Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterization of some nanostructured composite oxides for low temperature catalytic combustion of dilute propane
C. Doroftei *a and
L. Leontieab
*a and
L. Leontieab
aAlexandru Ioan Cuza University, Integrated Center for Studies in Environmental Science for North-East Region (CERNESIM), 11 Carol I Blvd, 7000506 Iasi, Romania. E-mail: docorneliug@gmail.com; docorneliu@yahoo.com; Tel: +40 730 719855
bAlexandru Ioan Cuza University, Faculty of Physics, 11 Carol I Blvd, 7000506 Iasi, Romania
First published on 25th May 2017
Abstract
Powders of five nanosized perovskites, SrMnO3, SrCoO3, GdAlO3, FeMnO3, La0.6Pb0.2Mg0.2MnO3, and four ferrospinels, MgFe2O4, Ni0.5Co0.5Fe2O4, Ni0.5Co0.5Fe1.9Sc0.1O4, Ni0.5Co0.5Fe1.8Sc0.2O4, were prepared by a sol–gel self-combustion technique. The powders were catalytically tested in the flameless combustion of dilute propane at atmospheric pressure. All samples had nanocrystalline structures and the crystallite sizes were in the range of 26–89 nm. Catalytic testing showed a significant dependence of the catalytic performance on the catalyst composition. As experiments revealed, the La0.6Pb0.2Mg0.2MnO3 perovskite catalyst, with a specific surface area of 8.6 m2 g−1, exhibits the best performance in propane conversion, of about 92% at 350 °C. The lowest catalytic efficiency was obtained for FeMnO3 and SrCoO3 catalysts. Different reactivities of active oxygen species involved in the catalytic oxidation reaction determine registered differences in catalytic activity of oxide compounds. The results suggest that the La0.6Pb0.2Mg0.2MnO3 perovskite can be a suitable catalyst for catalytic combustion of dilute propane at low temperatures (below 400 °C).
1. Introduction
The progressive increase of volatile organic compound (VOC) emissions requires the development of new technologies for their removal from the atmosphere.1–3 Compared to conventional thermal incineration, catalytic combustion is considered a far more efficient method for removing low concentrations of VOCs from gaseous streams.2,4 Catalytic combustion is also more economical because it can operate at low temperatures (<500 °C) with diluted effluent streams (<1% VOC). Catalytic combustion performance is strongly dependent on the type of catalyst used. Two categories of catalysts have been typically employed for VOC oxidation reactions: noble metals and transition metal oxides.5–11 Noble metal catalysts display, in general, high hydrocarbon combustion activity, but they are expensive and more prone to deactivation by poisoning. The transition metal oxide catalysts are, generally, less active than noble metals, but more resistant towards poisoning and bear a lower cost.7,11Recently, considerable attention has been directed towards catalysts based on mixed metal oxides such as perovskite-type compounds, ABO3 (A is usually a rare earth and B is a transition metal),12–16 and ferrospinel-type compounds, AFe2O4 (A is a divalent metal).17–20
The preparation of catalysts with nanosized particles is the key to an efficient catalytic performance. In nanostructured materials, the interface between nanoparticles and surrounding medium plays a more important role than in the bulk materials. Moreover, the strong curvature of nanoparticles due to their small radius leads to an increased number of the structural defects at the nanoparticle surface, enhancing the surface reactivity. Various synthesis methods have been tested to obtain catalyst materials with superior microstructure, e.g. sol–gel,21 coprecipitation,22 or combustion reaction.23 Oliva et al.24 found that the preparation procedure can exert a remarkable influence on the physico-chemical characteristics and catalytic properties of the obtained materials.
In the present work a nonconventional method, sol–gel self-combustion,25 followed by a thermal treatment, was used to prepare low cost catalysts – perovskite and ferrospinel nanopowders. In this processing technology, the heat generated by a rapid exothermic combustion reaction was used for the synthesis reaction of oxide ceramics. The method requires basic equipment and assures a high purity and homogeneous mixing on the atomic scale. The ceramic materials prepared by sol–gel self-combustion have superior properties with respect to those made by conventional methods and are cheaper to produce. We analyzed comparatively the catalytic performances of the obtained nanopowders utilized in the catalytic flameless combustion of the dilute propane. The influence of various parameters such as chemical composition of the catalysts, crystallite size, surface specific area, reaction temperature and conversion degree of the propane has been investigated. The structural and morphological characterization of the materials was performed using X-ray diffraction (XRD), Scanning Electron Microscopy (SEM), Brunauer–Emmett–Teller (BET) surface area analysis, Energy Dispersive X-ray spectroscopy (EDX) and X-ray photoelectron spectroscopy (XPS).
2. Experimental
2.1. Catalysts preparation and characterization
Five nanograined perovskite powders of nominal compositions: SrMnO3, SrCoO3, GdAlO3, FeMnO3, La0.6Pb0.2Mg0.2MnO3 and four nanograined ferrospinel powders of nominal compositions: MgFe2O4, Ni0.5Co0.5Fe2O4, Ni0.5Co0.5Fe1.9Sc0.1O4, Ni0.5Co0.5Fe1.8Sc0.2O4 were prepared by sol–gel self-combustion procedure followed by heat treatment at 900 °C or 1000 °C (depending on the nature of crystalline phase of materials). We used metal nitrates, ammonium hydroxide and polyvinyl alcohol (PVA) as starting materials. A solution containing metal nitrates was mixed with an aqueous solution (10% concentration) of polyvinyl alcohol. A small amount of NH4OH solution (10% concentration) was dropped to adjust the pH value to about 8. This produced a sol of metal hydroxides and ammonium nitrate. By drying at 100 °C for 12 h, the sol was turned into a dried gel. The dried gel was ignited in a corner and a combustion reaction spontaneously propagated through the whole gel. The obtained powders were calcinated at 500 °C to eliminate the residual organic compounds. Finally, the calcined powders were annealed in air at 900 °C (ferrite powders) or 1000 °C (perovskite powders). Some details on the preparation procedure can be found in earlier publications.26,27 A total of 9 different samples were synthesized by sol–gel self-combustion, as mentioned in Table 1.| Sample symbol | Chemical composition | Heat treatment |
|---|---|---|
| F1 | MgFe2O4 | 900 °C, 20 min |
| F2 | Ni0.5Co0.5Fe2O4 | 900 °C, 4 h |
| F3 | Ni0.5Co0.5Fe1.9Sc0.1O4 | 900 °C, 4 h |
| F4 | Ni0.5Co0.5Fe1.8Sc0.2O4 | 900 °C, 4 h |
| P1 | SrMnO3 | 1000 °C, 7 h |
| P2 | SrCoO3 | 1000 °C, 7 h |
| P3 | FeMnO3 | 1000 °C, 7 h |
| P4 | GdAlO3 | 1000 °C, 7 h |
| P5 | La0.6Pb0.2Mg0.2MnO3 | 1000 °C, 2 h |
The crystal structure and phase composition of the samples were analyzed by XRD. X-ray diffraction measurements of the powders were performed at room temperature using a PANALYTICAL X' PERT PRO MPD powder diffractometer with Cu-Kα1 radiation (λ = 0.15405 nm). The XRD patterns were registered between 20 and 80° (2θ) at a rate of 2° min−1. The average crystallite size was estimated based on XRD peak broadening, using the Scherrer equation:28
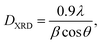 | (1) |
2.2. Catalytic activity measurements
Catalyst activity tests were performed at atmospheric pressure, in a laboratory scale with a flow type set-up (flow rate of 100 cm3 min−1, propane concentration in air of 1–2‰ and the gas hourly space velocity, GHSV, of 5100 h−1), previously described in ref. 30. The catalyst powder (0.3–0.5 g) was sandwiched between two layers of quartz wool in a quartz tubular microreactor (ϕ = 7 mm) placed in an electric furnace. The increase of the temperature was made in steps of 50 °C, from 50 to 550 °C. As a result of catalytic combustion, at every predetermined temperature, the gas concentration at the reactor exit was smaller than the inlet gas concentration. The catalytic activity of the nine oxide materials under study was evaluated in terms of propane conversion (to carbon dioxide) rate over these materials:26,30,31
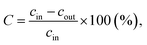 | (2) |
3. Results and discussion
3.1. Materials characterization
The XRD patterns at room temperature of the nine powdered samples are shown in Fig. 1. Well defined sharp peaks can be clearly seen in the diffraction patterns and show that as-synthesized materials have a good crystalline quality. Crystalline phases identified by comparing to a known standard, average crystallite size DXRD, derived from XRD data, specific surface area SBET and pore volume obtained from nitrogen adsorption/desorption isotherms at 77 K, are summarized in Table 2.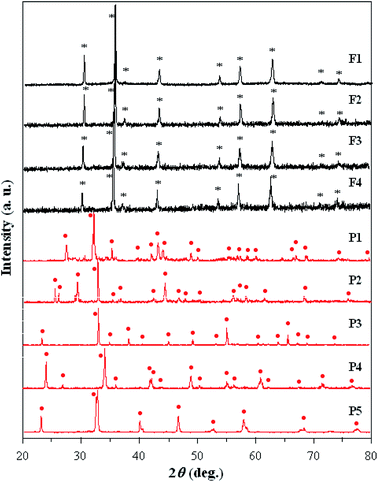 | ||
| Fig. 1 Powder X-ray diffraction patterns of the investigated samples. (*) – Main phases in studied ferrites; (•) – main phases in studied perovskites. | ||
| Sample symbol | Crystalline phases | DXRD (nm) | SBET (m2 g−1) | Pore volume (cm3 g−1) |
|---|---|---|---|---|
| F1 | Cubic spinel | 41.8 | 4.0 | 0.0060 |
| F2 | Cubic spinel | 41.7 | 26.5 | 0.0053 |
| F3 | Cubic spinel | 35.2 | 31.7 | 0.0072 |
| F4 | Cubic spinel | 34.8 | 32.3 | 0.0089 |
| P1 | Hexagonal perovskite | 88.9 | 2.2 | 0.0010 |
| P2 | Hexagonal perovskite | 59.9 | 1.9 | 0.0030 |
| P3 | Cubic perovskite | 59.2 | 3.2 | 0.0044 |
| P4 | Orthorhombic perovskite | 39.6 | 9.8 | 0.0018 |
| P5 | Cubic perovskite | 25.8 | 8.6 | 0.0021 |
On the basis of data presented in Fig. 1 and Table 2, the following remarks can be made:
(a) All samples were indexed as perovskite- or ferrospinel-type structures, without presence of any foreign phase;
(b) Irrespective of phase composition, all materials show nanoscale crystallinity. The average crystallite size, DXRD, was found to be in the range of 26–89 nm. The smallest crystallites were identified in La–Pb–Mg manganite perovskite powder;
(c) The average crystallite size decreased by scandium incorporation in Ni–Co ferrite (F3, F4 samples). This may be due to a structural disorder induced by the larger Sc-ions, which can lead to some delay in the growth of the ferrite crystallites;
(d) The SBET values are smaller for perovskites and ferrites containing only two cations and these results appear to be caused by an effective increase in the degree of crystallinity as a result of the heat treatment. These values are comparable to those reported by other authors,32 for similar chemical compositions.
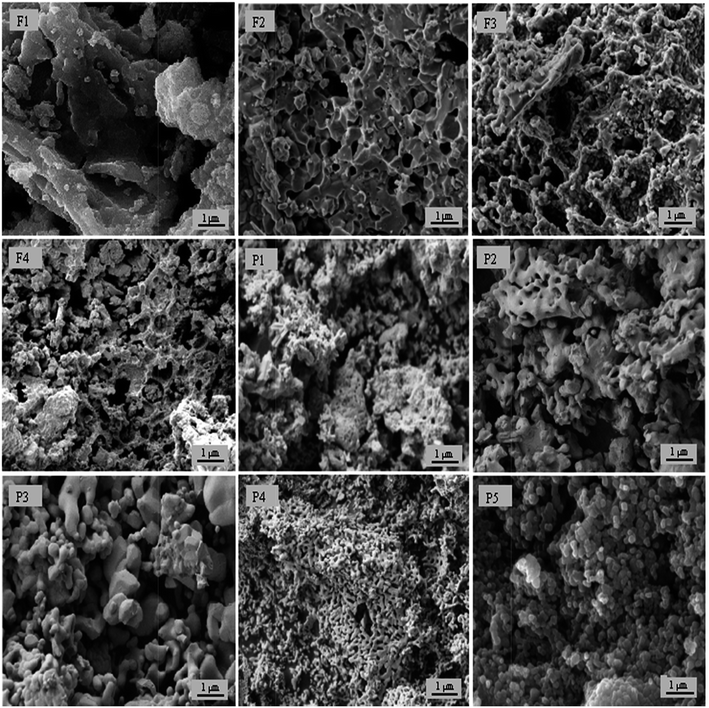
The microstructure plays a major role in the performance of a ceramic catalyst and for this reason it was examined by SEM. The surface morphology of the nine materials is shown in SEM micrographies given in Fig. 2. As can be seen, the grains have a rounded shape and the pores are not intragranular. These images also show that samples exhibit similar morphologies, being composed of aggregates of nanograins with irregular shapes and sizes. The images also reveal the presence of large interaggregate pores.
The surface elemental composition of the materials was inspected by EDX analysis. In Fig. 3 the EDX spectra for four catalyst materials are given and their elemental compositions are summarized in Table 3. Each provided composition represents the mean value of three determinations at different points of each sample. One can remark that the chemical composition of the samples is close to the nominal one, i.e. the M/Mt (see Table 3) ratios are close to the theoretical values given in parenthesis. This is proof of the homogeneous distribution of the elements in the solids prepared by sol–gel self-combustion technique.
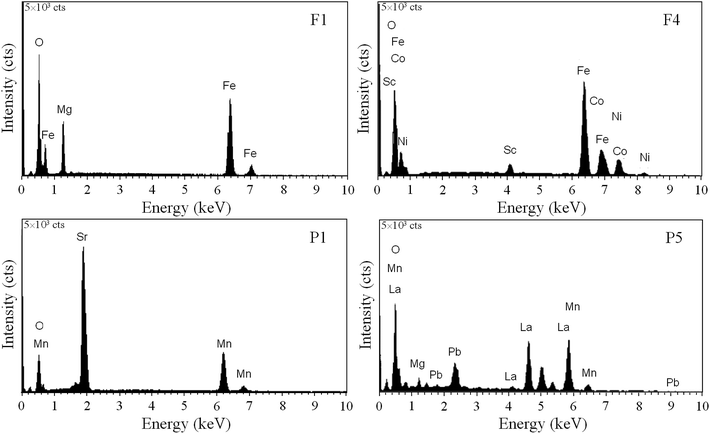 | ||
| Fig. 3 EDX spectra for MgFe2O4 (F1), Ni0.5Co0.5Fe1.8Sc0.2O4 (F4), SrMnO3 (P1) and La0.6Pb0.2Mg0.2MnO3 (P5). | ||
| MgFe2O4 | GdAlO3 | SrMnO3 | SrCoO3 | Ni0.5Co0.5Fe1.8Sc0.2O4 | La0.6Pb0.2Mg0.2MnO3 |
|---|---|---|---|---|---|
| a Mt is total amount of metallic composition on the surface.b In parenthesis are given theoretical values. | |||||
| O (at%) 54.44 | O (at%) 64.18 | O (at%) 62.11 | O (at%) 60.80 | O (at%) 49.39 | O (at%) 63.85 |
| Mg (at%) 15.08 | Gd (at%) 17.82 | Sr (at%) 21.50 | Sr (at%) 20.84 | Ni (at%) 8.09 | La (at%) 11.61 |
| Fe (at%) 30.48 | Al (at%) 18.00 | Mn (at%) 16.39 | Co (at%) 18.36 | Co (at%) 9.82 | Mn (at%) 18.04 |
| Mg/Mt 0.33 (0.33) | Gd/Mt 0.5 (05) | Sr/Mt 0.56 (0.5) | Sr/Mt 0.53 (0.5) | Sc (at%) 3.34 | Mg (at%) 4.02 |
| Fe/Mt 0.67 (0.66) | Al/Mt 0.5 (0.5) | Mn/Mt 0.44 (0.5) | Co/Mt 0.47 (0.5) | Fe (at%) 29.36 | Pb (at%) 2.47 |
| Ni/Mt 0.16 (0.17) | La/Mt 0.3 2 (0.3) | ||||
| Co/Mt 0.19 (0.17) | Mn/Mt 0.50 (0.5) | ||||
| Sc/Mt 0.07 (0.07) | Mg/Mt 0.11(0.1) | ||||
| Fe/Mt 0.58 (0.60) | Pb/Mt 0.07 (0.1) | ||||
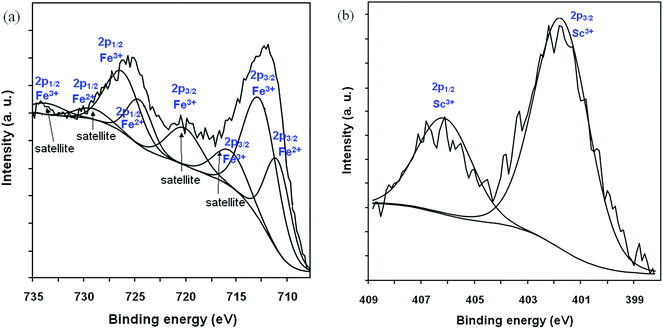
The XPS technique was used to distinguish the oxidation state of the cations present on the surfaces. Among the studied ferrites are presented the XPS spectra of the Ni0.5Co0.5Fe1.8Sc0.2O4 ferrite and among the studied perovskites are presented the XPS spectra of the La0.6Pb0.2Mg0.2MnO3 perovskite. Fig. 4(a) and (b) show the Fe 2p and Sc 2p, respectively, XPS spectra of Ni0.5Co0.5Fe1.8Sc0.2O4 ferrite. However, the analyses of Fe 2p spectra are relatively complex for ferrites. The divalent Fe 2p3/2 peak at 709.5 eV and the trivalent Fe 2p3/2 peak at 711.2 eV are respectively associated to satellite peaks at 715.5 eV and 719.0 eV.19,33–35 In the case of the sample with Sc0.2 the observed Fe 2p3/2 peaks with binding energies (BE) between 711.2 eV and 713.0 eV, and the associated satellite at around 716.3 eV and 720.0 eV (Fig. 4a), confirm the predominance of Fe3+ and a small fraction of Fe2+ present on the sample surface.19,35,36 The observed BE shifts can be attributed to the different surroundings of the Fe3+ ions in the A and B sites and of the Sc3+ ions (Fig. 4b) in the B (octahedral) site within the ferrite structure.
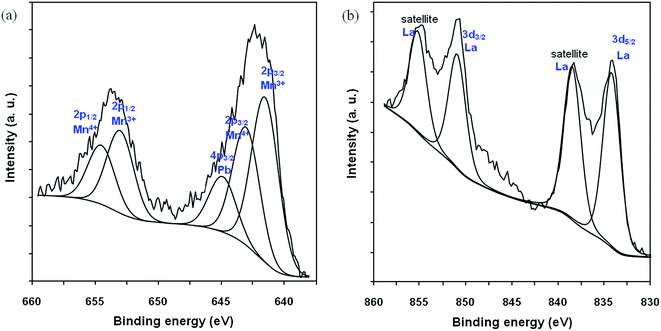
Fig. 5(a) and (b) show the Mn 2p and La 3d, respectively, XPS spectra of La0.6Pb0.2Mg0.2MnO3 perovskite. The La 3d level is characterised by a double peak for each components (La 3d3/2 and La 3d5/2). The Mn 2p peak was decomposed in 2 components. The full width at half maximum (FWHM) of which were imposed to be equal: a component at 653.6 attributed to Mn 2p1/2 and a component at 641.9 attributed to Mn 2p3/2.37 The binding energies of the elements present in the parent perovskite (LaMnO3) reported by Arendt et al.38 are the following: 641.9 eV for Mn 2p3/2, 834.0 eV for La 3d5/2, 851.0 eV for La 3d3/2, 529.1 eV for O(I) 1s and 530.6 eV for O(II) 1s. The binding energies of the elements present in the La0.67Pb0.33MnO3 reported by Kowalik et al.39 are the following: 641.1 eV for Mn3+ 2p3/2, 643.2 eV for Mn4+ 2p3/2, 645.7 eV for Pb 4p3/2 and 2.02 for the Mn3+/Mn4+ ratio. The binding energies of the perovskite catalyst used by us (La0.6Pb0.2Mg0.2MnO3) are characterised by values very close to those reported above: 641.5 eV for Mn3+ 2p3/2, 643.1 eV for Mn4+ 2p3/2, 644.9 eV for Pb 4p3/2, 653.1 eV for Mn3+ 2p1/2, 654.5 eV for Mn4+ 2p1/2, 834.2 eV for La 3d5/2 (838.4 eV for satellite), 850.9 eV for La 3d3/2 (855.2 eV for satellite) and 1.40 for the Mn3+/Mn4+ ratio.
3.2. Catalytic activity
The catalytic combustion tests of propane over the nine catalyst samples were performed as a function of temperature. Propane oxidation reaction on the catalyst surface takes place according to a radical mechanism consisting of several stages. Global oxidation reaction of propane, summing all stages of this mechanism, will be as follows:| C3H8 + 5O2 = 3CO2 + 4H2O. | (3) |
In one of these stages catalyst contribution occurs, which will speed up the process of combustion through its intervention from time to time, until all residues of hydrocarbon species are consumed, finally yielding CO2 and H2O.
Several repetitions of tests led to similar results, thus indicating reproducibility of actual experimental data. Some of these results were published in previous articles.30,40
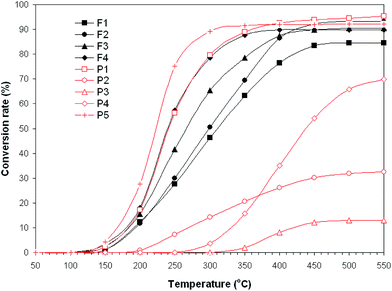
Results showing the temperature dependence of propane conversion in its combustion reaction over all catalysts are presented in Fig. 6. The catalysts exhibited substantial differences in catalytic activity, in the temperature range of 200–400 °C. The conversion data in Fig. 6 suggest a significant influence of the chemical composition of the catalyst on the propane conversion at different temperatures. It should be noted that no catalytic activity was observed at temperatures below 100 °C. Moreover, the catalytic activity of SrCoO3, GdAlO3 and FeMnO3 in propane combustion reaction started at temperatures over 200 °C and temperatures above that level had rather limited influence on their activity.
Among the nine catalysts, the La0.6Pb0.2Mg0.2MnO3 perovskite showed the highest propane combustion activity at low temperatures (below 350 °C). This catalyst was able to convert 50% of propane at temperature of 224 °C and 90% at 320 °C. The high improved catalytic activity may be attributed to the very small crystallites (26 nm), as well as to the oxygen vacancies generated by the presence of both manganese ions with variable valence,41,42 fact confirmed by us by analyzing XPS spectra in Fig. 5a. The partial substitution of La3+ ions by lower valence ions (as Pb2+–Mg2+) in LaMnO3 perovskite leads to the oxidation of Mn3+ to Mn4+ and the formation of oxygen vacancies (active sites for oxygen adsorption).41,42 The oxygen vacancies are essential for the adsorption/dissociation of oxygen molecules during the catalytic oxidation reaction and strongly affect the catalytic activity of the catalyst. More oxygen vacancies involve a larger density of adsorbed oxygen species (O−, O2−, O2−), weakly anchored on the catalyst surface, which favors the VOC oxidation. The larger the number of oxygen ions adsorbed, the faster the gas oxidation reaction would be.
From Fig. 6 one can also remark that SrMnO3 perovskite and Ni0.5Co0.5Fe1.8Sc0.2O4 ferrite show similar catalytic activities in the temperature range 150–350 °C, even if these catalysts display different compositions and crystallographic structures. Both catalysts can convert 50% of propane in CO2 and H2O at the same low temperature, of about 240 °C. Moreover, at 350 °C a high conversion degree of propane, of 88–89%, was obtained over the two catalysts.
Even at low conversions, CO was not observed among the final products and this indicates that propane was completely converted to CO2 and H2O.
The less efficient catalytic performance in propane conversion was registered for SrCoO3 (32% conversion rate at 500 °C) and FeMnO3 (13% conversion rate at 500 °C), although our previous work40 showed that these perovskites exhibit high catalytic activity towards acetone conversion. The reasons for such a selective catalytic activity of SrCoO3 and FeMnO3 are not yet clear. A dependence of activity on the surface area was not found. Other factors, such as structural defects or oxygen mobility, are likely to control the catalytic activity of oxide catalysts.
It is interesting to note, from the results presented in Fig. 6, that the La0.6Pb0.2Mg0.2MnO3 catalyst, which shows the highest propane combustion activity at low temperatures, is a pure cubic perovskite as is the FeMnO3 catalyst, which exhibits very low propane combustion activity. This suggests that the high catalytic activity of the first may be related to the presence of the structural defects (oxygen vacancies) created by the decreasing crystallite size (Table 2).
The main indicator of catalytic activity of a given catalyst is typically temperature T50 – the temperature required for 50% conversion of a gas. At T50 temperature the catalytic activity is sufficiently high and the interactions between catalyst surface and reactants are intense. The lower this parameter is, the higher the activity of the catalyst is. In Table 4 the values of T50 and T90 (the temperature required for 90% propane conversion) for all catalysts are listed. The T50 and T90 temperatures for the most active catalyst, La0.6Pb0.2Mg0.2MnO3, are much lower than those for the other catalysts. As present studies revealed, the degree of propane conversion for SrCoO3 and FeMnO3 perovskite catalysts is smaller than 50%. The catalytic performance of La0.6Pb0.2Mg0.2MnO3 catalyst in combustion reaction of propane is comparable to that of the Pt/Al2O3 noble metal catalyst, which could achieve 50% conversion of propane at 325 °C and 90% at 400 °C.43
| Symbol | T50 (°C) | T90 (°C) | Conv400 (%) | Reaction ratea (μmol s−1 m2) | Activation energyb (kJ mol−1) |
|---|---|---|---|---|---|
| a Reaction rate at low conversion per unit surface area of catalyst.b Apparent activation energy for low conversions. | |||||
| F1 | 311 | — | 76 | 8.14 × 10−2 | 75 |
| F2 | 300 | 418 | 86 | 4.57 × 10−2 | 82 |
| F3 | 267 | 432 | 88 | 4.66 × 10−2 | 65 |
| F4 | 240 | 380 | 90 | 5.53 × 10−2 | 50 |
| P1 | 240 | 400 | 90 | 9.8 × 10−2 | 31 |
| P2 | — | — | 26 | 3.8 × 10−2 | 48 |
| P3 | — | — | 8 | 6.1 × 10−2 | 80 |
| P4 | 438 | — | 35 | 7.8 × 10−2 | 71 |
| P5 | 224 | 320 | 92 | 11.0 × 10−2 | 28 |
It can be assumed that the catalytic combustion of propane vapor takes place in the presence of excess oxygen. The apparent activation energies (Ea) for the catalytic reactions were calculated by means of the Arrhenius-type plot of the natural logarithm of the reaction rate (k) at very low conversion, below 20%, versus inverse temperature (1/T).44–47 The Arrhenius equation can be given in the form:44,48
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT) exp(−Ea/RT)
| (4) |
Table 4 also includes the values of the kinetic parameters (apparent activation energy, Ea, and reaction rate, k) for propane oxidation over the nine catalysts. Reaction rates are normalized to the BET surface area, in order to compare the specific catalytic activities of the catalysts. One can observe that the normalized reaction rate changes from 3.8 × 10−2 μmol (s−1 m−2) to 11 × 10−2 μmol (s−1 m−2), in dependence of catalyst composition. The highest value of the reaction rate [11 × 10−2 μmol (s−1 m−2)] was obtained for propane combustion over La0.6Pb0.2Mg0.2MnO3 catalyst, which proved the best propane conversion rate, of 92%, at 350 °C (see Fig. 6).
In Table 4 one can observe the wide variation in determined activation energies from 28 kJ mol−1 to 85 kJ mol−1. The smallest value (28 kJ mol−1) of the activation energy was registered for La0.6Pb0.2Mg0.2MnO3 catalyst, for which the reaction rate was the highest. The differences observed between the activation energies of the nine catalysts allow one to speculate that the nature of the catalytic sites differs from one catalyst to another. We mention that the values of the kinetic parameters provided here are comparable to those presented by other authors for other oxides and VOCs.49,50
The present study suggests that La0.6Pb0.2Mg0.2MnO3 perovskite catalyst can be a promising candidate to substitute highly expensive noble metal catalysts used in propane combustion. Further investigations of other ferrite and perovskite compositions are required, in order to find new catalyst compositions with better performances.
4. Conclusions
Composite oxides catalysts (ferrites: MgFe2O4, Ni0.5Co0.5Fe2O4, Ni0.5Co0.5Fe1.9Sc0.1O4, Ni0.5Co0.5Fe1.8Sc0.2O4 and perovskites: SrMnO3, SrCoO3, GdAlO3, FeMnO3, La0.6Pb0.2Mg0.2MnO3) for propane combustion can be obtained by sol–gel self-combustion technique, followed by heat treatment. Among the nine studied compounds, La0.6Pb0.2Mg0.2MnO3 perovskite was found to exhibit the best catalytic performance with respect to propane combustion at low temperatures: a 92% conversion rate at 350 °C. This perovskite catalyst is a promising candidate to substitute expensive noble metal catalysts in propane combustion.References
- Z. Fan, P. Lioy, K. Veschler, N. Fiedler, H. Kipen and J. Zhang, Environ. Sci. Technol., 2003, 37, 1811–1821 CrossRef CAS PubMed.
- W. B. Li, W. B. Chu, M. Zhuang and J. Hua, Catal. Today, 2004, 93–95, 205–209 CrossRef CAS.
- W. B. Li, J. X. Wang and H. Gong, Catal. Today, 2009, 148, 81–87 CrossRef CAS.
- K. Everaert and J. Baeyens, J. Hazard. Mater., 2004, 109, 113–139 CrossRef CAS PubMed.
- H. G. Ahn, B. M. Choi and D. J. Lee, J. Nanosci. Nanotechnol., 2006, 6, 3599–3603 CrossRef CAS PubMed.
- L. van de Beld, M. C. van der Ven and K. R. Westerterp, Chem. Eng. Process., 1995, 34, 469–478 CrossRef CAS.
- S. C. Kim and W. G. Shim, Appl. Catal., B, 2010, 98, 180–185 CrossRef CAS.
- A. K. Sinha and K. Suzuki, Angew. Chem., Int. Ed., 2005, 44, 271–273 CrossRef CAS PubMed.
- D. Kim and S. Ihm, Environ. Sci. Technol., 2001, 35, 222–226 CrossRef CAS PubMed.
- K. Jirátová, J. Mikulová, J. Klempa, T. Grygar, Z. Bastl and F. Kovanda, Appl. Catal., A, 2009, 361, 106–116 CrossRef.
- M. R. Morales, B. P. Barbero and L. E. Cadús, Fuel, 2008, 87, 1177–1186 CrossRef CAS.
- L. G. Tejuca, J. L. G. Fierro and J. M. O. Tascon, Adv. Catal., 1989, 36, 327–328 Search PubMed.
- N. Yamazoe and Y. Teraoka, Catal. Today, 1990, 8, 175–199 CrossRef CAS.
- T. Seiyama, Catal. Rev.: Sci. Eng., 1992, 34, 281–300 CAS.
- A. Musialiak-Piotrowska and K. Syczewska, Catal. Today, 2000, 59, 269–278 CrossRef.
- M. A. Pena and J. L. G. Fierro, Chem. Rev., 2001, 101, 1981–2018 CrossRef CAS PubMed.
- A. Urda, A. Herraïz, A. Rédey and I. C. Marcu, Catal. Commun., 2009, 10, 1651–1655 CrossRef CAS.
- M. Florea, M. Alifanti, V. I. Parvulescu, D. Mihaila-Tarabasanu, L. Diamandescu, M. Feder, C. Negrila and L. Frunza, Catal. Today, 2009, 14, 361–366 CrossRef.
- A. S. Albuquerque, M. V. C. Tolentino, J. D. Alrdisson, F. C. C. Moura, R. de Mendonça and W. A. A. Macedo, Ceram. Int., 2012, 38, 2225–2231 CrossRef CAS.
- C. C. Ramankutty and S. Sugunan, Appl. Catal., A, 2001, 218, 39–51 CrossRef CAS.
- N. Chu, X. Wang, Y. Liu, H. Jin, Q. Wu, L. Li, Z. Wang and H. Ge, J. Alloys Compd., 2009, 470, 438–442 CrossRef CAS.
- A. B. Gadkari, T. J. Shinde and P. N. Vasambekar, Mater. Chem. Phys., 2009, 114, 505–510 CrossRef CAS.
- A. C. F. M. Costa, R. T. Lula, R. H. G. A. Kiminami, L. F. V. Gama, A. A. de Jesus and H. M. C. Andrade, J. Mater. Sci., 2006, 41, 4871–4875 CrossRef CAS.
- C. Oliva, L. Bonoldi, S. Cappelli, L. Fabbrini, I. Rossetti and L. Forni, J. Mol. Catal. A: Chem., 2005, 226, 33–40 CrossRef CAS.
- P. D. Popa, N. Rezlescu and G. Iacob, A new procedure for preparing ferrite powders, Romanian Patent No. 121300, OSIM, Bucharest, 2008.
- N. Rezlescu, E. Rezlescu, P. D. Popa, E. Popovici, C. Doroftei and M. Ignat, Mater. Chem. Phys., 2013, 137, 922–927 CrossRef CAS.
- C. Doroftei, P. D. Popa, F. Iacomi and L. Leontie, Sens. Actuators, B, 2014, 191, 239–245 CrossRef CAS.
- B. D. Cullity and R. S. Stock, Elements of X-Ray Diffraction, Prentice Hall, New Jersey, 3rd edn, 2001 Search PubMed.
- S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density, Kluwer Academic Publishers, Dordrecht-Boston-London, 2004 Search PubMed.
- N. Rezlescu, E. Rezlescu, P. D. Popa, C. Doroftei and M. Ignat, Ceram. Int., 2015, 41, 4430–4437 CrossRef CAS.
- C. Doroftei, P. D. Popa, E. Rezlescu and N. Rezlescu, J. Alloys Compd., 2014, 584, 195–198 CrossRef CAS.
- Y. I. Pyatnitskii, A. I. Bostan, L. N. Raevskaza, S. A. Nedil’ko, A. G. Dzyaz’ko and E. G. Zen’kovich, Theor. Exp. Chem., 2005, 41, 117–121 CrossRef CAS.
- V. K. Mittal, P. Chandramohan, S. Bera, M. P. Srinivasan, S. Velmurugan and S. V. Narasimhan, Solid State Commun., 2006, 137, 6–10 CrossRef CAS.
- T. Yamashita and P. A. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- H. J. Lee, M. H. Park, Y. J. Kim, C. S. Hwang, J. H. Kim, H. Funakubo and H. Ishiwara, J. Appl. Phys., 2011, 110, 074111–074118 CrossRef.
- N. S. McIntyre and D. G. Zetaruk, Anal. Chem., 1977, 49, 1521–1529 CrossRef CAS.
- J. F. Moulder, W. F. Stickel, P. E. Sobol and K. D. Bomben, in Handbook of X-ray Photoelectron Spectroscopy, ed. J. Chastain, 1992 Search PubMed.
- E. Arendt, A. Maione, A. Klisinska, O. Sanz, M. Montes, S. Suarez, J. Blanco and P. Ruiz, Appl. Catal., A, 2008, 339, 1–14 CrossRef CAS.
- M. Kowalik, R. Zalecki and A. Kolodziejczyk, Acta Phys. Pol., A, 2010, 117, 227–280 CrossRef.
- N. Rezlescu, E. Rezlescu, P. D. Popa, C. Doroftei and M. Ignat, Composites, Part B, 2014, 60, 515–522 CrossRef CAS.
- A. Kaddouri, P. Gelin and N. Dupont, Catal. Commun., 2009, 10, 1085–1089 CrossRef CAS.
- R. M. G. de la Cruz, H. Falcon, M. A. Pena and J. I. G. Fierro, Appl. Catal., B, 2001, 33, 45–55 CrossRef.
- R. Burch, E. Halpin, M. Hayes, K. Ruth and J. A. Sullivan, Appl. Catal., B, 1998, 19, 199–207 CrossRef CAS.
- L. Kundakovic and M. F. Stephanopoulos, J. Catal., 1998, 179, 203–221 CrossRef CAS.
- H. G. El-Shobaky and M. M. Mokhtar, Appl. Surf. Sci., 2007, 253, 9407–9413 CrossRef CAS.
- R. C. Everson, L. N. Mulay, O. P. Mahajan and P. L. Walker, J. Chem. Technol. Biotechnol., 1979, 29, 1–7 CrossRef CAS.
- V. G. Milt, M. A. Ulla and E. A. Lombardo, Catal. Lett., 2000, 65, 67–73 CrossRef CAS.
- H. Arandiyan, H. Dai, K. Ji, H. Sun and J. Li, ACS Catal., 2015, 5, 1781–1793 CrossRef CAS.
- V. G. Milt, R. Spretz, M. A. Ulla and E. A. Lombardo, Catal. Lett., 1996, 42, 57–63 CrossRef CAS.
- K. S. Song, D. Klvana and J. Kirchnerova, Appl. Catal., A, 2001, 213, 113–121 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |