Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lewis base-catalyzed diastereoselective [3 + 2] cycloaddition reaction of nitrones with electron-deficient alkenes: an access to isoxazolidine derivatives†
Honglei Liua,
Yan Zhaoa,
Zhen Lia,
Hao Jiaa,
Cheng Zhang a,
Yumei Xiaoa and
Hongchao Guo
a,
Yumei Xiaoa and
Hongchao Guo *ab
*ab
aDepartment of Applied Chemistry, China Agricultural University, Beijing 100193, China. E-mail: hchguo@cau.edu.cn
bKey Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, Guizhou University, Guiyang 550025, China
First published on 6th June 2017
Abstract
A Lewis base-catalyzed [3 + 2] cycloaddition reaction of nitrones with electron-deficient alkenes has been achieved under mild reaction conditions, affording various functionalized isoxazolidine derivatives as single diastereomers in moderate to excellent yields.
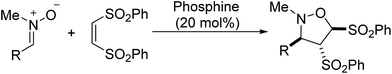
Nucleophilic phosphine-catalyzed cycloaddition reactions provide important access to various synthetically useful or biologically important carbo- and heterocyclic compounds1 and serve as the key step for the total synthesis of some natural products.2 During the past several decades a wide range of cycloaddition reactions have been developed.3–11 A variety of phosphine acceptors such as activated allenes, alkynes and alkenes and electrophilic coupling partners such as aldehydes, alkenes, imines, and aziridines have been exploited for these reactions.1 In the past five years, 1,3-dipoles such as N,N′ or C,N-cyclic azomethine imines and azomethine ylides have been used as versatile electrophilic coupling partners for phosphine-catalyzed [3 + 2],12 [3 + 3],12,13 [4 + 3]12,14 and [3 + 2 + 3]12 cycloadditions with activated allenes, alkynes, alkenes and MBH carbonates, producing biologically important nitrogen-containing heterocycles, such as tetrahydropyrazolopyrazolone, tetrahydropyranzolo-pyridazinone, tetrahydropyrazolodiazepinone, tetrahydropyrazolo-diazocinone, tricyclic dihydroisoquinoline and tetrahydro-isoquinoline derivatives.12–14 Although these dipoles have displayed diverse reactivities in the phosphine-catalyzed cycloadditions, the scope of 1,3-dipoles is still limited to azomethine imines and azomethine ylides. Other kinds of 1,3-dipoles have received little attention and have not been explored in phosphine-catalyzed cycloadditions. In this context, we tried to develop novel cycloaddition reactions based on other 1,3-dipoles, such as nitrones.15 Nitrones are readily accessible and stable compounds and worked as efficient 1,3-dipoles in various cycloadditions to provide diverse cyclic compounds,15 which are important precursors for synthesis of bioactive compounds, natural products and other useful compounds.16 Herein, we present the first phosphine-catalyzed [3 + 2] cycloaddition reaction of various nitrones with electron-deficient alkenes for synthesis of functionalized isoxazolidines, which are potential scaffolds for the synthesis of pharmacologically active molecules (Scheme 1).
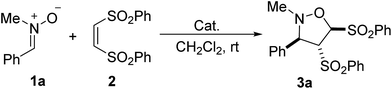
In our initial investigation, the reaction of N-methyl-1-phenylmethanimine oxide 1a with (Z)-1,2-bis(phenylsulfonyl)-ethylene 2 was chosen as the model reaction (Table 1). The reaction of 1a and 2 was carried out in dichloromethane at room temperature in the absence of catalyst for 48 h, no new spots was observed by TLC monitoring (Table 1, entry 1). In the presence of 20 mol% PPh3, the nitrone 1a was treated with the alkene 2 in dichloromethane at room temperature for 48 h, leading to a desired [3 + 2] cycloaddition product isoxazolidine derivative 3a as a single diastereomer in 99% yield (entry 2). The relative configuration of the product isoxazolidine derivative 3a was unequivocally determined through the related X-ray crystallographic data of the homologous compound 3b in Table 2.17 Several nucleophilic phosphines such as PBu3, Me2PPh, MePPh2, EtPPh2, n-PrPPh2, i-PrPPh2, t-BuPPh2 and CyPPh2 were next screened. Among these phosphines, both Me2PPh and MePPh2 were identified as the most effective catalysts for this reaction (entries 4 and 5). Other phosphines including PBu3, EtPPh2, n-PrPPh2, i-PrPPh2 and CyPPh2 could also promote the reaction, but giving the corresponding product in lower 37–89% yields (entries 3, 6–8, and 10). With the use of t-BuPPh2 as the catalyst, only trace of [3 + 2] cycloaddition product was obtained. Some tertiary amines, such as trimethylamine (Et3N), 1,4-diazobicyclo[2.2.2]octane (DABCO), 1,8-diazabicyclo-[5.4.0]-undec-7-ene (DBU), and 4-dimethylamino-pyridine (DMAP), have also been examined and displayed moderate to excellent catalytic activity (entries 11–14). The DBU and DMAP led to 99% yield of the product 3a (entries 13 and 14). In the presence of Ph3P, the catalyst loading was attempted to be decreased to 10 mol% and 5 mol%, but the yield was reduced to 50% yield and 32% yield, respectively (entries 15 and 16).
| Entry | Catalyst | Yieldb (%) |
|---|---|---|
| a Reactions of 1 (0.1 mmol), 2 (0.12 mmol) and catalyst (0.02 mmol) were carried out in 2.5 mL of CH2Cl2 at room temperature for 48 h.b Without catalyst.c 10 mol% catalyst was used.d 5 mol% catalyst was used. | ||
| 1b | — | 0 |
| 2 | PPh3 | 99 |
| 3 | PBu3 | 60 |
| 4 | Me2PPh | 95 |
| 5 | MePPh2 | 97 |
| 6 | EtPPh2 | 77 |
| 7 | n-PrPPh2 | 89 |
| 8 | i-PrPPh2 | 68 |
| 9 | t-BuPPh2 | Trace |
| 10 | CyPPh2 | 37 |
| 11 | Et3N | 66 |
| 12 | DABCO | 36 |
| 13 | DBU | 99 |
| 14 | DMAP | 99 |
| 15c | PPh3 | 50 |
| 16d | PPh3 | 32 |
| Entry | Cat. | R | t/h | 3 | Yield (%) |
|---|---|---|---|---|---|
| a Reactions of 1 (0.2 mmol), 2 (0.24 mmol) and the catalyst (0.04 mmol) were carried out in 5 mL of CH2Cl2 at room temperature. | |||||
| 1 | Ph3P | C6H5 (1a) | 48 | 3a | 99 |
| 2 | DMAP | 2-MeC6H4 (1b) | 48 | 3b | 81 |
| 3 | DMAP | 3-MeC6H4 (1c) | 48 | 3c | 74 |
| 4 | DMAP | 4-MeC6H4 (1d) | 48 | 3d | 69 |
| 5 | Ph3P | 2,4-Me2C6H3 (1e) | 48 | 3e | 90 |
| 6 | Ph3P | 3,4-Me2C6H3 (1f) | 48 | 3f | 87 |
| 7 | Ph3P | 2-MeOC6H4 (1g) | 120 | 3g | 76 |
| 8 | Ph3P | 3-MeOC6H4 (1h) | 120 | 3h | 80 |
| 9 | Ph3P | 4-MeOC6H4 (1i) | 120 | 3i | 63 |
| 10 | DMAP | 2,3-(OMe)2C6H3 (1j) | 120 | 3j | 77 |
| 11 | Ph3P | 2,4-(OMe)2C6H3 (1k) | 120 | 3k | 75 |
| 12 | Ph3P | 2,3,4-(OMe)3C6H2 (1l) | 120 | 3l | 78 |
| 13 | Ph3P | 4-NMe2C6H4 (1m) | 48 | 3m | 65 |
| 14 | Ph3P | 2-FC6H4 (1n) | 48 | 3n | 51 |
| 15 | Ph3P | 2-ClC6H4 (1o) | 48 | 3o | 91 |
| 16 | DMAP | 3-ClC6H4 (1p) | 48 | 3p | 62 |
| 17 | DMAP | 4-ClC6H4 (1q) | 48 | 3q | 61 |
| 18 | Ph3P | 2-BrC6H4 (1r) | 48 | 3r | 83 |
| 19 | DMAP | 3-BrC6H4 (1s) | 48 | 3s | 41 |
| 20 | Ph3P | 4-BrC6H4 (1t) | 48 | 3t | 62 |
| 21 | DMAP | 4-PhC6H4 (1u) | 48 | 3u | 75 |
| 22 | Ph3P | 2-Naphthyl (1v) | 48 | 3v | 99 |
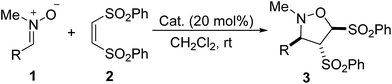
With the optimal reaction conditions in hand, we next investigated the scope of the Lewis base-catalyzed [3 + 2] cycloaddition of nitrones with alkenes. With 20 mol% of PPh3 or DMAP as the catalyst, various nitrones 1 underwent [3 + 2] cycloaddition reaction with (Z)-1,2-bis(phenylsulfonyl)ethylene 2 in dichloromethane at rt for 48–120 h, providing a variety of 4,5-bis(phenylsulfonyl)isoxazolidine derivatives (3a–3v) in moderate to excellent yields (Table 2, entries 1–22). Nitrones bearing whether electron-donating or withdrawing groups on the benzene ring worked smoothly to afford the corresponding products in satisfactory yields (entries 2–21). The methoxy-substituted nitrones were not very active, requiring longer reaction time (entries 7–12). Those nitrones having di and trisubstituted aryl groups were also tolerated, leading to good yields of the [3 + 2] cycloadducts (entries 5–6, 10–12). Particularly, the cycloaddition of 2-naphthyl-substituted nitrone (1v) proceeded efficiently to give the product 3v in 99% yield (entry 22).
The reaction of nitrone 1a with (E)-1,2-bis(phenylsulfonyl)-ethylene 2′ has also been performed, producing 76% yield of the identical product 3a with the reaction of (Z)-1,2-bis(phenylsulfonyl)-ethylene 2 (Scheme 2). It indicated that the stereoselectivity of the reaction was not influenced by the configuration of carbon–carbon double bond in the alkene 2 and 2′.
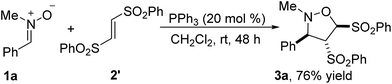 | ||
| Scheme 2 PPh3-catalyzed [3 + 2] cycloaddition of nitrone 1a with (E)-1,2-bis(phenylsulfonyl)ethylene 2′. | ||
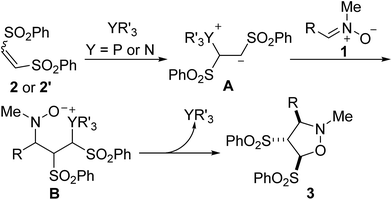
The proposed mechanism for [3 + 2] cycloaddition of the nitrone 1 with 1,2-bis(phenylsulfonyl)ethylene 2 is presented in Scheme 3. Conjugate addition of the phosphine or tertiary amine to the alkene 2 or 2′ gives the zwitterion intermediate A, which then attacks nitrone 1 to give the intermediate B. It undergoes an intramolecular nucleophilic attack to accomplish the [3 + 2] cyclization to give the product 3 with simultaneous regeneration of the catalyst. Since whether (Z)-alkene 2 or (E)-alkene 2′ produced the identical intermediate A, the stereochemistry of the reaction cannot be influenced by the configuration of the alkene.
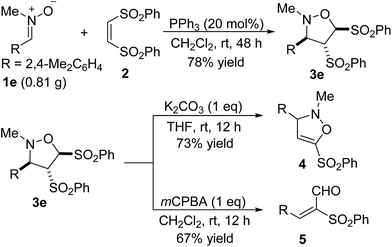
The present reaction is quite robust. The reaction of 0.81 g of nitrone 1e with alkene 2 still worked efficiently to produce the desired product 3e in 78% yield (Scheme 4). To further demonstrate the reaction could be a practical tool for the synthesis of other valuable compounds, some synthetic transformations of cycloadduct 3e were tried (Scheme 4). Treatment of the product 3e with 1 equiv. K2CO3 in THF resulted in elimination of one of two phenylsulfonyl groups, affording the derivative 4 in 73% yield. The oxidation of 3e with 1 equiv. of mCPBA in dichloromethane gave an α,β-unsaturated aldehyde 5 in 67% yield.
Conclusions
We have developed a Lewis base-catalyzed [3 + 2] cycloaddition reaction of nitrones with electron-deficient alkene, giving various functionalized isoxazolidine derivatives in moderate to excellent yields. A variety of nitrones underwent the reaction smoothly under the mild reaction conditions. The scaled-up reaction and further transformation of the cycloadducts demonstrated that the reaction could be a practical tool for organic synthesis.Acknowledgements
This work is supported by the NSFC (No. 21172253, 21372256 and 21572264).Notes and references
- For recent reviews, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) J. L. Methot and W. R. Roush, Adv. Synth. Catal., 2004, 346, 1035 CrossRef CAS; (c) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (d) C. E. Aroyan, A. Dermenci and S. J. Miller, Tetrahedron, 2009, 65, 4069 CrossRef CAS; (e) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (f) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (g) S.-X. Wang, X. Y. Han, F. R. Zhong, Y. Q. Wang and Y. X. Lu, Synlett, 2011, 2766 CAS; (h) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (i) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (j) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (k) Y. Xiao, Z. Sun, H. Guo and O. Kwon, Beilstein J. Org. Chem., 2014, 10, 2089 CrossRef PubMed; (l) P. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC; (m) Y. Xiao, H. Guo and O. Kwon, Aldrichimica Acta, 2016, 49, 3 CAS; (n) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed.
- For application in synthesis of natural products, see: (a) J. C. Wang and M. J. Krische, Angew. Chem., Int. Ed., 2003, 42, 5855 CrossRef CAS PubMed; (b) K. Agapiou and M. J. Krische, Org. Lett., 2003, 5, 1737 CrossRef CAS PubMed; (c) Y. S. Tran and O. Kwon, Org. Lett., 2005, 7, 4289 CrossRef CAS PubMed; (d) R. A. Jones and M. J. Krische, Org. Lett., 2009, 11, 1849 CrossRef CAS PubMed; (e) M. Sampath, P.-Y. B. Lee and T. P. Loh, Chem. Sci., 2011, 2, 1988 RSC; (f) I. P. Andrews and O. Kwon, Chem. Sci., 2012, 3, 2510 RSC; (g) R. A. Villa, Q. H. Xu and O. Kwon, Org. Lett., 2012, 14, 4634 CrossRef CAS PubMed; (h) G. A. Barcan, A. Patel, K. N. Houk and O. Kwon, Org. Lett., 2012, 14, 5388 CrossRef CAS PubMed; (i) L. Cai, K. Zhang and O. Kwon, J. Am. Chem. Soc., 2016, 138, 3298 CrossRef CAS PubMed.
- For [2 + 1] annulation, see: (a) S. Xu, L. Zhou, R. Ma, H. Song and Z. He, Org. Lett., 2010, 12, 544 CrossRef CAS PubMed; (b) A. A. Ibrahim, P. H. Wei, G. D. Harzmann and N. J. Kerrigan, J. Org. Chem., 2010, 75, 7901 CrossRef CAS PubMed; (c) S. Chen, E. C. Salo, K. A. Wheele and N. J. Kerrigan, Org. Lett., 2012, 14, 1784 CrossRef CAS PubMed. For [2 + 2] annulation, see: (d) Z. Yang, H. Yu, L. Zhang, H. Wei, Y. Xiao, L. Chen and H. Guo, Chem.–Asian J., 2014, 9, 313 CrossRef CAS PubMed.
- For representative examples on [3 + 2] annulation, see: (a) C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS; (b) G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Am. Chem. Soc., 1997, 119, 3836 CrossRef CAS; (c) J.-C. Wang, S.-S. Ng and M. J. Krische, J. Am. Chem. Soc., 2003, 125, 3682 CrossRef CAS PubMed; (d) J. E. Wilson and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1426 CrossRef CAS PubMed; (e) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (f) B. J. Cowen and S. J. Miller, J. Am. Chem. Soc., 2007, 129, 10988 CrossRef CAS PubMed; (g) A. Voituriez, A. Panossian, N. Fleury-Bregeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS PubMed; (h) Y. Q. Fang and E. N. Jacobsen, J. Am. Chem. Soc., 2008, 130, 5660 CrossRef CAS PubMed; (i) M. Sampath and T.-P. Loh, Chem. Sci., 2010, 1, 739 RSC; (j) H. Xiao, Z. Chai, C. W. Zheng, Y. Q. Yang, W. Liu, J. K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (k) Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed; (l) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (m) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed; (n) D. Duvvuru, N. Pinto, C. Gomez, J.-F. Betzer, P. Retailleau, A. Voituriez and A. Marinetti, Adv. Synth. Catal., 2012, 354, 408 CrossRef CAS; (o) Q. Zhao, X. Han, Y. Wei, M. Shi and Y. Lu, Chem. Commun., 2012, 48, 970 RSC; (p) X. Han, F. Zhong, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2012, 51, 767 CrossRef CAS PubMed; (q) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764 RSC; (r) F. Zhong, G. Chen, X. Han, W. Yao and Y. Lu, Org. Lett., 2012, 14, 3764 CrossRef CAS PubMed; (s) H.-P. Deng, Y. Wei and M. Shi, Adv. Synth. Catal., 2012, 354, 783 CrossRef CAS; (t) J. Marco-Martinez, V. Marcos, S. Reboredo, S. Filippone and N. Martin, Angew. Chem., Int. Ed., 2013, 52, 5115 CrossRef CAS PubMed; (u) H. Yu, L. Zhang, Z. Yang, Z. Li, Y. Zhao, Y. Xiao and H. Guo, J. Org. Chem., 2013, 78, 8427 CrossRef CAS PubMed; (v) X.-N. Zhang and M. Shi, ACS Catal., 2013, 3, 507 CrossRef CAS; (w) D. Wang, Y. Lei, Y. Wei and M. Shi, Chem.–Eur. J., 2014, 20, 15325 CrossRef CAS PubMed; (x) C. E. Henry, Q. H. Xu, Y. C. Fan, T. J. Martin, L. Belding, T. Dudding and O. Kwon, J. Am. Chem. Soc., 2014, 136, 11890 CrossRef CAS PubMed; (y) Z. Gao, C. Wang, C. Yuan, L. Zhou, Z. Sun, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 105359 RSC; (z) X. Han, W.-L. Chan, W. Yao, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2016, 55, 6492 CrossRef CAS PubMed.
- For [4 + 1] annulation, see: (a) X. Meng, Y. Huang and R. Chen, Org. Lett., 2009, 11, 137 CrossRef CAS PubMed; (b) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550 CrossRef CAS PubMed; (c) X. N. Zhang, H. P. Deng, L. Huang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 8664 RSC; (d) D. T. Ziegler, L. Riesgo, T. Ikeda, Y. Fujiwara and G. C. Fu, Angew. Chem., Int. Ed., 2014, 53, 13183 CrossRef CAS PubMed; (e) X. Han, W. Yao, T. Wang, Y. R. Tan, Z. Yan, J. Kwiatkowski and Y. Lu, Angew. Chem., Int. Ed., 2014, 53, 5643 CrossRef CAS PubMed; (f) S. Kramer and G. C. Fu, J. Am. Chem. Soc., 2015, 137, 3803 CrossRef CAS PubMed; (g) Z. Gao, C. Wang, C. Yuan, L. Zhou, Y. Xiao and H. Guo, Chem. Commun., 2015, 51, 12653 RSC.
- For [4 + 2] annulation, see: (a) M. P. S. Ishar, K. Kumar, S. Kaur, S. Kumar, N. K. Girdhar, S. Sachar, A. Marwaha and A. Kapoor, Org. Lett., 2001, 3, 2133 CrossRef CAS PubMed; (b) X.-F. Zhu, J. Lan and O. Kwon, J. Am. Chem. Soc., 2003, 125, 4716 CrossRef CAS PubMed; (c) R. P. Wurz and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 12234 CrossRef CAS PubMed; (d) X.-F. Zhu, A.-P. Schaffner, R. C. Li and O. Kwon, Org. Lett., 2005, 7, 2977 CrossRef CAS PubMed; (e) Y. S. Tran and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12632 CrossRef CAS PubMed; (f) S. Castellano, H. D. G. Fiji, S. S. Kinderman, M. Watanabe, P. de Leon, F. Tamanoi and O. Kwon, J. Am. Chem. Soc., 2007, 129, 5843 CrossRef CAS PubMed; (g) X. Meng, Y. Huang, H. Zhao, P. Xie, J. Ma and R. Chen, Org. Lett., 2009, 11, 991 CrossRef CAS PubMed; (h) H. Xiao, Z. Chai, H. F. Wang, X. W. Wang, D. D. Cao, W. Liu, Y. P. Lu, Y. Q. Yang and G. Zhao, Chem.–Eur. J., 2011, 17, 10562 CrossRef CAS PubMed; (i) F. Zhong, X. Han, Y. Wang and Y. Lu, Chem. Sci., 2012, 3, 1231 RSC; (j) H. Xiao, Z. Chai, D. Cao, H. Wang, J. Chen and G. Zhao, Org. Biomol. Chem., 2012, 10, 3195 RSC; (k) S. Takizawa, F. A. Arteaga, Y. Yoshida, M. Suzuki and H. Sasai, Asian J. Org. Chem., 2014, 3, 412 CrossRef CAS; (l) H. Yu, L. Zhang, Z. Li, H. Liu, B. Wang, Y. Xiao and H. Guo, Tetrahedron, 2014, 70, 340 CrossRef CAS; (m) W. J. Yao, X. W. Dou and Y. Lu, J. Am. Chem. Soc., 2015, 137, 54 CrossRef CAS PubMed; (n) W. Yang, Y. Zhang, S. Qiu, C. Zhao, L. Zhang, H. Liu, L. Zhou, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 62343 RSC; (o) H. Liu, Y. Liu, C. Yuan, G.-P. Wang, S.-F. Zhu, Y. Wu, B. Wang, Z. Sun, Y. Xiao, Q.-L. Zhou and H. Guo, Org. Lett., 2016, 18, 1302 CrossRef CAS PubMed; (p) C. Wang, Z. Gao, L. Zhou, C. Yuan, Z. Xun, Y. Xiao and H. Guo, Org. Lett., 2016, 18, 3418 CrossRef CAS PubMed; (q) C. Wang, H. Jia, C. Zhang, Z. Gao, L. Zhou, C. Yuan, Y. Xiao and H. Guo, J. Org. Chem., 2017, 82, 633 CrossRef CAS PubMed.
- For an example of [3 + 3] cycloaddition, see: H. Guo, Q. Xu and O. Kwon, J. Am. Chem. Soc., 2009, 131, 6318 CrossRef CAS PubMed.
- For [4 + 3] annulation, see: (a) K. Kumar, R. Kapoor, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 2023 CrossRef CAS PubMed; (b) S. Zheng and X. Lu, Org. Lett., 2009, 11, 3978 CrossRef CAS PubMed; (c) R. Zhou, J. Wang, C. Duan and Z. He, Org. Lett., 2012, 14, 6134 CrossRef CAS PubMed.
- For [2 + 2 + 2] annulation, see: X. Zhu, C. E. Henry, J. Wang, T. Dudding and O. Kwon, Org. Lett., 2005, 7, 1387 CrossRef CAS PubMed.
- For an example of [8 + 2] cycloaddition, see: K. Kumar, A. Kapur and M. P. S. Ishar, Org. Lett., 2000, 2, 787 CrossRef CAS PubMed.
- For selected examples on other annulations, see: (a) B. M. Trost and C.-J. Li, J. Am. Chem. Soc., 1994, 116, 10819 CrossRef CAS; (b) C. Lu and X. Lu, Org. Lett., 2002, 4, 4677 CrossRef CAS PubMed; (c) B. Liu, R. Davis, B. Joshi and D. W. Reynolds, J. Org. Chem., 2002, 67, 4595 CrossRef CAS PubMed; (d) H. Kuroda, I. Tomita and T. Endo, Org. Lett., 2003, 5, 129 CrossRef CAS PubMed; (e) Y. Du, X. Lu and C. Zhang, Angew. Chem., Int. Ed., 2003, 42, 1035 CrossRef CAS PubMed; (f) R. K. Thalji and W. R. Roush, J. Am. Chem. Soc., 2005, 127, 16778 CrossRef CAS PubMed; (g) F. Silva, M. Sawaki and V. Gouverneur, Org. Lett., 2006, 8, 5417 CrossRef CAS PubMed; (h) S. Gabillet, D. Lecercle, O. Loreau, M. Carboni, S. Dezard, J.-M. Gomis and F. Taran, Org. Lett., 2007, 9, 3925 CrossRef CAS PubMed; (i) V. Nair, S. C. Mathew, A. T. Biju and E. Suresh, Angew. Chem., Int. Ed., 2007, 46, 2070 CrossRef CAS PubMed; (j) L.-W. Ye, X.-L. Sun, Q.-G. Wang and Y. Tang, Angew. Chem., Int. Ed., 2007, 46, 5951 CrossRef CAS PubMed; (k) V. Sriramurthy, G. A. Barcan and O. Kwon, J. Am. Chem. Soc., 2007, 129, 12928 CrossRef CAS PubMed; (l) Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2009, 48, 2225 CrossRef CAS PubMed; (m) L. W. Ye, S. B. Wang, Q. G. Wang, X. L. Sun, Y. Tang and Y. G. Zhou, Chem. Commun., 2009, 45, 3092 RSC; (n) S. Takizawa, N. Inoue, S. Hirata and H. Sasai, Angew. Chem., Int. Ed., 2010, 49, 9725 CrossRef CAS PubMed; (o) Z. Shi, P. Yu, T. P. Loh and G. Zhong, Angew. Chem., Int. Ed., 2012, 51, 7825 CrossRef CAS PubMed; (p) F. Zhong, X. Dou, X. Han, W. Yao, Q. Zhu, Y. Meng and Y. Lu, Angew. Chem., Int. Ed., 2013, 52, 943 CrossRef CAS PubMed; (q) R. J. Lundgren, A. Wilsily, N. Marion, C. Ma, Y. K. Chung and G. C. Fu, Angew. Chem., Int. Ed., 2013, 52, 2525 CrossRef CAS PubMed.
- (a) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, Y. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard III, H. Guo and O. Kown, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed; (b) Z. Li, H. Yu, H. Liu, L. Zhang, H. Jiang, B. Wang and H. Guo, Chem.–Eur. J., 2014, 20, 1731 CrossRef CAS PubMed; (c) D. Wang, Y. Lei, Y. Wei and M. Shi, Chem.–Eur. J., 2014, 20, 15325 CrossRef CAS PubMed; (d) C. Yuan, L. Zhou, Z. Sun and H. Guo, RSC Adv., 2016, 6, 77931 RSC.
- (a) J. Liu, H. Liu, R. Na, G. Wang, Z. Li, H. Yu, M. Wang, J. Zhong and H. Guo, Chem. Lett., 2012, 41, 218 CrossRef CAS; (b) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316 CrossRef CAS PubMed; (c) L. Liang and Y. Huang, Org. Lett., 2016, 18, 2604 CrossRef CAS PubMed; (d) Z. Li, H. Yu, Y. Liu, L. Zhou, Z. Sun and H. Guo, Adv. Synth. Catal., 2016, 358, 1880 CrossRef CAS.
- (a) C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed; (b) Z. Li, H. Yu, Y. Feng, Z. Hou, L. Zhang, W. Yang, Y. Wu, Y. Xiao and H. Guo, RSC Adv., 2015, 5, 34481 RSC; (c) C. Yuan, L. Zhou, M. Xia, Z. Sun, D. Wang and H. Guo, Org. Lett., 2016, 18, 5644 CrossRef CAS PubMed.
- For some reviews on 1,3-dipolar cycloadditions of nitrones, see: (a) M. Frederikson, Tetrahedron, 1997, 53, 403 CrossRef; (b) K. V. Gothelf and K. A. Jørgensen, Chem. Commun., 2000, 1449 RSC; (c) R. C. F. Jones and J. N. Martin, The Chemistry of Heterocyclic Compounds, in Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, Wiley, New York, 2002, vol. 59, pp. 1–81 Search PubMed; (d) K. V. Gothelf, in Cycloaddition Reactions in Organic Synthesis, ed. S. Kobayashi and K. A. Jørgensen, Wiley-VCH, Weinheim, Germany, 2002, pp. 211–245 Search PubMed; (e) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry—Toward Heterocycles and Natural Products, ed. A. Padwa and W. H. Pearson, John Wiley and Sons, Hoboken, NJ, 2003 Search PubMed; (f) P. N. Confalone and E. M. Huie, The [3 + 2] Nitrone–Olefin Cycloaddition Reaction, in Organic Reactions, John Wiley & Sons, 2004, vol. 36, p. 1 Search PubMed; (g) H. Feuer, Nitrile Oxides, Nitrones, and Nitronates in Organic Synthesis, 2nd edn, John Wiley & Sons, Hoboken, NJ, 2008 Search PubMed; (h) T. Hashimoto and K. Maruoka, in Handbook of Cyclization Reactions, ed. S. Ma, Wiley-VCH, Weinheim, Germany, 2009, ch. 3, pp. 87–168 Search PubMed; (i) L. L. Anderson, M. A. Kroc, T. W. Reidl and J. Son, J. Org. Chem., 2016, 81, 9521 CrossRef CAS PubMed. For two examples on cycloaddition of nitrones with phenylsulfonyl alkenes, see: (j) M. Burdisso, R. Gandolfi and P. Grünanger, J. Org. Chem., 1990, 55, 3427 CrossRef CAS; (k) Y. Dürüst and C. Altuğ, J. Heterocycl. Chem., 2006, 43, 1267 CrossRef.
- (a) A. R. Minter, B. B. Brennan and A. K. Mapp, J. Am. Chem. Soc., 2004, 126, 10504 CrossRef CAS PubMed; (b) I. A. O'Neil, V. E. Ramos, G. L. Ellis, E. Cleator, A. P. Chorlton and S. B. Kalindjian, Tetrahedron Lett., 2004, 45, 3659 CrossRef; (c) A. T. Saito, T. Yamada, S. Miyazaki and T. Otani, Tetrahedron Lett., 2004, 45, 9581 CrossRef; (d) A. T. Saito, T. Yamada, S. Miyazaki and T. Otani, Tetrahedron Lett., 2004, 45, 9585 CrossRef; (e) N. R. Iralapati, J. E. Baldwin, R. M. Adlington, G. J. Pritchard and A. R. Cowley, Tetrahedron, 2005, 61, 1773 CrossRef; (f) J. K. Gallos, C. I. Stathakis, S. S. Kotoulas and A. E. Koumbis, J. Org. Chem., 2005, 70, 6884 CrossRef CAS PubMed; (g) S. Akai, K. Tanimoto, Y. Kanao, S. Omura and Y. Kita, Chem. Commun., 2005, 2369 RSC; (h) S. M. Lait, D. A. Rankic and B. A. Keay, Chem. Rev., 2007, 107, 767 CrossRef CAS PubMed; (i) M. S. Wilson and A. Padwa, J. Org. Chem., 2008, 73, 9601 CrossRef CAS PubMed; (j) K. Clinch, G. B. Evans, R. F. Fröhlich, R. H. Furneaux, P. M. Kelly, L. Legentil, A. S. Murkin, L. Li, V. L. Schramm, P. C. Tyler and A. D. Woolhouse, J. Med. Chem., 2009, 52, 1126 CrossRef CAS PubMed; (k) D. G. Piotrowska, J. Balzarini and I. E. Glowacka, Eur. J. Med. Chem., 2012, 47, 501 CrossRef CAS PubMed; (l) C.-Z. Yao, Z.-F. Xiao, X.-S. Ning, J. Liu, X.-W. Zhang and Y.-B. Kang, Org. Lett., 2014, 16, 5824 CrossRef CAS PubMed; (m) J. Hoogenboom, H. Zuilhof and T. Wennekes, Org. Lett., 2015, 17, 5550 CrossRef CAS PubMed.
- The crystallographic data for 3b has been deposited with the Cambridge Crystallographic Data Centre as supplementary number CCDC 1532199 (see ESI)..
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectral data and crystallographic data. CCDC 1532199. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra04264g |
| This journal is © The Royal Society of Chemistry 2017 |