cancer Chemother. Pharmacol., 2006, 58, 494–503 CrossRef CAS PubMed.
- A. Kurita, S. Kado, T. Matsumoto, N. Asakawa, N. Kaneda, I. Kato, K. Uchida, M. Onoue and T. Yokokura, Cancer Chemother. Pharmacol., 2011, 67, 201–213 CrossRef CAS PubMed.
- K. Takasuna, T. Hagiwara, M. Hirohashi, M. Kato, M. Nomura, E. Nagai, T. Yokoi and T. Kamataki, Cancer Res., 1996, 56, 3752–3757 CAS.
- A. M. Stringer, R. J. Gibson, R. M. Logan, J. M. Bowen, A. S. J. Yeoh and D. M. K. Keefe, Cancer Biol. Ther., 2008, 7, 1919–1925 CrossRef CAS PubMed.
- A. M. Stringer, R. J. Gibson, R. M. Logan, J. M. Bowen, A. S. J. Yeoh, J. Burns and D. M. K. Keefe, Exp. Biol. Med., 2007, 232, 96–106 CAS.
- T. Nakao, N. Kurita, M. Komatsu, K. Yoshikawa, T. Iwata, T. Utusnomiya and M. Shimada, J. Surg. Res., 2012, 173, 341–347 CrossRef CAS PubMed.
- A. Kato, J. Ueyama, F. Abe, K. Hotta, I. Tsukiyama, T. Oshima, F. Kondo, H. Saito and T. Hasegawa, Anticancer Res., 2011, 31, 2915–2922 CAS.
- B. D. Wallace, H. Wang, K. T. Lane, J. E. Scott, J. Orans, J. S. Koo, M. Venkatesh, C. Jobin, L. A. Yeh, S. Mani and M. R. Redinbo, Science, 2010, 330, 831–835 CrossRef CAS PubMed.
- Y. Q. Gao, Y. C. Si, X. N. Liu, Q. F. Luo and X. Niu, Beijing Zhongyiyao Daxue Xuebao, 2004, 27, 47–49 Search PubMed.
- G. Chen, Y. Yang, M. Liu, Z. Teng, J. Ye, Y. Xu, X. Cai, X. Cheng, J. Yang, C. Hu, M. Wang and P. Cao, J. Ethnopharmacol., 2015, 166, 149–156 CrossRef PubMed.
- L. Q. Jia and Y. N. Lou, The effect of Chinese herbs in prevention and treatment of side-effect associated with new anticancer agents, The 12th annual meeting of China association for science and technology: The function and status of traditional Chinese medicine in common health accident, Fujian, Nov 2010, pp. 113–116 Search PubMed.
- J. Wang, L. Q. Jia, H. Y. Tan, L. Pan, L. L. Yu and B. Deng, Chin. J. Integr. Tradit. West. Med., 2015, 35, 1236–1243 Search PubMed.
- C. Deng, B. Deng, L. Jia, H. Tan, P. Zhang, S. Liu, Y. Zhang, A. Song and L. Pan, J. Evidence-Based Complementary Altern. Med., 2017 DOI:10.1155/2017/7350251.
- K. Takasuna, Y. Kasai, Y. Kitano, K. Mori, R. Kobayashi, T. Hagiware, K. Kakihata, M. Hirohashi, M. Nomura, E. Nagai and T. Kamataki, Jpn. J. Cancer Res., 1995, 86, 978–984 CrossRef CAS PubMed.
- T. Itoh, S. Itagaki, Y. Sumi, T. Hirano, I. Takemoto and K. Iseki, Cancer Chemother. Pharmacol., 2005, 55, 420–424 CrossRef CAS PubMed.
- P. Huang, S. Tan, Y. X. Zhang, J. S. Li, C. Chai, J. J. Li and B. C. Cai, J. Ethnopharmacol., 2014, 155, 649–664 CrossRef CAS PubMed.
- L. Wang, S. Y. Zhang, L. Chen, X. J. Huang, Q. W. Zhang, R. W. Jiang, F. Yao and W. C. Ye, Phytochem. Lett., 2014, 7, 89–92 CrossRef CAS.
- R. Krausse, J. Bielenberg, W. Blaschek and U. Ullmann, J. Antimicrob. Chemother., 2004, 54, 243–246 CrossRef CAS PubMed.
- T. Akao, Biol. Pharm. Bull., 2000, 23, 1418–1423 CAS.
- D. H. Kim, I. S. Jang, H. K. Lee, E. A. Jung and K. Y. Lee, Arch. Pharmacal Res., 1996, 19, 292–296 CrossRef CAS.
- Q. C. Yang, W. H. Wu, F. M. Han and Y. Chen, J. Pharm. Pharmacol., 2009, 61, 647–652 CrossRef CAS PubMed.
- R. Feng, J. W. Shou, Z. X. Zhao, C. Y. He, C. Ma, M. Huang, J. Fu, X. S. Tan, X. Y. Li, B. Y. Wen, X. Chen, X. Y. Yang, G. Ren, Y. Lin, Y. Chen, X. F. You, Y. Wang and J. D. Jiang, Sci. Rep., 2015, 5, 12155 CrossRef CAS PubMed.
- S. Xing, M. Wang, Y. Peng, D. Chen and X. Li, J. Ethnopharmacol., 2014, 152, 183–189 CrossRef CAS PubMed.
- G. Peng, H. Guan, X. Wang and Y. Shi, Acta Pharm. Sin. B, 2017, 7, 193–201 CrossRef PubMed.
- Y. Wang, R. Xu, J. Xiao, J. Zhang, X. Wang, R. An and Y. Ma, J. Pharm. Biomed. Anal., 2014, 88, 525–535 CrossRef CAS PubMed.
- L. Tong, M. Wan, L. Zhang, Y. Zhu, H. Sun and K. Bi, J. Pharm. Biomed. Anal., 2012, 70, 6–12 CrossRef CAS PubMed.
- H. J. Chung, S. Lim, I. S. Kim, Y. Bu, H. Kim, D. H. Kim and H. H. Yoo, Bull. Korean Chem. Soc., 2012, 33, 177–182 CrossRef CAS.
- H. L. Li, W. D. Zhang, C. Zhang, R. H. Liu, X. W. Wang, X. L. Wang, J. B. Zhu and C. L. Chen, J. Pharm. Biomed. Anal., 2006, 41, 1342–1346 CrossRef CAS PubMed.
- L. Liu and Z. Chen, Anal. Chim. Acta, 2012, 737, 99–104 CrossRef CAS PubMed.
- Z. J. Lin, S. X. Qiu, A. Wufuer and L. Shum, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 814, 201–207 CrossRef CAS PubMed.
- Q. Zou, P. Wei, J. Li, Z. X. Ge and P. Ouyang, Biomed. Chromatogr., 2009, 23, 54–62 CrossRef CAS PubMed.
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP), Guideline on Bioanalytical Method Validation, July 2011 Search PubMed.
- J. Xu, M. Zhao, D. Qian, E. X. Shang, S. Jiang, J. Guo, J. A. Duan and L. Du, J. Ethnopharmacol., 2014, 153, 368–374 CrossRef CAS PubMed.
- R. Xu, Y. Peng, M. Wang, L. Fan and X. Li, J. Ethnopharmacol., 2014, 158, 338–344 CrossRef CAS PubMed.
- D. Tang, Y. Yu, X. Zheng, J. Wu, Y. Li, X. Wu, Q. Du and X. Yin, J. Pharm. Biomed. Anal., 2014, 100, 1–10 CrossRef CAS PubMed.
- L. Yan, X. Yang, Z. Meng, Y. Yuan, W. Xiao, Z. Wang, W. Huang, Z. Yang and C. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 971, 81–88 CrossRef CAS PubMed.
- J. Xu, D. Qian, S. Jiang, J. Guo, E. X. Shang, J. A. Duan and J. Yang, Chromatographia, 2013, 76, 975–983 CAS.
- W. Zhang, S. Jiang, D. Qian, E. Shang and J. Duan, Biomed. Chromatogr., 2014, 28, 1271–1277 CrossRef CAS PubMed.
- Y. S. Kim, J. J. Kim, K. H. Cho, W. S. Jung, S. K. Moon, E. K. Park and D. H. Kim, J. Microbiol. Biotechnol., 2008, 18, 1109–1114 CAS.
- Y. Zhang, W. Wu, F. Han and Y. Chen, Biomed. Chromatogr., 2008, 22, 1360–1367 CrossRef CAS PubMed.
- Y. Pan, Z. Zhang, D. Ding and X. Jia, China J. Chin. Mater. Med., 2013, 38, 3239–3245 CAS.
- T. Walle, Y. Otake, A. Galijatovic, J. K. Ritter and U. K. Walle, Drug Metab. Dispos., 2000, 28, 1077–1082 CAS.
- C. Chen, Z. Yu, Y. Li, J. Fichna and M. Storr, Am. J. Chin. Med., 2014, 42, 1053–1070 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03521g |
| This journal is © The Royal Society of Chemistry 2017 |