Open Access Article
Open Access ArticleConfinement of alcohols to enhance CO2 capture in MIL-53(Al)†
Gerardo A. González-Martínez‡
a,
J. Antonio Zárate‡a,
Ana Martínez*a,
Elí Sánchez-González a,
J. Raziel Álvareza,
Enrique Limaa,
Eduardo González-Zamora
a,
J. Raziel Álvareza,
Enrique Limaa,
Eduardo González-Zamora *b and
Ilich A. Ibarra
*b and
Ilich A. Ibarra *a
*a
aLaboratorio de Fisicoquímica y Reactividad de Superficies (LaFReS), Instituto de Investigaciones en Materiales, Universidad Nacional Autónoma de México, Circuito Exterior S/N, CU, Del. Coyoacán, 04510, Ciudad de México, Mexico. E-mail: argel@unam.mx; egz@xanum.uam.mx; martina@unam.mx; Fax: +52-55-5622-4595
bDepartamento de Química, Universidad Autónoma Metropolitana-Iztapalapa, San Rafael Atlixco 186, Col. Vicentina, Iztapalapa, C. P. 09340, Ciudad de México, Mexico
First published on 10th May 2017
Abstract
CO2 capture of MIL-53(Al) was enhanced by confining MeOH and i-PrOH within its micropores. Compared to MIL-53(Al), results showed an approximately 1.3 fold increase in CO2 capture capacity (kinetic isothermal CO2 adsorption experiments), via confining small amounts of both alcohols. Adsorption–desorption properties are investigated for MeOH and i-PrOH and the enthalpy of adsorption, for MeOH and i-PrOH, was measured by differential scanning calorimetry (DSC): ΔH = 50 and 56 kJ mol−1, respectively. Regeneration (CO2 adsorption–desorption cycles) of the sample MeOH@MIL-53(Al) exhibited a loss on the CO2 capacity of only 6.3% after 10 cycles and the desorption is accomplished by only turning the CO2 flow off. Static CO2 adsorption experiments (at 196 K) demonstrated a 1.25-fold CO2 capture increase (from 7.2 mmol g−1, fully activated MIL-53(Al) to 9.0 mmol g−1, MeOH@MIL-53(Al)). The CO2 enthalpy of adsorption for MIL-53(Al) and Me@OHMIL-53(Al) were estimated to be ΔH = 42.1 and 50.3 kJ mol−1, respectively. Computational calculations demonstrated the role of the hydrogen bonds formed between CO2 molecules and confined MeOH and i-PrOH molecules, resulting in the enhancement of the overall CO2 capture.
Introduction
The physicochemical properties (e.g. dynamics and structure) in nanometre confining porous scales of condensed matter are considerably different to what is observed at the macroscopic level. Typically, gas solubility in solvents, including those confined in macroporous solid materials, is normally defined by Henry's Law, which postulates a linear relationship between the concentration of a dissolved gas and its partial pressure above the solvent.1 Although, some recent investigations have shown that the confinement of solvents in porous materials, considerably enhances the gas solubility with respect to the values predicted by Henry's law. This striking improvement is known as “oversolubility”.2 The oversolubility of confined solvents considerably modifies their viscosity, density, dielectric constant and specific heat.3 For example, Garcia-Garibay et al.4 demonstrated in a MOF material (UCLA-R3) that the confinement of DMF molecules considerably enhanced (4 orders of magnitude) its dynamic viscosity which was comparable to that of honey.Focusing on gas oversolubility (or gas enhancement by confining solvents), Luzar and Bratko5 showed by computational simulations (molecular dynamics, MD) an increase of N2 and O2 solubility in water (from 5-fold to 10-fold) under confinement conditions in hydrophobic mesopores. By confining n-hexane, CHCl3, EtOH and H2O in mesostructured materials (MCM-41, SBA-15 and silica aerogel), remarkable H2 solubility enhancements, were reported by Pera-Titus and co-workers.1,6 Pellenq7 confined N-methyl-2-pyrrolydone (NMP) in MCM-41 and demonstrated a 6-fold increase in CO2 solubility. Interestingly, Llewellyn8 reported a 5-fold increase in CO2 capture by confining water in a mesoporous MOF material entitled MIL-100(Fe). This striking CO2 increase was achieved when approximately 40 wt% of H2O was pre-adsorbed in the mesopores of MIL-100(Fe).
In all the previous examples only mesoporous materials showed gas oversolubility properties. Interestingly, for microporous materials this phenomenon has not been observed as thoroughly demonstrated by Llewellyn and co-workers8 in a MOF microporous material UiO-66. Thus, in order to referring to gas oversolubility it is necessary to incorporate (pre-adsorb) considerably high amounts of solvent (e.g. H2O, n-hexane and EtOH) prior any gas adsorption. For example, Farrusseng et al.9 remarkably reported a 22-fold improvement on the H2 uptake (at 298 K and 30 bar) on MIL-101(Cr) by confining n-hexane. By a solvent wet impregnation method,9 they loaded n-hexane into the fully activated MOF material and this corresponded to the 60% of the pore volume. In other words, 60% of the pore volume of MIL-101(Cr), was filled with n-hexane.
However, it is possible to confine small amounts of solvents in micropores materials to enhance gas adsorption properties. Certainly, this phenomenon cannot be referred as gas oversolubility since there is not “enough” solvent to solubilise gas molecules. Walton et al.10 exhibited that small amounts of water can enhance CO2 capture in microporous MOF materials. These small amounts of H2O can interact with selected functional groups within the pores of these materials. In particular, they demonstrated that hydroxyl (–OH) functional groups act as a directing agent for water molecules inside the pores allowing a more efficient and ordered packing of H2O.11 In addition, Yaghi12 showed that these functional groups (–OH), significantly improve the affinity of MOF materials to water.
Previously, we have demonstrated that small amounts of pre-adsorbed H2O into microporous MOF materials considerably enhanced their CO2 capture properties.13 Additionally, our research group started to investigate the confinement of alcohols in MOFs to increase their CO2 capture capabilities and this was exemplified with the pre-adsorption of small amounts of EtOH in InOF-1.14 Therefore, we continue with the development of hybrid adsorbent MOF materials (via confining small amounts of alcohols within their micropores) which can contribute to new CO2 capture technologies.15 Among ‘the twelve principles of CO2 chemistry’ that Poliakoff16 proposed, CO2 capture constitutes one of these challenges (maximise integration). Therefore, the search for post-synthetically modified MOFs with high structural stability, adsorption capacity, solvent stability, fast sorption kinetics and mild regeneration conditions, is nowadays a very hot research field.17,18
Herein, we report the augmented CO2 capture properties of the microporous material MIL-53(Al), first reported by Serre and co-workers,19 upon confining small amounts of alcohols (methanol and isopropanol), together with MeOH and i-PrOH adsorption–desorption properties of MIL-53(Al).
Experimental section
Synthetic preparations
MIL-53(Al), [Al(OH)(BDC)], was synthesised via a continuous flow process,20 using merely water as the reaction medium. After the Al(III)-MOF material was synthesised, it went through a calcination process (extraction of terephthalic acid from within the pores of MIL-53(Al)) by heating the material up to 330 °C for 3 days. Thermogravimetric analysis (calcined MIL-53(Al)) (see Fig. S1, ESI†) and bulk powder X-ray diffraction patterns (see Fig. S2, ESI†) of the calcined MIL-53(Al) confirmed the retention of its structural integrity upon terephthalic acid removal. It is important to clarify, as previously reported,19b,c that the calcined MIL-53(Al), at room temperature, corresponds to the lt form (water molecules are located inside the channels of the material).19b,c When the calcined MOF sample is activated (180 °C and 10−3 bar for 2 h) there is a transformation from the lt form to the ht form.19b,c This characteristic structural transformation is very well known as the breathing effect of MIL-53.19d N2 adsorption isotherms for activated MIL-53(Al), vide supra, at 77 K were used to estimate the BET surface area (0.01 < P/P0 < 0.04) of 1098 m2 g−1.Adsorption isotherms for N2, CO2, MeOH and i-PrOH (isopropanol)
N2 isotherms (up to 1 bar and 77 K) were recorded on a Belsorp mini II analyser under high vacuum in a clean system with a diaphragm pumping system. CO2 isotherms up to 1 bar at 196 K, 212 K and 231 K, were recorded on a Belsorp HP (High Pressure) analyser. MeOH and i-PrOH isotherms were recorded in a DVS Advantage 1 instrument from Surface Measurement System. Ultra-pure grade (99.9995%) N2 and CO2 gases were purchased from PRAXAIR.Kinetic CO2 uptake experiments
Kinetic experiments were performed by using a thermobalance (Q500 HR, from TA) at 30 °C with a constant CO2 flow (60 mL min−1).Results and discussion
Methanol and isopropanol adsorption studies
Methanol (MeOH) and isopropanol (i-PrOH) adsorption properties were investigated for MIL-53(Al). First, a sample of the calcined MIL-53(Al) was placed in an analyser cell (DVS Advantage 1 instrument) and activated at 180 °C for 2 h and under a flow of N2. When the sample was fully degassed and cooled down to 30 °C, a methanol adsorption–desorption isotherm was carried out from %P/P0 = 0 to 85 (Fig. 1). The adsorbed amount of MeOH gradually increased with increasing pressure up to %P/P0 = 10. Then, a rapid MeOH uptake was observed in the pressure range from %P/P0 = 10 to 42. Finally, from %P/P0 = 12 to 85 there was a slow but gradual weight increase, and the maximum MeOH uptake was ∼8.1 mmol g−1 (26.1 wt%). The overall MeOH isotherm exhibited a sigmoidal shape and a strong hysteresis loop (at %P/P0 = 2–12) was observed with marked stepped profiles in the desorption phase (Fig. 1, open circles). The pore dimensions of activated MIL-53(Al), ht form,19b,c are approximately 2.6 Å × 13.6 Å which are considerably much larger than the kinetic diameter of methanol (∼3.6 Å). Thus, the observed hysteresis is most likely due to moderately strong host–guest interactions, which result in an enhanced affinity of MIL-53(Al) for MeOH. These interactions are the result of methanol molecules forming hydrogen bonds with the bridging hydroxo functional groups (μ2-OH), within the pores. This phenomenon was previously observed in a remarkable work by Maurin et al.19e They studied the methanol adsorption properties of MIL-53(Cr), an isostructural material to MIL-53(Al), finding a very similar MeOH adsorption–desorption isotherm and a strong and localised hydrogen bond between MeOH and the hydroxo functional group (μ2-OH).19e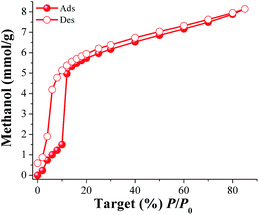 | ||
| Fig. 1 Methanol adsorption isotherm at 30 °C of MIL-53(Al) from %P/P0 = 0 to 85. Solid circles represent adsorption, and open circles show desorption. | ||
Later, a new calcined MIL-53(Al) sample was activated (as previously described) and an isopropanol adsorption–desorption isotherm was performed from %P/P0 = 0 to 85 at 30 °C (Fig. 2). To the best of our knowledge, this is the first time that the adsorption properties of isopropanol (i-PrOH) are described in MIL-53(Al). From 0 to 10 (%P/P0) the uptake of i-PrOH was rapidly increased (indicative of favourable host–guest interactions) achieving an i-PrOH uptake of approximately 2.5 mmol g−1 (15.3 wt%). Then, from %P/P0 = 10 to 85 a much slower (but constant) alcohol uptake was observed with a maximum of approximately 3.8 mmol g−1 (23.2 wt%). A relatively strong hysteresis was observed in the desorption phase (Fig. 2, open symbols). This hysteresis occurred mainly in the low pressure range from %P/P0 = 0 to 10. As in the previous case (MeOH), the kinetic diameter of i-PrOH is 4.6 Å which is considerably smaller than the pore openings of activated MIL-53(Al), (2.6 Å × 13.6 Å, ht form),19b,c suggesting again, the formation of hydrogen bonds with the bridging hydroxo functional groups (μ2-OH).19e
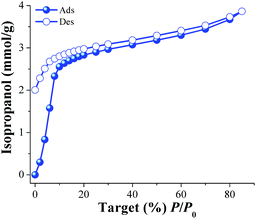 | ||
| Fig. 2 Isopropanol adsorption isotherm at 30 °C of MIL-53(Al) from %P/P0 = 0 to 85. Solid circles represent adsorption, and open circles show desorption. | ||
When both alcohols (MeOH and i-PrOH) uptakes in MIL-53(Al) are compared at low loadings (%P/P0 = 0 to 10), there is a clear preference for i-PrOH over MeOH (2.5 vs. 1.4 mmol g−1, respectively) which can be attributed to a stronger interaction of i-PrOH with the material. In order to confirm this, the enthalpy of adsorption (ΔH) for i-PrOH was experimentally measured by differential scanning calorimetry (DSC) from room temperature to 600 °C (with a ramp of 5 °C min−1). The ΔH value was equal to 56 kJ mol−1 (see Fig. S3 ESI†). When the same experimental determination was performed for MeOH, the ΔH value was equal to 50 kJ mol−1 (see Fig. S4 ESI†), in good correlation with the alcohol uptakes (low loadings). When the total uptake of these alcohols is compared (MeOH = 8.1 mmol g−1 and i-PrOH = 3.8 mmol g−1), MIL-53(Al) can adsorb considerably more MeOH than i-PrOH, presumably, due to the size of the alcohol molecules (methanol is smaller than isopropanol; kinetic diameters = 3.6 Å and 4.6 Å, respectively).
CO2 capture studies
Isothermal and dynamic CO2 adsorption experiments (kinetic) were performed on desolvated MIL-53(Al). First, a sample of the calcined MIL-53(Al) was placed inside a thermobalance (Q500 HR) and activated by heating from room temperature to 180 °C for 2 h and under a flow of pure N2 gas flow. Then, the sample was cooled down to 30 °C under N2 flow. After the sample had reached 30 °C, the N2 purge flow was switched to 60 mL min−1 of CO2. Fig. 3 (MIL-53(Al)) exhibits the kinetic CO2 uptake experiment at 30 °C. At this temperature, the material showed the maximum weight percentage gain, which indicates the maximum amount of CO2 captured. This amount corresponds to 3.5 wt%, which was rapidly reached after only 4 min remains constant until the end of the experiment (12 min), Fig. 3 (MIL-53(Al)).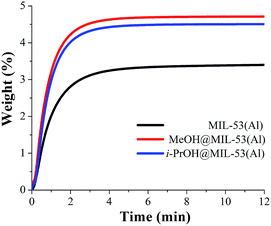 | ||
| Fig. 3 Kinetic CO2 uptake experiments performed at 30 °C with a CO2 flow of 60 mL min−1 in MIL-53(Al), (black curve); MeOH@MIL-53(Al), (red curve) and i-PrOH@MIL-53(Al), (blue curve). | ||
Later, a calcined sample of MIL-53(Al) was activated (180 °C for 2 h and under a flow of N2), cooled down to 30 °C (under N2) and saturated with MeOH (see ESI†). After an activation protocol (see ESI†) the residual amount of MeOH was equal to 2 wt%. The reproducibility of this protocol was confirmed by performing 5 independent experiment (see ESI†). Hereinafter, this sample will be referred as MeOH@MIL-53(Al), [Al(OH)(BDC)]·MeOH0.08. Similar procedure was carried out for sample i-PrOH@MIL-53(Al), [Al(OH)(BDC)]·iPrOH0.05, as it is described in the ESI.†
It was decided to only pre-adsorbed small amounts of alcohols (MeOH and i-PrOH) in MIL-53(Al) samples, based on the investigation of confined H2O in the micropores of MIL-53(Cr),21 (a MOF material which is isostructural to MIL-53(Al)). Then, Paesani and co-workers21 demonstrated by computational infrared spectroscopy that at low water loadings, these water molecules interact strongly with the hydroxo (μ2-OH) functional groups, via hydrogen bonding, which are located inside the pore walls of MIL-53(Cr). Continuing with the investigation of MIL-53, by different calculation methodologies, Haigis22 employed molecular dynamics (MD), to show that H2O molecules can form strong hydrogen bonds with the μ2-OH functional groups, in MIL-53(Cr), as a function of water loading. In addition, Maurin et al.23 corroborated by GCMC computational simulations, in MIL-53(Cr), that at low water loadings, these H2O molecules are regularly accommodated inside all the pores of the material.
Certainly, all the previous computational approaches indicated the role of the μ2-OH functional groups (inside the micropores of MIL-53(Cr)) to “pin” small amounts of water via hydrogen bonding. Complementing to these calculations, we experimentally demonstrated that small amounts of EtOH (2.6 wt%), confined within the micropores of InOF-1 (ref. 14) can: (i) hydrogen-bond to the similar hydroxo functional (In2(μ2-OH)) which was visualised by single crystal X-ray diffraction; and (ii) significantly enhance CO2 capture (2.7 fold).
Therefore, we rationalised the hypothesis of low MeOH and i-PrOH loadings where the micro-porous channels of MIL-53(Al) could efficiently accommodate these alcohols molecules. As a result of this very well ordered positioning of MeOH and i-PrOH, these confined alcohol molecules could help to pack more efficiently CO2 molecules and finally enhance the total CO2 capture.
Then, a kinetic CO2 experiment, at 30 °C, was carried out on the MeOH@MIL-53(Al) sample. The maximum amount of CO2 captured corresponded to 4.7 wt%, which was reached at approximately 4 min and it was constant until the end of the experiment (12 min), Fig. 3 (MeOH@MIL-53). It is important to mention that samples of MeOH@MIL-53(Al) were prepared with anhydrous methanol (<0.005% water) and methanol (reagent alcohol, 95%). The kinetic CO2 experiments showed no difference in the maximum amount of CO2 captured. Additionally, we also tried different small residual-amounts of MeOH: 3%, 4% and 5% and the best result was obtained with 2 wt% of MeOH.
Therefore, the CO2 capture was approximately 1.3-fold improved (from 3.5 wt% to 4.7 wt%), when small amounts of MeOH were pre-adsorbed in MIL-53(Al). Moreover, the 1.3-fold increase was reached at the same time (∼4 min) than the MIL-53(Al) sample, showing that the CO2 adsorption kinetics were highly improved due to the MeOH presence.
Later, the sample i-PrOH@MIL-53(Al) was prepared as previously described (vide supra and see ESI†) where the amount of pre-adsorbed isopropanol was equal to 2 wt%. Fig. 3 exhibits the kinetic CO2 experiment performed on i-PrOH@MIL-53(Al) and the maximum amount of adsorbed CO2 (captured) was equal to 4.5 wt%. This value is slightly smaller to the one observed for sample MeOH@MIL-53(Al), indicating that the pre-adsorption of MeOH in MIL-53(Al) favours the overall capture of CO2. It is worth to emphasise that the confinement of small amounts of both alcohols (2 wt%) enhances the CO2 capture properties of this MOF material.
Long-term regeneration capacity is a fundamental parameter for any CO2 capture material and it is desirable to show very low energy requirements for CO2 release.24 In industrial separation processes this step is typically very expensive and complicated.25 Among many current methodologies to this target, perhaps, the most common is the use of vacuum and temperature swing adsorption. For example, Long and co-workers26 reported a working CO2 capacity (total CO2 adsorption) of ∼7 wt% at room temperature (25 °C) on mmen-CuBTTri. This MOF material was regenerated by switching the flow (15% CO2 in N2) to a pure N2 stream followed by increasing the temperature up to 60 °C. Denayer and co-workers27 reported a total CO2 adsorption of 3.7 wt% for NH2-MIL-53 and the regeneration of this material was performed under purge flow at 159 °C.
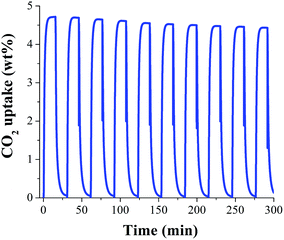
In order to evaluate the regeneration properties of MeOH@MIL-53(Al), a new sample was prepared (by confining 2 wt% of MeOH in MIL-53(Al)) and kinetic CO2 adsorption–desorption experiments, at 30 °C, were carried on (Fig. 4). We decided to only evaluate the regeneration properties of MeOH@MIL-53(Al) since it showed the best CO2 capture result. Each cycle consists of an adsorption step (15 min) and a desorption step (15 min), providing a cycling time of only 30 min without the use of N2 purge nor increasing the temperature.
Simply, by turning off the CO2 flow (corresponding to the desorption step) and keeping the adsorption temperature (30 °C), the total regeneration of the MeOH@MIL-53(Al) sample was accomplished. Form the first cycle (4.7 wt% CO2 adsorption) to the tenth cycle (4.4 wt% CO2 adsorption) it was observed a loss of the CO2 capacity (from 4.7 to 4.4 wt%) which represents a loss on the CO2 capacity of only 6.3% (Fig. 4). Although there was a small loss in the CO2 capacity, this result is noteworthy since there is no need to use a purge gas (e.g. N2) and more significantly no thermal re-activation of the sample is required, resulting in a very low cost separation process.
In order to describe the CO2 adsorption properties of MeOH@MIL-53(Al), we performed static (increasing the partial pressure from 0 to 1 bar) and isothermal (196 K) CO2 adsorption experiments on MeOH@MIL-53(Al) samples. It was decided to perform these experiments at 196 K since the adsorption of CO2 at 30 °C (303 K) is problematic due to proximity to the critical temperature of CO2.28 The uncertainly of the δCO2 (density) of the CO2 adsorbed, and since at that temperature the CO2 saturation pressure is extremely high, the range of P/P0 is limited to 0.02 at sub-atmospheric pressures.29 It has been proposed that adsorption in well-defined micropores occurs by a pore-filling mechanism rather than surface coverage.29,30 As an example, N2 molecules at 77 K can fill these micropores in a liquid-like fashion at very low relative pressures (below 0.01). On the other hand, CO2 adsorbed at approximately ambient temperatures (298 or 303 K) can only form a monolayer on the walls of the micropores.30 Therefore, to accomplish pore-filling within the micropores of MOFs and a more accurate description of the CO2 adsorption properties of these microporous materials, CO2 gas adsorption experiments at 196 K are preferred.31
First, a CO2 adsorption experiment at 196 K was carried on a fully activated (180 °C for 1 h and 10−3 bar) sample of MIL-53(Al) exhibiting a total CO2 uptake of 7.2 mmol g−1 (31.7 wt%), (see Fig. 5, MIL-53(Al)). Later, a MeOH@MIL-53(Al) sample was placed in a high-pressure cell (Belsorp HP) and gently evacuated to remove any absorbed moisture. The CO2 uptake was measured from 0 to 1 bar at 196 K and the resultant CO2 adsorption exhibited a characteristic Type-I isotherm with a total CO2 capture of 9.0 mmol g−1 (39.6 wt%), (Fig. 5, MeOH@MIL-53(Al)). Then, at 1 bar and 196 K, the CO2 capture was approximately 1.25-fold increased (from 7.2 to 9.0 mmol g−1) when small amounts of MeOH are present within the micropores of MIL-53(Al). The evaluation of the BET surface area of MeOH@MIL-53(Al) was equal to 762 m2 g−1 with a pore volume of 0.39 cm3 g−1 (lower values than for the fully activated MIL-53(Al), BET = 1096 m2 g−1 and pore volume = 0.56 cm3 g−1). In addition, we measured CO2 adsorption isotherms at 212 K (dry ice and chloroform bath) and 231 K (dry ice and acetonitrile bath), in order to calculate the enthalpy of adsorption (ΔH) by isosteric method for both samples: MIL-53(Al) and MeOH@MIL-53(Al), (see Fig. S5–S8 ESI†). The values were estimated to be 42.1 and 50.3 kJ mol−1 for MIL-53(Al) and MeOH@MIL-53(Al), respectively. Thus, ΔH for CO2 was enhanced when small quantities of MeOH are confined within the material MIL-53(Al).
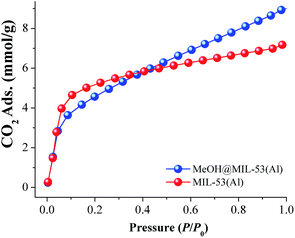 | ||
| Fig. 5 Static CO2 adsorption performed from 0 to 1 bar at 196 K on MIL-53(Al), (red circles) and MeOH@MIL-53(Al), (blue circles). | ||
Computational studies
In order to investigate the relationship between the presence of methanol and isopropanol (confined into MIL-53(Al)) and their affinity towards CO2, a model of the binuclear [Al2(μ2-OH)] building block (reactive section) was taken and the geometry was optimised (see ESI,† computational details). Since the most representative section of the model is related to the μ2-OH group, we decided to study the interactions between μ2-OH and the incorporation of different analytes (MeOH, i-PrOH and CO2). Fig. 6 shows the optimised structure of the proposed model (A) and the optimised structures for the model interacting with MeOH (B) and i-PrOH (C). As it was expected, both alcohols form hydrogen bonds with the μ2-OH group. Bond distances of these hydrogen bonds are very similar (1.39 and 1.40 Å). Our computations suggest that the interaction of both alcohol molecules (MeOH and i-PrOH) with the μ2-OH group of the model is very similar, since the distance of the hydrogen bond is practically the same. This result correlates with the small CO2 capture difference on confining small amounts of MeOH vs. i-PrOH within MIL-53(Al), see Fig. 3.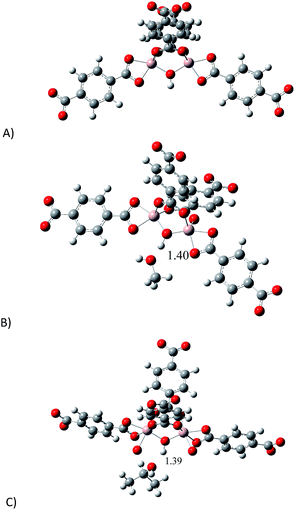 | ||
| Fig. 6 Optimised structures of: (A) proposed model to represent the active site; (B) model interacting with MeOH and (C) model interacting with i-PrOH. | ||
Later, we introduced a single CO2 molecule in all the optimised structures: proposed model (empty) and the models interacting with MeOH and i-PrOH. In Fig. 7 is presented the optimised structures of all the models interacting with CO2. As expected, the single CO2 molecule forms hydrogen bonds in all the structures. It is very well studied that the hydrogen bond distance is an indication on the strength of the interaction between molecules.32 Thus, If we compare the μ2-OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances, it is possible to observe that the structure containing MeOH establishes a shorter hydrogen bond (1.79 Å, Fig. 7A) than the structure with i-PrOH (1.85 Å, Fig. 7B). This slight difference, in the bond length, can actually explain the small dissimilarity in the overall capture of CO2 when small amounts of the alcohols are confined within the MOF material MIL-53(Al), see Fig. 3. Therefore, our computational calculations highlighted the significance of the hydrogen bonds formed between CO2 molecules and confined alcohol molecules: on increasing the strength (diminishing the distance) of the hydrogen bond between the μ2-OH group and the confined alcohol, the overall ability to capture CO2 increases.
O bond distances, it is possible to observe that the structure containing MeOH establishes a shorter hydrogen bond (1.79 Å, Fig. 7A) than the structure with i-PrOH (1.85 Å, Fig. 7B). This slight difference, in the bond length, can actually explain the small dissimilarity in the overall capture of CO2 when small amounts of the alcohols are confined within the MOF material MIL-53(Al), see Fig. 3. Therefore, our computational calculations highlighted the significance of the hydrogen bonds formed between CO2 molecules and confined alcohol molecules: on increasing the strength (diminishing the distance) of the hydrogen bond between the μ2-OH group and the confined alcohol, the overall ability to capture CO2 increases.
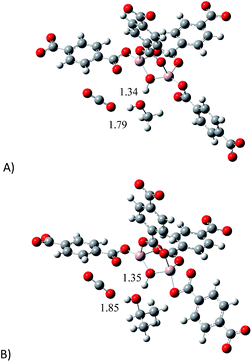 | ||
| Fig. 7 Optimised structures of: (A) proposed model interacting with MeOH and CO2 and (B) proposed model interacting with i-PrOH and CO2. | ||
Conclusions
The microporous Al(III)-based MOF material MIL-53(Al) exhibited, by kinetic isotherm CO2 experiments, a total CO2 uptake of 3.5 wt%. When confining small amounts of methanol (MeOH) and isopropanol (i-PrOH) (2 wt%) within MIL-53(Al), the CO2 capture incremented to 4.7 and 4.5 wt%, respectively, which approximately corresponds to a 1.3-fold increase. MeOH and i-PrOH adsorption properties were investigated for MIL-53(Al). Uptakes (at low loadings: %P/P0 = 0 to 10) of both alcohols in MIL-53(Al) showed a clear preference for i-PrOH over MeOH (2.5 vs. 1.4 mmol g−1, respectively) which was attributed to a stronger interaction of i-PrOH with the material. Enthalpy of adsorption (ΔH) for i-PrOH and MeOH were experimentally measured by differential scanning calorimetry (DSC) with values of ΔH = 56 and 50 kJ mol−1, respectively. MeOH@MIL-53(Al) can reversibly adsorb/desorb CO2 with a loss on the CO2 capacity of only 6.3% after 10 cycles and the desorption is performed by turning the CO2 flow off without the need to change the temperature or use inert gas. Static and isothermal CO2 experiments (from 0 to 1 bar of CO2 at 196 K) also demonstrated a 1.25-fold CO2 capture increase (from 7.2 mmol g−1, MIL-53(Al) fully activated to 9.0 mmol g−1, MeOH@MIL-53(Al)). The CO2 enthalpy of adsorption for MIL-53(Al) and MeOHMIL-53(Al) were calculated from CO2 adsorption isotherms at 212 K and 231 K to be ΔH = 42.1 and 50.3 kJ mol−1, respectively. Quantum calculations demonstrated the role of the hydrogen bonds formed between CO2 molecules and confined MeOH and i-PrOH molecules: on increasing the strength (shorting the distance) of the hydrogen bond between the hydroxo functional group (μ2-OH) group and the confined alcohol, the overall result is the enhancement of the CO2 capture. We are currently exploring other MOF materials and the confinement of different solvents (polar and non-polar) which could enhance CO2 capture in MOFs.Acknowledgements
The authors thank Dr A. Tejeda-Cruz (X-ray; IIM-UNAM), CONACyT Mexico (212318), PAPIIT UNAM Mexico (IN100415) for financial support. E. G.-Z. thanks CONACyT (236879), Mexico for financial support. Thanks to U. Winnberg (ITAM) for scientific discussions. Thanks to Eriseth R.-Morales for acces to Laboratorio de Análisis Térmico. NES supercomputer, provided by DGTIC-UNAM. Oralia L Jiménez, María Teresa Vázquez and Caín González for their technical support.Notes and references
- S. Miachon, V. V. Syakaev, A. Rakhmatullin, M. Pera-Titus, S. Caldarelli and J.-A. Dalmon, ChemPhysChem, 2008, 9, 78 CrossRef CAS PubMed.
- (a) S. Clauzier, L. N. Ho, M. Pera-Titus, D. Farrusseng and B. Coasne, J. Phys. Chem. C, 2014, 118, 10720 CrossRef CAS; (b) L. N. Ho, Y. Schuurman, D. Farrusseng and B. Coasne, J. Phys. Chem. C, 2015, 119, 21547 CrossRef CAS.
- (a) F. Volino, H. Gérard and S. Miachon, Ann. Phys., 1997, 22, 43 CrossRef CAS; (b) K. Morishige and M. Shikimi, J. Chem. Phys., 1998, 108, 7821 CrossRef CAS; (c) M. O. Kimball and F. M. Gasparini, Phys. Rev. Lett., 2005, 95, 165701 CrossRef PubMed; (d) U. Zammit, M. Marinelli, F. Mercuri and S. Paoloni, J. Phys. Chem. B, 2009, 113, 14315 CrossRef CAS PubMed.
- X. Jiang, H.-B. Duan, S. I. Khan and M. A. Garcia-Garibay, ACS Cent. Sci., 2016, 2, 608 CrossRef CAS PubMed.
- (a) A. Luzar and D. Bratko, J. Phys. Chem. B, 2005, 109, 22545 CrossRef CAS PubMed; (b) D. Bratko and A. Luzar, Langmuir, 2008, 24, 1247 CrossRef CAS PubMed.
- (a) M. Pera-Titus, R. El-Chahal, V. Rakotovao, S. Miachon and J.-A. Dalmon, ChemPhysChem, 2009, 10, 2082 CrossRef CAS PubMed; (b) M. Pera-Titus, S. Miachon and J.-A. Dalmon, AIChE J., 2009, 55, 434 CrossRef CAS; (c) V. Rakotovao, R. Ammar, S. Miachon and M. Pera-Titus, Chem. Phys. Lett., 2010, 485, 299 CrossRef CAS.
- (a) N. L. Ho, J. Perez-Pellitero, F. Porcheron and R. J.-M. Pellenq, J. Phys. Chem. C, 2012, 116, 3600 CrossRef CAS; (b) N. L. Ho, J. Perez-Pellitero, F. Porcheron and R. J.-M. Pellenq, Langmuir, 2011, 27, 8187 CrossRef PubMed; (c) N. L. Ho, F. Porcheron and R. J.-M. Pellenq, Langmuir, 2010, 26, 13287 CrossRef CAS PubMed.
- E. Soubeyrand-Lenoir, C. Vagner, J. W. Yoon, P. Bazin, F. Ragon, Y. K. Hwang, C. Serre, J.-S. Chang and P. L. Llewellyn, J. Am. Chem. Soc., 2012, 134, 10174 CrossRef CAS PubMed.
- S. Clauzier, L. N. Ho, M. Pera-Titus, B. Coasne and D. Farrusseng, J. Am. Chem. Soc., 2012, 134, 17369 CrossRef CAS PubMed.
- (a) H. Jasuja, Y.-G. Huang and K. S. Walton, Langmuir, 2012, 28, 16874 CrossRef CAS PubMed; (b) H. Jasuja, J. Zang, D. S. Sholl and K. S. Walton, J. Phys. Chem. C, 2012, 116, 23526 CrossRef CAS; (c) J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642 RSC; (d) N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575 CrossRef CAS PubMed.
- G. E. Cmarik, M. Kim, S. M. Cohen and K. S. Walton, Langmuir, 2012, 28, 15606 CrossRef CAS PubMed.
- H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yahgi, J. Am. Chem. Soc., 2014, 136, 4369 CrossRef CAS PubMed.
- (a) M. R. Gonzalez, J. H. González-Estefan, H. A. Lara-García, P. Sánchez-Camacho, E. I. Basaldella, H. Pfeiffer and I. A. Ibarra, New J. Chem., 2015, 39, 2400 RSC; (b) H. A. Lara-García, M. R. Gonzalez, J. H. González-Estefan, P. Sánchez-Camacho, E. Lima and I. A. Ibarra, Inorg. Chem. Front., 2015, 2, 442 RSC; (c) R. A. Peralta, B. Alcántar-Vázquez, M. Sánchez-Serratos, E. González-Zamora and I. A. Ibarra, Inorg. Chem. Front., 2015, 2, 898 RSC; (d) J. R. Álvarez, R. A. Peralta, J. Balmaseda, E. González-Zamora and I. A. Ibarra, Inorg. Chem. Front., 2015, 2, 1080 RSC; (e) M. Sánchez-Serratos, P. A. Bayliss, R. A. Peralta, E. González-Zamora, E. Lima and I. A. Ibarra, New J. Chem., 2016, 40, 68 RSC; (f) A. Zárate, R. A. Peralta, P. A. Bayliss, R. Howie, M. Sánchez-Serratos, P. Carmona-Monroy, D. Solis-Ibarra, E. González-Zamora and I. A. Ibarra, RSC Adv., 2016, 6, 9978 RSC; (g) E. Sánchez-González, J. R. Álvarez, R. A. Peralta, A. Campos-Reales-Pineda, A. Tejeda-Cruz, E. Lima, J. Balmaseda, E. González-Zamora and I. A. Ibarra, ACS Omega, 2016, 1, 305 CrossRef; (h) E. González-Zamora and I. A. Ibarra, Mater. Chem. Front., 2017 10.1039/C6QM00301J.
- R. A. Peralta, A. Campos-Reales-Pineda, H. Pfeiffer, J. R. Álvarez, J. A. Zárate, J. Balmaseda, E. González-Zamora, A. Martínez, D. Martínez-Otero, V. Jancik and I. A. Ibarra, Chem. Commun., 2016, 52, 10273 RSC.
- (a) D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058 CrossRef PubMed; (b) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, Z. T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724 CrossRef CAS PubMed.
- M. Poliakoff, W. Leitner and E. S. Streng, Faraday Discuss., 2015, 183, 9 RSC.
- (a) S. Yang, G. S. B. Martin, J. J. Titman, A. J. Blake, D. R. Allan, N. R. Champness and M. Schröder, Inorg. Chem., 2011, 50, 9374 CrossRef CAS PubMed; (b) S. Yang, X. Lin, A. J. Blake, G. S. Walker, P. Hubberstey, N. R. Champness and M. Schröder, Nat. Chem., 2009, 1, 487 CrossRef CAS PubMed; (c) A. J. Nuñez, L. N. Shear, N. Dahal, I. A. Ibarra, J. W. Yoon, Y. K. Hwang, J.-S. Chang and S. M. Humphrey, Chem. Commun., 2011, 47, 11855 RSC; (d) I. A. Ibarra, J. W. Yoon, J.-S. Chang, S. K. Lee, V. M. Lynch and S. M. Humphrey, Inorg. Chem., 2012, 51, 12242 CrossRef CAS PubMed; (e) X. Lin, A. J. Blake, C. Wilson, X. Z. Sun, N. R. Champness, M. W. George, P. Hubberstey, R. Mokaya and M. Schröder, J. Am. Chem. Soc., 2006, 128, 10745 CrossRef CAS PubMed; (f) P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80 CrossRef CAS PubMed; (g) O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 522 Search PubMed; (h) W. M. Bloch, A. Burgun, C. J. Coghlan, R. Lee, M. L. Coote, C. J. Doonan and C. J. Sumby, Nat. Chem., 2014, 6, 906 CrossRef CAS PubMed; (i) K. Okada, R. Ricco, Y. Tokudome, M. J. Styles, A. J. Hill, M. Takahashi and P. Falcaro, Adv. Funct. Mater., 2014, 24, 1969 CrossRef CAS; (j) H. Li, M. R. Hill, R. Huang, C. Doblin, S. Lim, A. J. Hill, R. Babarao and P. Falcaro, Chem. Commun., 2016, 52, 5973 RSC; (k) K. Sumida, N. Moitra, J. Reboul, S. Fukumoto, K. Nakanishi, K. Kanamori, S. Furukawa and S. Kitagawa, Chem. Sci., 2015, 6, 5938 RSC; (l) K. Hirai, S. Furukawa, M. Kondo, H. Uehara, O. Sakata and S. Kitagawa, Angew. Chem., Int. Ed., 2011, 50, 8057 CrossRef CAS PubMed.
- (a) T. Tozawa, J. T. A. Jones, S. I. Swamy, S. Jiang, D. J. Adams, S. Shakespeare, R. Clowes, D. Bradshaw, T. Hasell, S. Y. Chong, C. Tang, S. Thompson, J. Parker, A. Trewin, J. Bacsa, A. M. Z. Slawin, A. Steiner and A. I. Cooper, Nat. Mater., 2009, 8, 973 CrossRef CAS PubMed; (b) R. Dawson, D. J. Adams and A. I. Cooper, Chem. Sci., 2011, 2, 1173 RSC; (c) W. M. Bloch, R. Babarao, M. R. Hill, C. J. Doonan and C. J. Sumby, J. Am. Chem. Soc., 2013, 135, 10441 CrossRef CAS PubMed; (d) J. A. Mason, K. Sumida, Z. R. Herm, R. Krishna and J. R. Long, Energy Environ. Sci., 2011, 4, 3030 RSC; (e) R. Babarao, C. J. Coghlan, D. Rankine, W. M. Bloch, G. K. Gransbury, H. Sato, S. Kitagawa, C. J. Sumby, M. R. Hill and C. J. Doonan, Chem. Commun., 2014, 50, 3238 RSC; (f) I. A. Ibarra, A. Mace, S. Yang, J. Sun, S. Lee, J.-S. Chang, A. Laaksonen, M. Schröder and X. Zou, Inorg. Chem., 2016, 55, 7219 CrossRef CAS PubMed; (g) A. López-Olvera, E. Sánchez-González, A. Campos-Reales-Pineda, A. Aguilar-Granda, I. A. Ibarra and B. Rodríguez-Molina, Inorg. Chem. Front., 2017, 4, 56 RSC.
- (a) G. Férey, M. Latroche, C. Serre, F. Millange, T. Loiseau and A. Percheron-Guégan, Chem. Commun., 2003, 2976 RSC; (b) T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulell, M. Henry, T. Batailleand and G. Férey, Chem.–Eur. J., 2004, 10, 1373 CrossRef CAS PubMed; (c) P. L. Llewellyn, G. Maurin, T. Devic, S. Loera-Serna, N. Rosenbach, C. Serre, S. Bourrelly, P. Horcajada, Y. Filinchuk and G. Férey, J. Am. Chem. Soc., 2010, 132, 9488 CrossRef PubMed; (d) G. Férey and C. Serre, Chem. Soc. Rev., 2009, 38, 1380 RSC; (e) S. Bourrelly, B. Moulin, A. Rivera, G. Maurin, S. Devautour-Vinot, C. Serre, T. Devic, P. Horcajada, A. Vimont, G. Clet, M. Daturi, J.-C. Lavalley, S. Loera-Serna, R. Denoyel, P. L. Llewellyn and G. Férey, J. Am. Chem. Soc., 2010, 132, 9488 CrossRef CAS PubMed.
- P. A. Bayliss, I. A. Ibarra, E. Pérez, S. Yang, C. C. Tang, M. Poliakoff and M. Schörder, Green Chem., 2014, 16, 3796 RSC.
- G. R. Medders and F. Paesani, J. Phys. Chem. Lett., 2014, 5, 2897 CrossRef CAS PubMed.
- V. Haigis, F.-X. Coudert, R. Vuilleumier and A. Boutin, Phys. Chem. Chem. Phys., 2013, 15, 19049 RSC.
- F. Salles, S. Bourrelly, H. Jobic, T. Devic, V. Guillerm, P. Llewellyn, C. Serre, G. Férey and G. Maurin, J. Phys. Chem. C, 2011, 115, 10764 CAS.
- R. A. Khatri, S. S. C. Chuang, Y. Soong and M. Gray, Ind. Eng. Chem. Res., 2005, 44, 3702 CrossRef CAS.
- B. Metz, O. Davidson, H. de Coninck, M. Loos and L. Meyer, IPCC Special Report: Carbon Dioxide Capture and Storage, 2005 Search PubMed.
- T. M. Mcdonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022 RSC.
- S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef CAS PubMed.
- D. R. Lide, Handbook of Chemistry and Physics, CRC Press LLC, 2004 Search PubMed.
- F. Rouquerol, J. Rouquerol, K. S. W. Sing, P. Llewellyn and G. Maurin, Adsorption by Powders and Porous Solids; Principles, Methodology and Applications, Elsevier Press, 2014 Search PubMed.
- J. Garrido, A. Linares-Solano, J. M. Martín-Martínez, M. Molina-Sabio, F. Rodríguez-Reinoso and R. Torregrosa, Langmuir, 1987, 3, 76 CrossRef CAS.
- (a) H. J. Park and M. P. Sun, Chem.–Eur. J., 2008, 14, 8812 CrossRef CAS PubMed; (b) I. A. Ibarra, K. E. Tan, V. M. Lynch and S. M. Humphrey, Dalton Trans., 2012, 41, 3920 RSC; (c) S. M. Humphrey, J.-S. Chang, S. H. Jhung, J. W. Yoon and P. T. Wood, Angew. Chem., Int. Ed, 2007, 46, 272 CrossRef CAS PubMed; (d) J. Lee, N. W. Waggoner, L. Polanco, G. R. You, V. M. Lynch, S. K. Kim, S. M. Humphrey and J. L. Sessler, Chem. Commun., 2016, 52, 8514 RSC.
- R. Vargas, J. Garza, D. A. Dixon and B. Hay, J. Am. Chem. Soc., 2000, 122, 4750 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: TGA data, PXRD data, DSC data, activation protocol, isosteric enthalpy of adsorption data and theoretical calculations. See DOI: 10.1039/c7ra03608f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |