Open Access Article
Open Access ArticleNew sorbents based on poly(methacrylic acid-TRIM) and poly(vinylimidazole-TRIM) for simultaneous preconcentration of herbicides in water samples with posterior determination by HPLC-DAD
César Ricardo Teixeira Tarley *ab,
Mariana Gava Segatellia,
Juliana Casarina and
Raquel Justo da Fonsecaac
*ab,
Mariana Gava Segatellia,
Juliana Casarina and
Raquel Justo da Fonsecaac
aUniversidade Estadual de Londrina (UEL), Departamento de Química, Centro de Ciências Exatas, Rodovia Celso Garcia Cid, PR 445, km 380, Londrina, PR 86050-482, Brazil. E-mail: tarley@uel.br; Fax: +55 43 3371 4286; Tel: +55 43 3371 4366
bInstituto Nacional de Ciência e Tecnologia (INCT) de Bioanalítica, Universidade Estadual de Campinas (UNICAMP), Instituto de Química, Departamento de Química Analítica, Cidade Universitária Zeferino Vaz, s/n, Campinas, SP 13083-970, Brazil
cUniversidade Tecnológica Federal do Paraná (UTFPR), Campus Apucarana, CEP 86812-460, Apucarana, PR, Brazil
First published on 1st August 2017
Abstract
In the present study, new sorbents for solid-phase extraction (SPE) based on poly(methacrylic acid-trimethylolpropane trimethacrylate) (PMA-TRIM) and poly(vinylimidazole-TRIM) (PV-TRIM) for simultaneous extraction of tebuthiuron (TBT), hexazinone (HEX), diuron (DIU) and ametryn (AME) and posterior determination by HPLC-DAD were developed. The method was based on the preconcentration of 50.0 mL of water at pH 6.0 through 200 mg of each sorbent packed into a cartridge with further elution by 3.0 mL of methanol. The analytical curves were linear for the herbicides in the range 1.0–150.0 μg L−1 with limits of detection (LOD) of 0.40, 0.31, 0.12, 0.34 μg L−1 for TBT, HEX, DIU and AME, respectively, when using PMA-TRIM as the sorbent and 0.14, 0.44, 0.17 and 0.39 μg L−1, respectively, when using PV-TRIM. The precision in terms of repeatability assessed as RSD (relative standard deviation, n = 10) for the multiresidue analysis ranged from 1.23% to 1.91% and 0.97% to 1.79% for respective concentrations of 10.0 and 100 μg L−1 using the PV-TRIM polymer and 2.13% to 3.31% and 1.43% to 2.91% using PMA-TRIM. As observed, better analytical performance was achieved using PV-TRIM as the sorbent. The obtained analytical performance from the polymers, mainly PV-TRIM in terms of LOD and preconcentration factor (PF) was much higher compared to commercial sorbents octadecyl-C18-silica (C18) and Amberlite® XAD4 poly(styrene-divinylbenzene) (DVB). Moreover, exceptional reusability was achieved (>161 recycles) without loss of adsorption capacity. To assess the accuracy of the preconcentration method, analyses of surface waters (well and river water), collected from an area near the cultivation of sugar cane, were carried out. From the recovery test ranging from 96% to 101%, it was concluded that the solid-phase extraction prior to HPLC-DAD analysis is appropriate and it is an alternative for multiresidue analysis in natural water.
Introduction
Herbicides have been widely applied over the years on different crops aimed at increasing the agricultural production. However, due to their persistence in the environment and the release into environmental water bodies, these compounds may be the source of contamination affecting human beings.1 Human health effects related to the exposure to some herbicides include endocrine disruption, neural disorders and cancer.2,3The sugarcane industry has been one of the most important products of the Brazilian economy, where Brazil is the biggest worldwide producer of sugarcane, whose crops are concentrated mainly in the São Paulo state region.4,5 The herbicides widely used in weed control and other plagues in sugarcane crops include the members of the substituted urea class, tebuthiuron (TBT) (N-t5-(1,1-dimethylethyl)-1,3,4-thiadazol-2-yll-N,N-dimethylurea) and diuron (DIU) (N′-(3,4-dichlorophenyl)-N,N-dimethylurea); hexazinone (HEX) (3-cyclohexyl-6-dimethylamino-1-methyl-1,3,5-triazine-2,4-(1H,3H)-dione) belongs to chemical group of triazinones and ametryn (AME) ((2-ethylamino)-4-(isopropylamino)-6-(methylthio)-s-triazine), which belongs to the triazine family.6,7
Tebuthiuron exhibits high leaching through soils and contaminates groundwater due to its high solubility in water (2.5 g L−1) and low absorption coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 1.79).8 Diuron, in turn, is classified by the EPA as a likely human carcinogen,9 and due to its low solubility in water of 42 mg L−1 and relatively low adsorption coefficient (log
Kow = 1.79).8 Diuron, in turn, is classified by the EPA as a likely human carcinogen,9 and due to its low solubility in water of 42 mg L−1 and relatively low adsorption coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 2.68), it presents low tendency to sorb to soils and sediments. However, considering that its hydrolysis and aqueous photolysis half-lives are relatively long, diuron can be considered both mobile and relatively persistent.10 Hexazinone and ametryn have low coefficient of adsorption (log
Kow = 2.68), it presents low tendency to sorb to soils and sediments. However, considering that its hydrolysis and aqueous photolysis half-lives are relatively long, diuron can be considered both mobile and relatively persistent.10 Hexazinone and ametryn have low coefficient of adsorption (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow = 1.2 and 2.98, respectively), and due to their high toxicity and high resistance in soil and surface water, they are considered one of the most important classes of chemical pollutants.11 Taking into account that these herbicides may be considered a contamination source affecting human beings, some international regulations have established the maximum allowed levels of these pesticides in treated water. The maximum allowed level of diuron is 0.5 and 10.0 μg L−1 according to European Community (EC) and Environmental Protection Agency (EPA), respectively.12–14 In Brazil, the Brazilian Ministry of Health (BMH) has established the maximum limit of diuron in drinking water to 90 μg L−1.15 Australian regulations allow a maximum level of 2.0 μg L−1 and 5.0 μg L−1 for hexazinone and ametryn, respectively.16 For tebuthiuron, EPA set 0.27 μg L−1 as the maximum contaminant level for irrigation water.16 As observed, the regulatory agencies around the world do not follow a pattern to define the maximum amount allowed of a specific herbicide in water samples. Nevertheless, the levels are extremely low, which makes the analysis of aforementioned herbicides in water samples, mainly natural waters containing high concentrations of interfering compounds, a difficult task.17 Liquid and gas chromatography have been widely used for pesticide analysis, particularly when coupled with mass spectrometry (MS). However, even while using mass spectrometry for detection, successful analysis of residual contaminants at trace levels requires previous sample preparation in order to promote sample clean-up or enrichment.18–20
Kow = 1.2 and 2.98, respectively), and due to their high toxicity and high resistance in soil and surface water, they are considered one of the most important classes of chemical pollutants.11 Taking into account that these herbicides may be considered a contamination source affecting human beings, some international regulations have established the maximum allowed levels of these pesticides in treated water. The maximum allowed level of diuron is 0.5 and 10.0 μg L−1 according to European Community (EC) and Environmental Protection Agency (EPA), respectively.12–14 In Brazil, the Brazilian Ministry of Health (BMH) has established the maximum limit of diuron in drinking water to 90 μg L−1.15 Australian regulations allow a maximum level of 2.0 μg L−1 and 5.0 μg L−1 for hexazinone and ametryn, respectively.16 For tebuthiuron, EPA set 0.27 μg L−1 as the maximum contaminant level for irrigation water.16 As observed, the regulatory agencies around the world do not follow a pattern to define the maximum amount allowed of a specific herbicide in water samples. Nevertheless, the levels are extremely low, which makes the analysis of aforementioned herbicides in water samples, mainly natural waters containing high concentrations of interfering compounds, a difficult task.17 Liquid and gas chromatography have been widely used for pesticide analysis, particularly when coupled with mass spectrometry (MS). However, even while using mass spectrometry for detection, successful analysis of residual contaminants at trace levels requires previous sample preparation in order to promote sample clean-up or enrichment.18–20
Thus, the implementation of sample pretreatment based on preconcentration methods has been highly recommended for a reliable analysis. Different preconcentration methods have been reported for analysis of pesticides, such as in tube solid-phase extraction,21 supramolecular system-based extraction,22 dispersive liquid–liquid microextraction,23 cloud point extraction,24 single-drop microextraction,25 and magnetic solid-phase extraction.26 The solid-phase extraction methods are still the most employed ones due to large amount of available sorbents with different properties.27
The literature reports some sorbent materials including molecularly imprinted polymers,27 montmorillonite,28 octadecyl-C18-silica,6,29 graphene,30 poly(methyloctadecylsiloxane) immobilized on silica,31 magnetic nanoporous carbon (MNC)32 and poly(styrene-divinylbenzene) sorbent (DVB),33 for solid-phase extraction of herbicides that are herein investigated with further determination by HPLC. Although the sorbents have been considered efficient for herbicides adsorption, in general, they are applied only in purified water samples or drinking water and are limited to environmental samples. Notwithstanding this, the reusability of some of them is much too low, mainly octadecyl-C18-silica, and to the best of our knowledge neither of them was applied to multiresidue analysis containing tebuthiuron, diuron, hexazinone and ametryn. Therefore, considerable efforts have been made to deal with the issue of development of new sorbent materials, which present fast adsorption towards the multiresidues, high chemical stability in a wide pH range, high adsorption capacity, negligible swelling effect in different fluids, exceptional reusability and low cost of synthesis.34 In this context, cross-linked polymers containing functional monomer with acid or base properties seem to be very useful for multiresidue preconcentration, due to their structural rigidity, high surface area depending upon synthesis process and the possibility to establish different interactions among binding sites of polymers.
Therefore, the aim of the current study has been the development of a new solid-phase method for the simultaneous preconcentration and detection by HPLC-DAD of tebuthiuron, diuron, hexazinone and ametryn using two cross-linked polymers synthesized with different functional monomers, methacrylic acid and 1-vinylimidazole, and trimethylolpropane trimethacrylate (TRIM) as the cross-linking reagent. These polymers have already been synthesized by our research group35 and characterized using FT-IR, SEM, TGA, textural data, elemental analysis, kinetic studies and adsorption isotherms for tebuthiuron. The highlight and novelty of present study relies on the evaluation of analytical performance of poly(methacrylic acid-TRIM) and poly(vinylimidazole-TRIM) towards multiresidues column solid-phase extraction and their validated applicability in the analysis of surface water (well and river water).
Experimental
Reagents and instruments
All herbicides (tebuthiuron, diuron, hexazinone and ametryn) were used as received without further purification from Sigma-Aldrich (PESTANAL, analytical standard, USA). Methanol (Sigma-Aldrich, HPLC grade, ≥99.9%, USA), chloroform (Sigma-Aldrich, analytical grade, ≥99.8%, USA), hexane (Sigma-Aldrich, analytical grade, ≥95%), 1-vinylimidazole (Sigma-Aldrich, ≥99.8%), methacrylic acid (Acros Organics, 99.5%), trimethylolpropanetrimethacrylate (TRIM) (Sigma-Aldrich, ≥99%), 2,2′-azoisobutilnitrile (Sigma-Aldrich, 98%), acetic acid (JTBaker, 99.9%), ethanol (JTBaker, 99.7%), acetonitrile (Sigma-Aldrich, HPLC grade, ≥99.9%), dichloromethane (Sigma-Aldrich, analytical grade, 99.5%) were used in the study. The water used to prepare the mobile phase was ultrapure from a Milli Q (Millipore) system and filtered through a 0.45 μm nylon membrane daily. The water and solvents used in mobile phase were also degassed using an ultrasonic Bath model USC 1400 (Marconi®, Piracicaba, Brazil).The chromatographic determinations were performed on a liquid chromatograph model LC-20AD/T LPGE kit, Shimadzu, operating isocratically. The chromatographic separation of tebuthiuron was carried out on a CLC-ODS column (250 mm × 4.6 mm id, 5 mm in particle size) containing a guard column Phenomenex (4.0 mm × 3.0 mm i.d., 5 mm inparticle size). The flow rate of the mobile phase (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 65
water, 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) was 0.7 mL min−1 and the injection volume was 20 μL. For multiresidue analysis, the separation of herbicides was carried out on a Luna 5 μm C8(2) column (250 mm × 4.6 mm id, 5 mm in particle size) and a guard column Phenomenex (4.0 mm × 3.0 mm i.d., 5 mm in particle size) using acetonitrile
35, v/v) was 0.7 mL min−1 and the injection volume was 20 μL. For multiresidue analysis, the separation of herbicides was carried out on a Luna 5 μm C8(2) column (250 mm × 4.6 mm id, 5 mm in particle size) and a guard column Phenomenex (4.0 mm × 3.0 mm i.d., 5 mm in particle size) using acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (35
water (35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65, v/v) as mobile phase at 1.0 mL min−1 flow rate. The temperature of chromatographic separation (25 °C) was controlled using a column oven. The peaks' purity was determined on a diode-array detector (DAD) and monitored at λmáx 254 nm. A manifold system (Bio-Rad) with a capacity for 12 cartridges, coupled to a vacuum pump (Marconi MA 2057) was used in the SPE procedure.
65, v/v) as mobile phase at 1.0 mL min−1 flow rate. The temperature of chromatographic separation (25 °C) was controlled using a column oven. The peaks' purity was determined on a diode-array detector (DAD) and monitored at λmáx 254 nm. A manifold system (Bio-Rad) with a capacity for 12 cartridges, coupled to a vacuum pump (Marconi MA 2057) was used in the SPE procedure.
Synthesis of polymers and SPE procedure
The syntheses of poly(methacrylic acid-TRIM) and poly(vinylimidazole-TRIM) have been reported by our research group.35 The SPE procedure was based on preconcentration of 50.0 mL of multiresidues solution at pH 6.0 through 200 mg of polymers packed into empty SPE cartridges and capped with fritted polyethylene disks at the top and bottom. The cartridge was placed in a manifold system coupled to a vacuum pump and operated under flow rate of 2.0 mL min−1. Before analysis, the cartridges were conditioned with 6.0 mL of ultrapure water. The elution was performed with 3.0 mL of methanol and analyzed by HPLC-DAD. It is worth mentioning that the adsorption pH (pH 6) was obtained from batch experiments as already reported in literature.35Breakthrough curve
In order to obtain the maximum adsorption capacity of polymers towards herbicides under dynamic conditions, a breakthrough curve was obtained, choosing tebuthiuron as target analyte. The experiments were performed by percolating aliquots of 5.0 mL of 100.0 mg L−1 solution of tebuthiuron through a SPE cartridge packed with 200 mg of polymers, previously conditioned with ultrapure water, until saturation was reached. Each aliquot was collected and analyzed by HPLC-UV, and the amount of tebuthiuron adsorbed on the polymers for each aliquot was determined according to eqn (1):
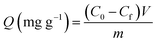 | (1) |
Results and discussion
Optimization of SPE procedure
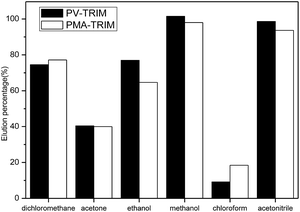
As can be seen, the polar and protic solvents were capable to disrupt the binding between the polymers and tebuthiuron. Higher elution percentage was also obtained using acetonitrile, which can be justified by high solubility of tebuthiuron, while a much lower desorption was observed for chloroform most likely due to its apolar properties, thus making it very difficult to disrupt the binding between the polymers and tebuthiuron. Therefore, methanol as the elution solvent was adopted in SPE procedure.
According to data presented in Table 1, 3.0 mL of methanol was sufficient to provide quantitative elution of tebuthiuron from both polymers, since the theoretical PF were very similar to the ones obtained experimentally. These findings reveal that SPE procedure may greatly improve the detectability of herbicides.
Breakthrough curve
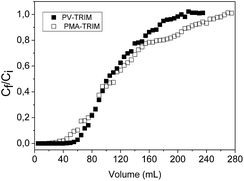
The breakthrough curve is shown in Fig. 2. It was observed, under experimental procedure previously mentioned, that the breakthrough volume for PV-TRIM was found to be 60.0 mL, which corresponds to the amount of tebuthiuron adsorbed on the polymer of 29.15 mg g−1. The column saturation was achieved by loading 220.0 mL of 100.0 mg L−1 tebuthiuron solution, yielding a maximum adsorption capacity (MAC) of 55.18 mg g−1. For the PMA-TRIM, a breakthrough volume of 35.0 mL was observed, corresponding to the amount of tebuthiuron adsorbed on the polymer of 12.45 mg g−1, while the column saturation was achieved by loading 265.0 mL of tebuthiuron solution, with a maximum adsorption capacity (MAC) of 62.48 mg g−1.From the analytical point of view, the values of breakthrough volume play an important role on the preconcentration method. Therefore, the quantitative adsorption of tebuthiuron for PV-TRIM and PMA-TRIM was found to be 5.83 mg and 2.49 mg, respectively. Such outcomes show that larger sample volume of tebuthiuron solution at very low concentration can be loaded in the cartridges packed with polymers, without loss of adsorption capacity, consequently yielding higher preconcentration factors. For instance, if a tebuthiuron solution of 100 μg L−1 concentration is used, 58.3 and 24.9 liters can be preconcentrated on the PV-TRIM and PMA-TRIM, respectively. One should note still that PV-TRIM shows higher adsorption towards tebuthiuron, most likely due to its higher porosity.35
Analytical features of SPE for tebuthiuron determination by HPLC-DAD
Fig. 3 shows the analytical curves for herbicide tebuthiuron after preconcentration (50.0 mL) on PV-TRIM, PMA-TRIM, and commercial sorbents octadecyl-C18-silica (C18) and Amberlite® XAD4 poly(styrene-diviylbenzene) (DVB) as well as the analytical curve without preconcentration step. The obtained linear correlation coefficients were found to be 0.998, 0.995, 0.990 and 0.998 for PV-TRIM, PMA-TRIM, C18 and DVB, respectively. The improvement on the sensitivity could be clearly observed by implementing the preconcentration step, mainly when PV-TRIM was used as sorbent. The preconcentration factor was determined as the ratio of slope of the calibration curve with and without preconcentration. Therefore, under optimized conditions, the preconcentration factor was found to be 17.1, 12.2, 8.6 and 7.8 for PV-TRIM, PMA-TRIM, C18 and DVB, respectively. The limits of detection (LOD) and quantification (LOQ) were determined as 3 × Sb (standard deviation of ten blank measurements) m−1 and 10 × Sb m−1, respectively, according to IUPAC recommendation, where m is the slope of analytical curve.36 The obtained values of LOD were found to be 0.07 μg L−1, 0.12 μg L−1, 0.16 μg L−1 and 0.57 μg L−1 and LOQ values were 0.25 μg L−1, 0.39 μg L−1, 0.53 μg L−1 and 1.91 μg L−1 for PV-TRIM, PMA-TRIM, C18 and DVB respectively. The precision of method for tebuthiuron determination was evaluated in terms of repeatability (n = 10) by preconcentrating solutions of 10.0 μg L−1 and 100.0 μg L−1, yielding relative standard deviation (RSD) of 1.7% and 1.4% for the polymer PV-TRIM and 4.6% and 1.9% for the polymer PMA-TRIM.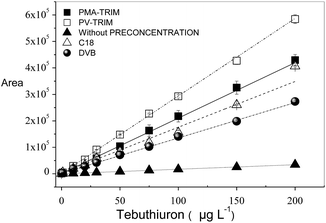 | ||
| Fig. 3 Calibration curves obtained for tebuthiuron after preconcentration step on PMA-TRIM, PV-TRIM, C18, DVB and without preconcentration. | ||
Evaluation of PV-TRIM and PMA-TRIM for adsorption of other herbicides
In order to evaluate the potential of polymers for multiresidue adsorption, particularly those used in sugarcane crops, adsorption studies with diuron, hexazinone and ametryn were carried out. First, a competitive adsorption experiment in batch mode of tebuthiuron in the presence of diuron was performed. For this task, a binary solution of tebuthiuron and diuron at 20.0 mg L−1 and 10.0 mg L−1 was stirred with 50 mg of each polymer for 20 min. Then, the supernatant was analyzed by HPLC. From the obtained results, the distribution coefficient (Kd) was calculated according to eqn (2)
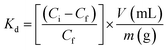 | (2) |
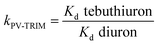 | (3) |
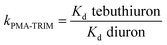 | (4) |
Table 2 shows comparison of Kd and k values. As observed, the Kd values of diuron were considerably higher compared to those of tebuthiuron for both polymers, thus showing a better affinity for diuron, which can be explained due to smaller size of the molecule.
| Sorbent | Initial concentration (mg L−1) | Kd (mLg−1) | k | ||
|---|---|---|---|---|---|
| Tebuthiuron | Diuron | Tebuthiuron | Diuron | ||
| PV-TRIM | 20 | 10 | 1577.78 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 184.62 184.62 |
0.10 |
| PMA-TRIM | 2163.79 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 812.01 812.01 |
0.18 | ||
Upon achieving outstanding adsorption of diuron in the presence of tebuthiuron, the potential of polymers for multiresidues adsorption was evaluated. In this case, adsorption experiments on column were carried out by loading 6.0 mL of quaternary mixture (tebuthiuron, hexazinone, diuron and ametryn) at 10.0 mg L−1 concentration of each herbicide through 200 mg of packed polymers under pH 6.0 and flow rate of 2.0 mL min−1. After loading, the elution was carried out with 3.0 mL of methanol. As shown in Fig. 4a, the obtained outcomes for adsorption of ametryn, diuron, tebuthiuron and hexazinone indicate the high potential of polymers for multiresidues adsorption considering the great adsorption rate above 97%.
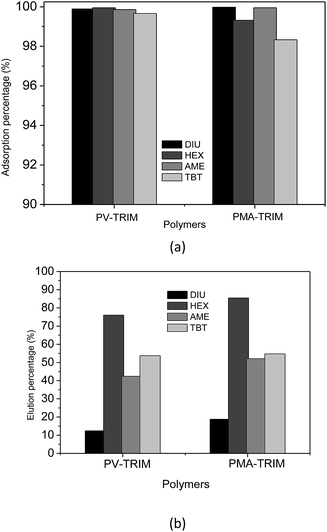 | ||
| Fig. 4 Adsorption percentage (%) of herbicides by PV-TRIM and PMA-TRIM (a) and elution percentage (%) of herbicides from PV-TRIM and PMA-TRIM using 3.0 mL of methanol as eluent (b). | ||
Although the experimental condition optimized for tebuthiuron adsorption has been satisfactory for adsorption of hexazinone, ametryn and diuron, the elution with 3.0 mL of methanol was not satisfactory (Fig. 4b). Such outcome can be attributed to high concentration of herbicides (10.0 mg L−1) and, as a consequence, 3.0 mL of methanol were not sufficient to strip off the herbicides quantitatively. It is important to point out that even though quantitative elution had not been achieved, the applicability of method was possible as will be further demonstrated, since in real samples, the concentrations of herbicides are much lower than 10.0 mg L−1.
Multiresidue analysis of herbicides
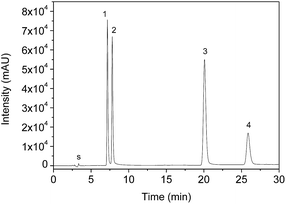
Taking into account the potential of polymers for multiresidues adsorption of urea, triazine and triazione classes, analytical curves in the range 1–150.0 μg L−1 under optimized conditions were built after preconcentration of 50.0 mL and elution with 3.0 mL of methanol (Fig. 5). The chromatographic profile for the separation of herbicides is shown in Fig. 6.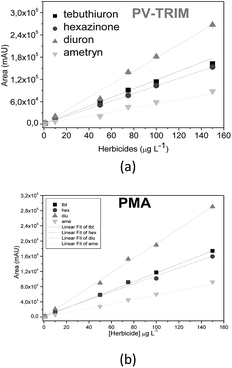 | ||
| Fig. 5 Analytical curves of quaternary solution of herbicides obtained after preconcentration on PV-TRIM (a) and PMA-TRIM (b). | ||
Tables 3 and 4 show the linear regression and the LOD and LOQ for herbicides achieved using the polymers. The values of LOD and LOQ for hexazinone, ametryn and diuron were similar, as found by comparing the polymers. However, lower LOD and LOQ for tebuthiuron were observed when the PV-TRIM was used as the sorbent. Moreover, the detectability for tebuthiuron in the quaternary mixture was lower than the one achieved in a single solution, thus indicating competitive adsorption of herbicides towards binding sites of polymers.
| Herbicide | Linear regression | R2 | LOD (μg L−1) | LOQ (μg L−1) |
|---|---|---|---|---|
| TBT | Area = 522.71–1158.03[TBT (μg L−1)] | 0.999 | 0.40 | 1.35 |
| HEX | Area = 3147.08–1051.60[HEX (μg L−1)] | 0.997 | 0.31 | 1.04 |
| DIU | Area = 422.87–1931.33[DIU (μg L−1)] | 0.999 | 0.12 | 0.40 |
| AME | Area = 522.96–573.70[AME (μg L−1)] | 0.997 | 0.34 | 1.14 |
| Herbicide | Linear regression | R2 | LOD (μg L−1) | LOQ (μg L−1) |
|---|---|---|---|---|
| TBT | Area = 1008.32–1176.31[TBT (μg L−1)] | 0.998 | 0.14 | 0.47 |
| HEX | Area = 346.115–1022.45[HEX (μg L−1)] | 0.999 | 0.44 | 1.49 |
| DIU | Area = 661.70–1779.20[DIU (μg L−1)] | 0.999 | 0.17 | 0.58 |
| AME | Area = 425.02–541.84[AME (μg L−1)] | 0.986 | 0.39 | 1.32 |
The analytical precision of multiresidues analysis using the polymers is shown in Table 5. The outcomes show that the method is precise owing to the low relative standard deviations. Furthermore, the reusability of sorbents was exceptional since adsorption/desorption cycles were performed repeatedly (161 recycles) without any significant loss in the initial binding affinity.
| PV-TRIM | ||||||||
|---|---|---|---|---|---|---|---|---|
| 10 μg L−1 | 100 μg L−1 | |||||||
| Herbicides | TBT | HEX | DIU | AME | TBT | HEX | DIU | AME |
Mean(![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ) ) |
10.0 | 10.5 | 10.2 | 10.1 | 100.4 | 100.3 | 100.8 | 101.5 |
| R.S.D (%) | 1.2 | 1.9 | 1.8 | 1.9 | 1.1 | 1.3 | 1.0 | 1.8 |
| PMA-TRIM | ||||||||
|---|---|---|---|---|---|---|---|---|
| 10 μg L−1 | 100 μg L−1 | |||||||
| Herbicide | TBT | HEX | DIU | AME | TBT | HEX | DIU | AME |
Mean (![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ) ) |
10.1 | 9.7 | 9.84 | 9.8 | 99.3 | 102.1 | 101.2 | 103.2 |
| R.S.D (%) | 3.0 | 3.3 | 2.1 | 3.2 | 2.0 | 1.4 | 2.4 | 2.9 |
The applicability of method in real water samples (well and river water) by using PV-TRIM as sorbent is shown in Table 6.
| Water samples | Herbicides | |||||
|---|---|---|---|---|---|---|
| Tebuthiuron | Hexazinone | |||||
| Added (μg L−1) | Concentration foundb (μg L−1) | Rec. (%) | Added (μg L−1) | Concentration foundb (μg L−1) | Rec. (%) | |
| a ND (not detected) = below the limit of detection.b The results are expressed as mean value ± S.D. based on two replicates (n = 3) determinations. | ||||||
| Well | 0 | ND | — | 0 | ND | — |
| 8 | 8.0 ± 0.1 | 100 | 8 | 8.1 ± 0.1 | 101 | |
| 16 | 16.1 ± 0.1 | 100 | 16 | 16.2 ± 0.1 | 101 | |
| River 1 | 0 | ND | — | 0 | ND | — |
| 8 | 8.0 ± 0.1 | 100 | 8 | 8.1 ± 0.1 | 100 | |
| 16 | 16.0 ± 0.1 | 100 | 16 | 16.1 ± 0.7 | 100 | |
| River 2 | 0 | ND | — | 0 | ND | — |
| 8 | 8.0 ± 0.1 | 100 | 8 | 7.9 ± 0.1 | 99 | |
| 16 | 16.1 ± 0.1 | 100 | 16 | 15.9 ± 0.1 | 99 | |
| River 3 | 0 | ND | — | 0 | ND | — |
| 8 | 7.9 ± 0.1 | 99 | 8 | 7.9 ± 0.1 | 99 | |
| 16 | 15.7 ± 0.3 | 98 | 16 | 15.8 ± 0.2 | 98 | |
| River 4 | 0 | ND | — | 0 | ND | — |
| 8 | 8.0 ± 0.2 | 100 | 8 | 7.7 ± 0.2 | 96 | |
| 16 | 16.0 ± 0.2 | 100 | 16 | 15.5 ± 0.3 | 97 | |
| Sample | Herbicides | |||||
|---|---|---|---|---|---|---|
| Diuron | Ametryn | |||||
| Added (μg L−1) | Concentration foundb (μg L−1) | Rec. (%) | Added (μg L−1) | Concentration foundb (μg L−1) | Rec. (%) | |
| Well | 0 | ND | — | 0 | ND | — |
| 8 | 8.0 ± 0.1 | 100 | 8 | 7.9 ± 0.1 | 98 | |
| 16 | 16.0 ± 0.1 | 100 | 16 | 15.9 ± 0.1 | 99 | |
| River 1 | 0 | ND | — | 0 | ND | — |
| 8 | 7.9 ± 0.1 | 99 | 8 | 8.1 ± 0.1 | 101 | |
| 16 | 15.9 ± 0.1 | 99 | 16 | 15.9 ± 0.1 | 99 | |
| River 2 | 0 | ND | — | 0 | ND | — |
| 8 | 7.9 ± 0.1 | 98 | 8 | 7.9 ± 0.1 | 98 | |
| 16 | 16.0 ± 0.1 | 100 | 16 | 16.0 ± 0.1 | 100 | |
| River 3 | 0 | ND | — | 0 | ND | — |
| 8 | 7.9 ± 0.3 | 99 | 8 | 8.0 ± 0.3 | 100 | |
| 16 | 15.5 ± 0.4 | 96 | 16 | 16.2 ± 0.3 | 101 | |
| River 4 | 0 | ND | — | 0 | ND | — |
| 8 | 7.8 ± 0.4 | 98 | 8 | 7.8 ± 0.2 | 98 | |
| 16 | 15.6 ± 0.3 | 98 | 16 | 15.7 ± 0.4 | 98 | |
Water was collected from rivers and wells situated near sugarcane crops in the São Paulo and Paraná States – Brazil. Sample collection was carried out in amber flasks, and the samples were acidified with 0.5 mol L−1 H2SO4 and the pH was adjusted to 6.0. From the data, the herbicides were not found in the samples and after spiking sample with known amount of herbicides, satisfactory recovery in the range 96–101% was obtained, indicating that the polymers were prone to sorb the herbicides free of interferences from natural water. Although herbicides were not determined naturally in the analyzed water samples, the detectability of method may be considerably improved by increasing the sample volume, according to breakthrough curve.
Conclusions
A solid-phase extraction method for simultaneous preconcentration of tebuthiuron, diuron, hexaxinone and ametryn using PVI-TRIM and PMA-TRIM as sorbents was developed. The suitability of PVI-TRIM for interference-free multiresidues analysis in natural waters was demonstrated by high recovery values and good precision. In general, PV-TRIM adsorbs higher amounts of tebuthiuron either in single or quaternary solutions compared to other herbicides. However, bearing in mind the analytical performance of SPE procedure using PMA-TRIM as sorbent, most likely this material can also be successfully applied in real samples. The polymers were advantageous relative to the commercial sorbents C18 and DVB, due to exceptional reusability (161-recycles) and a better detectability by loading larger volumes of samples (>50.0 mL). For final remarks and from the results, it can be concluded that new applications of cross-linked polymers synthesized with different functional monomers deserve to be expanded to other analytes aiming at developing of new analytical methods for multiresidue analysis.Acknowledgements
The authors would like to thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant No. 481669/2013-2, 305552/2013-9, 472670/2012-3), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (25/2014), Fundação Araucária do Paraná, Secretaria da Ciência, Tecnologia e Ensino Superior do Paraná (SETI), UTFPR-APUCARANA, SANEPAR, Laboratório de Espectroscopia da Central de Multiusuário da PROPPG and Instituto Nacional de Ciência e Tecnologia de Bioanalítica (INCT) (Grant No. 573672/2008-3) for their financial support and fellowships.References
- S. S. Caldas, F. F. Gonçalves, E. G. Primel, O. D. Prestes, M. L. Martins and R. Zanella, Quim. Nova, 2011, 9, 1604–1617 CrossRef.
- C. S. Machado, R. I. S. Alves, B. M. Fregonesi, K. A. A. Tonani, B. S. Martinis, J. Sierra, M. Nadal, J. L. Domingo and S. Segura-Muñoz, Procedia Eng., 2016, 162, 230–237 CrossRef CAS.
- J. Vymazal and T. Brezinová, Environ. Int., 2015, 75, 11–20 CrossRef CAS PubMed.
- FAOSTAT – Food and Agriculture Organization of the United Nations, Statistics Division, Word crop production, http://faostat3.fao.org/browse/Q/*/E, 2016, accessed April, 11, 2017.
- IBGE – Brazilian Institute of Geography and Statistics, Statistics of agricultural production, ftp://ftp.ibge.gov.br/Producao_Agricola/Producao_Agricola_Municipal_[anual]/2013/pam2013.pdf, 2013, accessed April, 11, 2017.
- S. H. G. Brondi and F. M. Lanças, J. Braz. Chem. Soc., 2005, 16, 650–653 CrossRef CAS.
- S. C. N. Queiroz, V. L. Ferracini, M. A. F. Gomes and M. A. Rosa, Quim. Nova, 2009, 32, 378–381 CrossRef CAS.
- P. Y. Caux, R. A. Kent, V. Bergeron, J. E. Warner and J. Busharda, Environ. Toxicol., 1997, 12, 61–65 CAS.
- A. Saleh, N. S. Fumani and S. Molaei, J. Chromatogr., A, 2014, 1356, 32–37 CrossRef CAS PubMed.
- S. Giacomazzi and N. Cochet, Chemosphere, 2004, 56, 1021–1032 CrossRef CAS PubMed.
- T. Santos, G. Cancian, D. N. R. Neodini, D. R. S. Mano, C. Capucho, F. S. Predes, R. Barbieri, C. A. Oliveira, A. A. Pigoso, H. Dolder and G. D. C. Severi-Aguiar, Exp. Toxicol. Pathol., 2015, 67, 525–550 CrossRef CAS PubMed.
- World Health Organization (WHO), Guidelines for drinking-water quality, Third edition incorporating the first and second addenda.volume 1, 2008, http://www.who.int/water_sanitation_health/dwq/fulltext.pdf, accessed April, 11 2017.
- European Commission, “Priority substances under the water framework directive”, http://ec.europa.eu/environment/water/waterframework/priority_substances.html, accessed April, 11 2017.
- A. Wong, M. R. V. Lanza and M. D. P. T. Sotomayor, J. Electroanal. Chem., 2013, 690, 83–88 CrossRef CAS.
- Brazilian Ministry of Health; Act No. 2.914 of December 12, http://bvsms.saude.gov.br/bvs/saudelegis/gm/2011/prt2914_12_12_2011.html, 2011, accessed April 11, 2017.
- D. J. Hamilton, Á. Ambrus, R. M. Dieterlie, A. S. Felsot, C. A. Harris, P. T. Holland, A. Katayama, Kurihara, N. J. Linders, J. Unsworth and S. S. Wong, Pure Appl. Chem., 2003, 75, 1123–1155 CrossRef CAS.
- S. H. G. Brondi and F. M. Lanças, J. Liq. Chromatogr. Relat. Technol., 2004, 27, 171–179 CrossRef CAS.
- S. Saha, R. Mistri and B. C. Ray, J. Chromatogr. A, 2010, 1217, 307–3011 CrossRef CAS PubMed.
- S. Saha, R. Mistri and B. B. C. Ray, Anal. Bioanal. Chem., 2013, 405, 9265–9272 CrossRef CAS PubMed.
- W. T. Ma, K. K. Fu, Z. Cai and G. B. Jiang, Chemosphere, 2003, 52, 1627–1632 CrossRef CAS PubMed.
- J. Wang, F. Wu and Q. Zhao, Chin. J. Chromatogr., 2015, 33, 849–855 CrossRef CAS.
- G. L. Sheel and C. R. T. Tarley, Microchem. J., 2017, 133, 650–657 CrossRef.
- C. Wang, S. Ju, Q. Wu and Z. Wang, J. Chromatogr. Sci., 2011, 49, 689–694 CAS.
- J. Wang, Y. Cui, W. Liu, M. Yang and J. Chen, Chin. J. Chromatogr., 2007, 25, 853–856 CAS.
- M. Saraji and B. Farajmand, J. Chromatogr., A, 2008, 1178, 17–23 CrossRef CAS PubMed.
- J. Ma, L. Jiang, G. Wu, Y. Xia, W. Lu, J. Li and L. Chen, J. Chromatogr., A, 2016, 1466, 12–20 CrossRef CAS PubMed.
- A. R. Koophaei, S. J. Shahtaheri, M. R. Ganjali, A. R. Forushani and F. Golbabaei, J. Hazard. Mater., 2009, 170, 1247–1255 CrossRef PubMed.
- E. I. P. Rezende, P. G. Peralta-Zamora, W. F. Jardim, C. Vidal and G. Abate, Anal. Lett., 2013, 46, 439–451 CrossRef.
- J. M. Pozzebon, S. C. N. Queiroz and I. C. S. F. Jardim, J. Liq. Chromatogr. Relat. Technol., 2003, 26, 78–790 CrossRef.
- X. L. Wu, L. Meng, Y. Wu, Y.-Y. Luk, Y. Ma and Y. Du, J. Braz. Chem. Soc., 2015, 26, 131–139 CAS.
- S. C. Queiroz, L. F. Melo and I. C. Jardim, J. Chromatogr. A, 2002, 948, 171–176 CrossRef CAS PubMed.
- X. Liu, C. Wang, Q. Wu and Z. Wang, Anal. Chim. Acta, 2015, 22, 870 Search PubMed.
- G. Mendas, V. Drevenkar and L. Zupancic-Kralj, J. Chromatogr., 2001, 918, 351–359 CrossRef CAS PubMed.
- V. Pichon, J. Chromatogr. A, 2000, 885, 195–215 CrossRef CAS PubMed.
- R. J. Fonseca, M. G. Segatelli, K. C. Borges and C. R. T. Tarley, React. Funct. Polym., 2015, 93, 1–9 CrossRef.
- G. L. Long and J. D. Winefordner, Anal. Chem., 1983, 55, 712–722 CrossRef.
| This journal is © The Royal Society of Chemistry 2017 |