Open Access Article
Open Access ArticlePyrolysis of n-butane investigated using synchrotron threshold photoelectron photoion coincidence spectroscopy
Xiaofeng Tang *a,
Xiaoxiao Lina,
Yupeng Zhuab,
Xiangkun Wubc,
Zuoying Wenab,
Lidong Zhangd,
Fuyi Liud,
Xuejun Gua and
Weijun Zhang*ae
*a,
Xiaoxiao Lina,
Yupeng Zhuab,
Xiangkun Wubc,
Zuoying Wenab,
Lidong Zhangd,
Fuyi Liud,
Xuejun Gua and
Weijun Zhang*ae
aLaboratory of Atmospheric Physico-Chemistry, Anhui Institute of Optics and Fine Mechanics, Chinese Academy of Sciences, Hefei, 230031 Anhui, China. E-mail: tangxf@aiofm.ac.cn; wjzhang@aiofm.ac.cn
bGraduate School, University of Science and Technology of China, Hefei, 230026 Anhui, China
cHefei National Laboratory for Physical Sciences at the Microscale, Department of Chemical Physics, University of Science and Technology of China, Hefei 230026, China
dNational Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230029, China
eSchool of Environmental Science and Optoelectronic Technology, University of Science and Technology of China, Hefei, 230026 Anhui, China
First published on 31st May 2017
Abstract
We present here the coupling of a flash pyrolysis micro-reactor with threshold photoelectron photoion coincidence (TPEPICO) spectroscopy at Hefei synchrotron to investigate thermal decomposition of n-butane. Primary products in the pyrolysis were determined with TPEPICO time-of-flight mass spectra and mass-selected threshold photoelectron spectra (TPES). Vibrational structures in the TPES of the CH3, C2H5 and C3H6 products were observed and their ionization energies were measured and compared satisfactorily to literature data. Moreover, the potential energy surface involved in the decomposition was calculated by using high-level quantum chemistry and several transition states were recognized. Then the detailed mechanisms of the pyrolysis of n-butane have been discussed and revealed.
1. Introduction
Thermal decomposition of hydrocarbons at high temperature plays an essential role in combustion and strongly links to atmospheric reactions to produce polycyclic aromatic hydrocarbons, soot and other toxic compounds.1–3 In the past, shock tubes, Knudsen cells and other pyrolysis reactors combing with a great number of analytical methods such as photo-absorption spectroscopy,4,5 gas chromatography (GC)6,7 and photoionization mass spectrometry (PIMS)3,8 have been adopted to elucidate the underlying chemistry. Typically, spectroscopic methods are usually used to probe products that only contain a few atoms, whereas unstable reactive species such as free radicals can be hardly observed by GC. PIMS as a universal method especially coupled with tunable vacuum ultraviolet (VUV) synchrotron radiation has been successfully utilized to identify various reaction products. Photoionization efficiency (PIE) spectra were adopted to discriminate isomers based on their different ionization energies.2,3 Photoelectron photoion coincidence spectroscopy (PEPICO) analyzes both electrons and ions simultaneously and offers the potential to provide additional and complementary information encoded in the electrons in the photoionization.9–12 In particular, the mass-selected threshold photoelectron spectrum (TPES) for each species can be obtained with PEPICO by scanning photon energy and collecting electrons with near zero kinetic energy and offer more detailed spectral information than PIE. In recent years PEPICO as an efficient analytical approach has started to be applied in complex systems such as combustion and atmosphere chemistry to identify products and isomers.13–15The objective of the present study, n-butane is the prototype of hydrocarbons and an essential model fuel to study combustion behaviors. The thermal decomposition reactions of n-butane are important initiation steps in the high-temperature oxidation and strongly influence on the ignition time,16 which have attracted a lot of attention in the past decades. A series of products from both the primary and the secondary pyrolysis reactions of n-butane have been identified and analyzed by using the methods of GC, Raman spectroscopy, electron impact ionization mass spectrometry and PIMS.6,7,17–20 For example, for the major stable products of CH4, C2H4, C2H6 and C3H6, it was believed that the thermal decomposition reactions responsible for the production were mostly proceeded through free-radical chain reactions.6 But, these stable products may also be formed from the direct decomposition of n-butane and in recent quantum chemical calculations several transition states were determined on the potential energy surfaces of the dissociation of n-butane and can correlate to the production of the stable products.21
Recently, we have developed a molecular beam flash pyrolysis micro-reactor22–24 in combination with a threshold PEPICO (TPEPICO) spectrometer10 at Hefei synchrotron to explore VUV spectroscopy of organic radicals25 and thermal decomposition of hydrocarbons. Benefited from its high temperature and short contact time, the flash pyrolysis micro-reactor allows for the thermal decomposition with high efficiency and minimizes secondary reactions.26–28 The TPEPICO scheme provides a strategy to obtain pure spectra of products without contamination from other byproducts. As a representative example, thermal decomposition of n-butane has been selected and investigated by using the flash pyrolysis micro-reactor and the TPEPICO spectrometer. The primary pyrolysis products were identified and determined from TPEPICO time-of-flight (TOF) mass spectra and mass-selected TPES. In addition, the potential energy surface involved in the decomposition was also calculated by using high-level quantum chemistry and several transition states were recognized. With the results of experiments and theoretical calculations, a clear picture of the dissociation mechanisms has been revealed.
2. Experimental setup
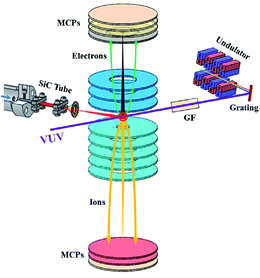
The experiments were carried out on the Atomic and Molecular Physics beamline at National Synchrotron Radiation Laboratory (NSRL) in Hefei, China. Fig. 1 gives a schematic diagram of the whole setups including the VUV synchrotron beamline, the TPEPICO spectrometer and the molecular beam flash pyrolysis micro-reactor. The setups have already been introduced in detail in our previous papers and so only a brief description will be presented here.10,25Synchrotron photons emitted from an undulator of 800 MeV electron ring at NSRL were dispersed by a 6 m monochromator equipped with three gratings (370, 740 and 1250 grooves per mm) covering the photon energy range of 7.5–124 eV. To maximize photon flux, presently the slits of the monochromator were expanded with widths of 200 μm and the photon energy resolution is about 20 meV (full width at half maximum, FWHM) at hν = 10 eV with the 370 grooves per mm grating. A gas filter filled with argon was installed to suppress the high-order harmonic photons emitted from the undulator. The absolute photon energy of the monochromator was calibrated on-line with the absorption resonant lines of Ar in the gas filter. The photons were softly focused onto a spot with ∼1 mm (V) × 2 mm (H) size in the photoionization region. A photodiode just located behind the photoionization region was used to measure the photon flux for normalizing signals in the photon energy scans.
The TPEPICO spectrometer10 is mainly composed of two TOF analyzers with double velocity map imaging29 designs for ions and electrons respectively operated in coincidence. A mask with a small central hole and a concentric ring was installed and located at the front of electron micro-channel plates (MCPs) with a chevron configuration. The small central hole and the concentric ring were used to collect near zero kinetic energy photoelectrons (threshold photoelectrons) and hot electrons, respectively, which were velocity focused as shown by the black and green trajectories in Fig. 1.30 After amplified, the electron signals were transferred to the start connector of a multiple-event time digitizers (P7888-2, FAST Comtec, Germany) and the ion signals to the stop connector. Then the electron signals provide start to record ion events in coincidence. The contribution of hot electrons that happen to the center of the electron detector was corrected by using the method of subtraction.30 TPES, especially mass-selected TPES corresponding to each species can be acquired in the coincidence mode by scanning synchrotron photon energy.
The flash pyrolysis micro-reactor25 is similar to the design of Chen et al.26 and primarily consists an orifice nozzle (50 μm diameter), a ceramic insulation plate and a silicon carbide tube (SiC, 1 mm inner diameter). The jet of n-butane seeded in argon with a stagnation pressure of 2 atm in the nozzle was adiabatically expanded into the SiC tube in vacuum and a continuous molecular beam with a subsonic speed was formed. The SiC tube was electrically connected to a DC power supply via a vacuum feedthrough port and the pyrolysis power can be controlled outside. The temperature inside the SiC tube was measured off-line with a thermocouple and can be approached at ∼1600 K. The high temperature and the short contact time (∼several tens of microseconds) due to the short heating length of the SiC tube (∼15 mm) and the fast speed of molecular beam allow for the thermal decomposition with high efficiency. Moreover, the flash pyrolysis micro-reactor was mounted on a manually-controlled XYZ manipulator in the source chamber of the TPEPICO spectrometer and its position can be tuned real-time to optimize the signals. The source and ionization chambers of the TPEPICO spectrometer were separated by a skimmer (1 mm diameter) and their backing pressures were 4 × 10−2 Pa and 5 × 10−5 Pa respectively with the molecular beam on.
3. Results and discussion
3.1 TPEPICO time-of-flight mass spectra
TPEPICO TOF mass spectrum, showing ions detected in coincidence with threshold electrons, of n-butane with no pyrolysis was measured at fixed photon energy of hν = 10.60 eV, just above the ionization energy of n-butane (IE = 10.53 ± 0.02 eV),31 and is presented in Fig. 2(a). One peak at m/z = 58 is observed with a narrow width and ascribed to C4H10+ parent ion in the mass spectrum. When the pyrolysis power was turned on, as shown in Fig. 2(b) and (c) observed at hν = 10.60 eV with the powers of P = 29 W and 36 W, corresponding to the temperatures of ∼1200 K and ∼1400 K in the SiC tube, the intensities of the C4H10+ peak in the mass spectra declined and dropped steadily with the power, meaning that n-butane precursor molecules had been thermally decomposed. At the same time, several other peaks at m/z = 15, 28, 29, 42 and 43 can be identified in the mass spectra and have been assigned as CH3+, C2H4+, C2H5+, C3H6+ and C3H7+, whose ionization energies cited from references have been listed in Table 1.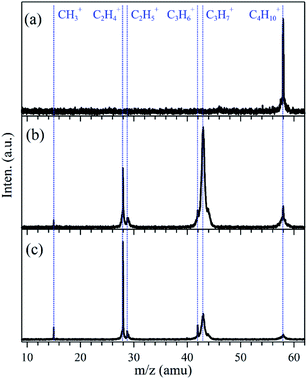 | ||
| Fig. 2 TPEPICO TOF mass spectra recorded at fixed photon energy of hν = 10.60 eV with (a) no pyrolysis, (b) pyrolysis power P = 29 W, and (c) pyrolysis power P = 36 W. | ||
| m/z | Species | IE (eV) | Methods | Ref. |
|---|---|---|---|---|
| a PES: photoelectron spectroscopy; PFI-ZEKE: pulsed-field-ionization zero-kinetic-energy photoelectron spectroscopy. | ||||
| 58 | n-C4H10 | 10.53 ± 0.02 | Thermochemistry | 31 |
| 57 | 1-C4H9 | 8.02 ± 0.04 | PES | 31 |
| 43 | 1-C3H7 | 8.09 ± 0.01 | PES | 33 |
| 42 | C3H6 | 9.7452 ± 0.0005 | PFI-ZEKE | 34 |
| 30 | C2H6 | 11.5 ± 0.1 | TPES | 35 |
| 29 | C2H5 | 8.117 ± 0.008 | PIMS | 36 |
| 28 | C2H4 | 10.51 ± 0.02 | PES | 37 |
| 16 | CH4 | 12.618 ± 0.004 | PFI-ZEKE | 38 |
| 15 | CH3 | 9.8387 ± 0.0018 | PFI-ZEKE | 39 |
In the mass spectra the widths of the CH3+, C2H4+, C2H5+, and C3H6+ peaks are very narrow, similar to the peak of C4H10+ parent ion, indicating that they were also from photoionization of their neutral molecules. Their intensities increase with pyrolysis power. The peaks of C3H7+ are much wider than those of the other ions and the C3H7+ ions may be produced from different processes. Photoionization and dissociative photoionization of n-butane have been studied well previously by the method of photoionization mass spectrometry and C3H7+ is the major fragment ion with the largest intensity in the dissociation.32 The appearance energy of C3H7+ in dissociative photoionization of n-butane was measured at 11.18 eV (ref. 32) which is above the present photon energy of hν = 10.60 eV. But, in the present experimental condition to arrive the photoionization region n-butane needed to go past the high-temperature SiC tube and the increased temperature can shift the onset of the dissociative photoionization. This effect of hot band has also been observed in the TPES of CH3, as shown in Fig. 4(a). Some kinetic energies should have been released in the dissociative photoionization and contribute to the wide width of the C3H7+ peak in the mass spectra.
The pyrolysis of n-butane may also produce the neutral C3H7 radical through the direct fission of the C1–C2 bond of n-butane in the SiC tube and the above observed CH3 can be its accompanied product. The signal of the C3H7 product is very weak, as demonstrated by the weak CH3+ signal in the mass spectra of Fig. 2, and may be buried in the C3H7+ wide peak. So we tried to change synchrotron photon energy and look for the evidence of the C3H7 product in TPEPICO TOF mass spectra. For example, with the fixed pyrolysis power of P = 36 W, TPEPICO TOF mass spectra were measured at fixed photon energies of hν = 9.50, 9.90 and 10.20 eV, all of which are far below the appearance energy of C3H7+ in dissociative photoionization of n-butane, and are displayed in Fig. 3. Except the absence of the C4H10+ parent ion, the peaks of CH3+, C2H4+, C2H5+, C3H6+ and C3H7+ ions can be determined in Fig. 3. All of them are very narrow in the mass spectra. Especially, in contrary to the case of Fig. 2, presently the peaks of C3H7+ in the mass spectra do not take a wide width again indicating that they were only from photoionization of the propyl radicals.
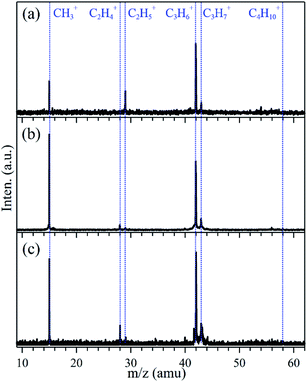 | ||
| Fig. 3 TPEPICO TOF mass spectra recorded at different photon energies with pyrolysis power of P = 36 W. (a) hν = 9.50 eV, (b) hν = 9.90 eV and (c) hν = 10.20 eV. | ||
3.2 Mass-selected TPES
The mass-selected TPES can be measured by scanning photon energy and provide detailed spectral fingerprint for each species. Especially rather than the steps in the PIE spectra, electronic states even fine vibrational structures of ions can appear as distinct peaks in the mass-selected TPES and offer great selectivity and sensitivity.3,13–15 As a representative, the mass-selected TPES of the methyl radical, ethyl radical and propylene products from pyrolysis of n-butane were recorded with a 15 meV scanning step size in the energy range of 8.0–10.0 eV and are depicted in Fig. 4. For the methyl radical, several peaks can be observed in the TPES and ascribed to the vibrational excitations in the photoionization. The adiabatic ionization energy of the methyl radical is measured at hν = 9.84 eV, which agrees well with the existing literature data,22,25,39,40 and is contributed to the most intense peak in Fig. 4(a). Taken into account the different symmetries of the neutral methyl radical (C3V) and its corresponding ion (D3h), the umbrella vibrational mode of CH3+ had been excited in the photoionization and contributes to the other peaks. Some other peaks with very weak intensities below the adiabatic ionization energy can also be observed in the TPES and are from the contributions of hot band due to the high temperature in the pyrolysis.22,25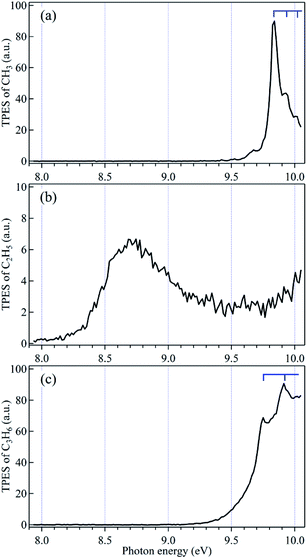 | ||
| Fig. 4 Mass-selected threshold photoelectron spectra (TPES) of (a) CH3, (b) C2H5 and (c) C3H6 products from pyrolysis of n-butane. | ||
The TPES of ethyl radical is exhibited with a broad band in Fig. 4(b). The electron signal appears at ∼8.2 eV and its intensity approaches the maximal at hν = 8.7 eV, contributing to the adiabatic and vertical ionization energies of ethyl radical, respectively. For propene product, as shown in Fig. 4(c), two vivid vibrational peaks can be observed in the TPES. The adiabatic ionization energy of propene is measured at 9.75 eV and corresponds to the first peak. The other peak with a spacing of 165 meV (1330 cm−1) to the first peak is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrational population of C3H6+.34,41 We had also tried to measure the TPES of the propyl radical from pyrolysis of n-butane. But, as we discussed in the above part of 3.1, the signals of C3H7+ were from two processes, photoionization of the propyl radical and dissociative photoionization of n-butane, and presently it is very difficult for us to discriminate them due to the restriction of the detectors. This problem can be overcome by employing position sensitive detectors (PSDs) to collect electrons and ions in coincidences and then the pure TPES of the propyl radical could be acquired with an ion-kinetic-energy-filtered method.42,43
C stretching vibrational population of C3H6+.34,41 We had also tried to measure the TPES of the propyl radical from pyrolysis of n-butane. But, as we discussed in the above part of 3.1, the signals of C3H7+ were from two processes, photoionization of the propyl radical and dissociative photoionization of n-butane, and presently it is very difficult for us to discriminate them due to the restriction of the detectors. This problem can be overcome by employing position sensitive detectors (PSDs) to collect electrons and ions in coincidences and then the pure TPES of the propyl radical could be acquired with an ion-kinetic-energy-filtered method.42,43
3.3 Pyrolysis mechanisms
In previous studies, the stable products such as CH4, C2H4, C2H6 and C3H6 from pyrolysis of n-butane had been observed by the methods of GC, GC-mass spectrometer (GC-MS), Raman spectroscopy and synchrotron VUV PIMS.6,16–20,44 It was suggested that these stable products were most likely to be produced through the following free-radical chain reactions:6| C4H10 + Δ → C4H9 + H | (1) |
| C4H9 → CH3 + C3H6 | (2) |
| C4H9 → C2H5 + C2H4 | (3) |
| CH3 + C4H10 → CH4 + C4H9 | (4) |
| C2H5 + C4H10 → C2H6 + C4H9 | (5) |
CH3, C2H5 and C4H9 free radicals are the intermediates of the reactions. But, the above stable products can be formed directly via the primary decomposition reactions of n-butane too, not involving the secondary reactions.
| C4H10 + Δ → CH4 + C3H6 | (6) |
| C4H10 + Δ → C2H6 + C2H4 | (7) |
In addition, the primary decomposition reactions of n-butane can also produce CH3, C2H5 and C3H7 radical products.
| C4H10 + Δ → CH3 + C3H7 | (8) |
| C4H10 + Δ → C2H5 + C2H5 | (9) |
The successful identification of the reaction intermediates in experiment, especially the reactive radicals, is the key to reveal and demonstrate its decomposition mechanisms. The thermal decomposition of n-butane via the reactions (8) and (9) had been approved in shock tube by Hanson et al.4 In previous flow reactor experiment CH3 and C2H5 radical products, with no C3H7 and C4H9 radicals, were also detected by synchrotron VUV PIMS.19
As shown in Fig. 2 and 3, in the present flash pyrolysis study of n-butane the products including the stable species of C2H4 and C3H6 and the radicals of CH3, C2H5 and C3H7 have been observed in the TPEPICO TOF mass spectra. C3H7 radical products are directly and successfully identified in the mass spectra for the first time. No C4H9 radical products, whose ionization energy is located at 8.02 eV,31 was observed in our experiments. Due to the high temperature and the short contact time in the SiC tube, the thermal decomposition of n-butane can be taken place with high efficiency. So we believe that all the products observed in our experiments were mainly formed from the reactions of (6)–(9).
To elucidate the present experimental results, the decomposition of n-butane was also investigated by using quantum chemical calculations. The method of Becke three-parameter exchange functional with the Lee, Yang, and Parr correlation functional (B3LYP) with the 6-311+G(d,p) basis set was employed for molecular geometry optimizations and harmonic vibrational frequency analysis.45 The intrinsic reaction coordinate (IRC) calculations were also carried out to probe transition states between the reactants and products and to characterize the reaction profiles and elucidate the decomposition channels.46,47 Then the high-level method of coupled cluster method with single, double, and non-iterative triple excitations (CCSD(T))48 with the aug-cc-pVTZ basis set was adopted to refine electronic energies based on the optimized B3LYP/6-311+G(d,p) structures. The corrections of zero point vibrational energies (ZPE) had been taken into account with the method of B3LYP/6-311+G(d,p). All the calculations were performed with the Gaussian 09 program.49
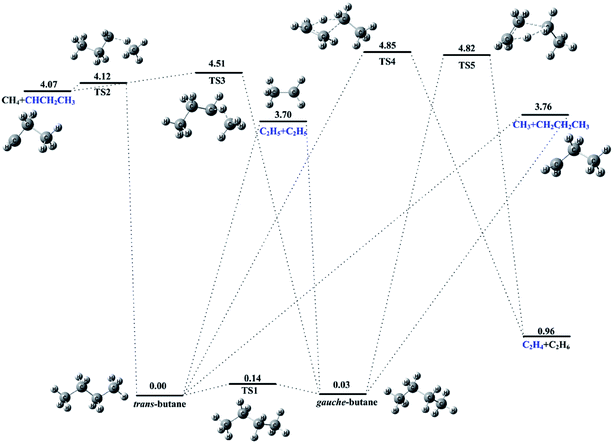
The potential energy surface together with the optimized molecular structures and their relative energies to the ground state of n-butane is presented in Fig. 5. It is shown that n-butane has two conformers with trans and gauche structures. The energy of the trans conformer is a litter smaller than that of the gauche and has a more stable configuration. The isomerization between the trans and gauche conformers can be proceeded via a transition state (TS1) with a small height barrier of 0.14 eV. The barrier is so small that the products in the high temperature pyrolysis can be formed from the both conformers.
The pyrolysis pathways observed in the present experiments were calculated and then the decomposition mechanisms of n-butane can be inferred. As shown in Fig. 5, CH3 and its accompanying C3H7 radical products corresponding to the reaction (8) were produced from the direct fission of the C1–C2 bond of n-butane and no barrier was found on the decomposition route. The C1–C2 bond energy of n-butane is calculated to be 3.76 eV. Similarly, two C2H5 radical products were formed directly through the C2–C3 bond fission of n-butane and the bond energy is 3.70 eV. But, the dissociation mechanisms to produce the C2H4 or C3H6 products observed in experiments are a litter complicated than those of the CH3 and C2H5 products. The calculations show that the process of H atom migration should be involved in the decomposition of n-butane to produce CH4 and C3H6 species. As shown in Fig. 5, this speculation is attested and the two transition states (TS2 and TS3) with a complex structure of CH4–CHCH2CH3 were also identified along the decomposition routes. For the productions of C2H4 and C2H6 species, two four-member-ring transition states of TS4 and TS5 are determined along the routes. The energy barriers for this production channel from the trans and gauche conformers of n-butane are calculated at 4.85 and 4.82 eV, respectively.
4. Conclusion
A molecular beam flash pyrolysis micro-reactor coupled with a threshold photoelectron photoion coincidence spectrometer has been constructed at Hefei synchrotron and utilized to investigate thermal decomposition of hydrocarbons. The high temperature and the short contact time in the micro-reactor allow for the thermal decomposition with high efficiency. The pyrolysis of n-butane has been selected as a representative example and analyzed. The primary pyrolysis products including CH3, C2H5 and C3H7 free radicals were detected and determined with TPEPICO TOF mass spectra and mass-selected TPES. Vibrational structures in the TPES of CH3, C2H5 and C3H6 products were identified and assigned and their ionization energies were measured, which compared satisfactorily to the existing literature data. The fine structures of the mass-selected TPES offer great selectivity and sensitivity in comparable to the traditional PIE spectra.The potential energy surface related to the thermal decomposition of n-butane has been calculated with the high-level theoretical method of CCSD(T)/aug-cc-pVTZ//B3LYP/6-311+G(d,p) + ZPE and the detailed dissociation mechanisms have been discussed and revealed. All the products observed in our experiments were mainly formed from the primary decomposition reactions of n-butane. The trans and gauche conformers of n-butane should be involved in the pyrolysis. The productions of the CH3 radical and its accompanying C3H7 products, and two C2H5 radical products, are simple and just through the direct bond fission of n-butane. But, the dissociation mechanisms to produce C3H6 or C2H4 fragments are a litter more complicated than those of the CH3 and C2H5 products. Transition states with four-member-ring structures were determined in the production of C2H4 and C2H6. In addition, to explain the pyrolysis to the CH4 and C3H6 products, a mechanism of H atom migration should be taken into account.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (91644109, 91544105), the National Key Research and Development Program of China (2016YFC0200300, 2016YFC0202205), National Key Scientific Instruments and Equipment Development Program of China (2012YQ220113) and the Natural Science Foundation of Anhui Province (1608085MB35). The authors are grateful to Dr Xiaoguo Zhou for helpful discussion in preparation of the manuscript.References
- B. S. Haynes and H. G. Wagner, Soot formation, Prog. Energy Combust. Sci., 1981, 7(4), 229–273 CrossRef CAS.
- K. Kohse-Hoinghaus, P. Osswald, T. A. Cool, T. Kasper, N. Hansen, F. Qi, C. K. Westbrook and P. R. Westmoreland, Biofuel combustion chemistry: from ethanol to biodiesel, Angew. Chem., Int. Ed., 2010, 49(21), 3572–3597 CrossRef PubMed.
- F. Qi, Combustion chemistry probed by synchrotron VUV photoionization mass spectrometry, Proc. Combust. Inst., 2013, 34, 33–63 CrossRef CAS.
- M. A. Oehlschlaeger, D. F. Davidson and R. K. Hanson, High-temperature thermal decomposition of isobutane and n-butane behind shock waves, J. Phys. Chem. A, 2004, 108(19), 4247–4253 CrossRef CAS.
- A. V. Friderichsen, J. G. Radziszewski, M. R. Nimlos, P. R. Winter, D. C. Dayton, D. E. David and G. B. Ellison, The infrared spectrum of the matrix-isolated phenyl radical, J. Am. Chem. Soc., 2001, 123(9), 1977–1988 CrossRef CAS PubMed.
- J. H. Purnell and C. P. Quinn, Nature of the reactions involved in the pyrolysis of n-butane inhibited by propylene, Nature, 1961, 189(4765), 656–658 CrossRef CAS.
- E. Goos, H. Hippler, K. Hoyermann and B. Jurges, Laser powered homogeneous pyrolysis of butane initiated by methyl radicals in a quasi-wall-free reactor at 750–1000 K, Phys. Chem. Chem. Phys., 2000, 2(22), 5127–5132 RSC.
- B. Shukla, A. Susa, A. Miyoshi and M. Koshi, In situ direct sampling mass spectrometric study on formation of polycyclic aromatic hydrocarbons in toluene pyrolysis, J. Phys. Chem. A, 2007, 111(34), 8308–8324 CrossRef CAS PubMed.
- T. Baer, in Gas phase ion chemistry, ed. M. T. Bowers, Academic, New York, 1979, ch. 5, vol. 1, pp. 153–196 Search PubMed.
- X. F. Tang, X. G. Zhou, M. L. Niu, S. L. Liu, J. D. Sun, X. B. Shan, F. Y. Liu and L. S. Sheng, A threshold photoelectron–photoion coincidence spectrometer with double velocity imaging using synchrotron radiation, Rev. Sci. Instrum., 2009, 80(11), 113101 CrossRef PubMed.
- A. Bodi, P. Hemberger, T. Gerber and B. Sztaray, A new double imaging velocity focusing coincidence experiment: i2PEPICO, Rev. Sci. Instrum., 2012, 83(8), 083105 CrossRef PubMed.
- G. A. Garcia, B. K. C. de Miranda, M. Tia, S. Daly and L. Nahon, DELICIOUS III: a multipurpose double imaging particle coincidence spectrometer for gas phase vacuum ultraviolet photodynamics studies, Rev. Sci. Instrum., 2013, 84, 053112 CrossRef CAS PubMed.
- A. Bodi, P. Hemberger, D. L. Osborn and B. Sztaray, Mass-resolved isomer-selective chemical analysis with imaging photoelectron photoion coincidence spectroscopy, J. Phys. Chem. Lett., 2013, 4(17), 2948–2952 CrossRef CAS.
- J. Kruger, G. A. Garcia, D. Felsmann, K. Moshammer, A. Lackner, A. Brockhinke, L. Nahon and K. Kohse-Hoinghaus, Photoelectron–photoion coincidence spectroscopy for multiplexed detection of intermediate species in a flame, Phys. Chem. Chem. Phys., 2014, 16(41), 22791–22804 RSC.
- G. A. Garcia, X. F. Tang, J. F. Gil, L. Nahon, M. Ward, S. Batut, C. Fittschen, C. A. Taatjes, D. L. Osborn and J. C. Loison, Synchrotron-based double imaging photoelectron/photoion coincidence spectroscopy of radicals produced in a flow tube: OH and OD, J. Chem. Phys., 2015, 142(16), 164201 CrossRef PubMed.
- M. A. Oehlschlaeger, D. F. Davidson, J. T. Herbon and R. K. Hanson, Shock tube measurements of branched alkane ignition times and OH concentration time histories, Int. J. Chem. Kinet., 2004, 36(2), 67–78 CrossRef CAS.
- S. L. K. Wittig, Study of the thermal decomposition of n-butane, Phys. Fluids, 1969, 12(5), I133–I135 CrossRef.
- D. M. Golden, Z. B. Alfassi and P. C. Beadle, Very low-pressure pyrolysis (VLPP) of alkanes: n-butane, 2,3-dimethylbutane, 2,2′,3,3′-tetramethylbutane, and isobutane, Int. J. Chem. Kinet., 1974, 6(3), 359–370 CrossRef CAS.
- Y.-J. Zhang, W.-H. Yuan, J.-H. Cai, L.-D. Zhang, F. Qi and Y.-Y. Li, Product identification and mass spectrometric analysis of n-butane and i-butane pyrolysis at low pressure, Chin. J. Chem. Phys., 2013, 26(2), 151–156 CrossRef CAS.
- M. S. Brogan, J. A. Cairns and T. J. Dines, Raman spectroscopic study of the thermal and catalytic decomposition of butane, J. Raman Spectrosc., 1994, 25(12), 927–932 CrossRef CAS.
- C.-H. Chin and S.-H. Lee, Comparison of two-body and three-body decomposition of ethanedial, propanal, propenal, n-butane, 1-butene, and 1,3-butadiene, J. Chem. Phys., 2012, 136(2), 024308 CrossRef PubMed.
- B. K. C. de Miranda, C. Alcaraz, M. Elhanine, B. Noller, P. Hemberger, I. Fischer, G. A. Garcia, H. Soldi-Lose, B. Gans, L. A. V. Mendes, S. Boye-Peronne, S. Douin, J. Zabka and P. Botschwina, Threshold photoelectron spectroscopy of the methyl radical isotopomers, CH3, CH2D, CHD2 and CD3: synergy between VUV synchrotron radiation experiments and explicitly correlated coupled cluster calculations, J. Phys. Chem. A, 2010, 114(14), 4818–4830 CrossRef PubMed.
- P. Hemberger, M. Steinbauer, M. Schneider, I. Fischer, M. Johnson, A. Bodi and T. Gerber, Photoionization of three isomers of the C9H7 radical, J. Phys. Chem. A, 2010, 114(14), 4698–4703 CrossRef CAS PubMed.
- F. Zhang, R. I. Kaiser, V. V. Kislov, A. M. Mebel, A. Golan and M. Ahmed, A VUV photoionization study of the formation of the indene molecule and its isomers, J. Phys. Chem. Lett., 2011, 2(14), 1731–1735 CrossRef CAS.
- Y. Zhu, X. Wu, X. F. Tang, Z. Wen, F. Liu, X. Zhou and W. Zhang, Synchrotron threshold photoelectron photoion coincidence spectroscopy of radicals produced in a pyrolysis source: the methyl radical, Chem. Phys. Lett., 2016, 664, 237–241 CrossRef CAS.
- D. W. Kohn, H. Clauberg and P. Chen, Flash pyrolysis nozzle for generation of radicals in a supersonic jet expansion, Rev. Sci. Instrum., 1992, 63(8), 4003–4005 CrossRef CAS.
- J. A. Blush, H. Clauberg, D. W. Kohn, D. W. Minsek, Z. Xu and P. Chen, Photoionization mass and photoelectron spectroscopy of radicals, carbenes, and biradicals, Acc. Chem. Res., 1992, 25(9), 385–392 CrossRef CAS.
- Q. Guan, K. N. Urness, T. K. Ormond, D. E. David, G. B. Ellison and J. W. Daily, The properties of a micro-reactor for the study of the unimolecular decomposition of large molecules, Int. Rev. Phys. Chem., 2014, 33(4), 447–487 CrossRef CAS.
- A. T. J. B. Eppink and D. H. Parker, Velocity map imaging of ions and electrons using electrostatic lenses: application in photoelectron and photofragment ion imaging of molecular oxygen, Rev. Sci. Instrum., 1997, 68(9), 3477–3484 CrossRef CAS.
- B. Sztaray and T. Baer, Suppression of hot electrons in threshold photoelectron photoion coincidence spectroscopy using velocity focusing optics, Rev. Sci. Instrum., 2003, 74(8), 3763–3768 CrossRef CAS.
- NIST Chemistry WebBook, http://webbook.nist.gov/chemistry/, accessed December, 2016.
- W. A. Chupka and J. Berkowit, Photoionization of ethane, propane, and n-butane with mass analysis, J. Chem. Phys., 1967, 47(8), 2921–2933 CrossRef CAS.
- J. Dyke, A. Ellis, N. Jonathan and A. Morris, Vacuum ultraviolet photoelectron spectroscopy of transient species. Part 18. The cyclopropyl, isopropyl and n-propyl radicals, J. Chem. Soc., Faraday Trans. 2, 1985, 81, 1573–1586 RSC.
- K. Vasilatou and F. Merkt, Torsional vibrational structure of the propene radical cation studied by high-resolution photoelectron spectroscopy, J. Chem. Phys., 2011, 135(12), 124310 CrossRef CAS PubMed.
- R. A. Mackie, A. M. Sands, S. W. J. Scully, D. M. P. Holland, D. A. Shaw, K. F. Dunn and C. J. Latimer, The molecular and dissociative photoionization of ethane in the inner and outer valence energy regions, J. Phys. B: At., Mol. Opt. Phys., 2002, 35(4), 1061–1069 CrossRef CAS.
- B. Ruscic, J. Berkowitz, L. A. Curtiss and J. A. Pople, The ethyl radical – Photoionization and theoretical studies, J. Chem. Phys., 1989, 91(1), 114–121 CrossRef CAS.
- G. R. Branton, D. C. Frost, T. Makita, C. A. McDowell and I. A. Stenhouse, Photoelectron spectra of some polyatomic molecules, Philos. Trans. R. Soc., A, 1970, 268(1184), 77–85 CrossRef CAS.
- R. Signorell and F. Merkt, The first rotationally resolved spectrum of CH4+, J. Chem. Phys., 1999, 110(5), 2309–2311 CrossRef CAS.
- A. M. Schulenburg, C. Alcaraz, G. Grassi and F. Merkt, Rovibrational photoionization dynamics of methyl and its isotopomers studied by high-resolution photoionization and photoelectron spectroscopy, J. Chem. Phys., 2006, 125(10), 104310 CrossRef CAS PubMed.
- J. A. Blush, P. Chen, R. T. Wiedmann and M. G. White, Rotationally resolved threshold photoelectron spectrum of the methyl radical, J. Chem. Phys., 1993, 98(4), 3557–3559 CrossRef CAS.
- A. Katrib and J. W. Rabalais, Electronic interaction between the vinyl group and its substituents, J. Phys. Chem., 1973, 77(19), 2358–2363 CrossRef CAS.
- B. Gans, G. A. Garcia, S. Boye-Peronne, J.-C. Loison, S. Douin, F. Gaie-Levrel and D. Gauyacq, Absolute photoionization cross section of the ethyl radical in the range 8–11.5 eV: synchrotron and vacuum ultraviolet laser measurements, J. Phys. Chem. A, 2011, 115(21), 5387–5396 CrossRef CAS PubMed.
- X. F. Tang, G. A. Garcia and L. Nahon, Adiabatic ionization energies of the overlapped A2A1 and B2E electronic states in CH3Cl+/CH3F+ measured with double imaging electron/ion coincidence, Phys. Chem. Chem. Phys., 2015, 17(26), 16858–16863 RSC.
- S. H. Yoon, N.-K. Park, T. J. Lee, K. J. Yoon and G. Y. Han, Hydrogen production by thermocatalytic decomposition of butane over a carbon black catalyst, Catal. Today, 2009, 146(1–2), 202–208 CrossRef CAS.
- A. D. Becke, Density functional thermochemistry. 3. The role of exact exchange, J. Chem. Phys., 1993, 98(7), 5648–5652 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, An improved algorithm for reaction path following, J. Chem. Phys., 1989, 90(4), 2154–2161 CrossRef CAS.
- C. Gonzalez and H. B. Schlegel, Reaction path following in mass-weighted internal coordinates, J. Phys. Chem., 1990, 94(14), 5523–5527 CrossRef CAS.
- J. A. Pople, M. Headgordon and K. Raghavachari, Quadratic configuration interaction. A general technique for determining electron correlation energies, J. Chem. Phys., 1987, 87(10), 5968–5975 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara and R. F. K. Toyota, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
| This journal is © The Royal Society of Chemistry 2017 |