Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrothermal conversion of high-concentrated glycerol to lactic acid catalyzed by bimetallic CuAux (x = 0.01–0.04) nanoparticles and their reaction kinetics†
Lingqin Shen,
Xin Zhou,
Aili Wang *,
Hengbo Yin
*,
Hengbo Yin *,
Haixu Yin and
Wanjing Cui
*,
Haixu Yin and
Wanjing Cui
Faculty of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, China. E-mail: yin@ujs.edu.cn; alwang@ujs.edu.cn; Tel: +86-0-511-88787591
First published on 14th June 2017
Abstract
Catalytic hydrothermal conversion of highly concentrated glycerol to lactic acid was investigated over bimetallic CuAux (x = 0.01–0.04) nanoparticle catalysts. The bimetallic CuAux nanoparticles were prepared by the wetness chemical reduction method and characterized by XRD, TEM, HRTEM, XPS, and atomic absorption spectrophotometry techniques. Metallic Cu and Au nanoparticles coalesced to form secondary bimetallic nanoparticles with an alloy trend. The bimetallic CuAux nanoparticles exhibited higher catalytic activity for the conversion of highly concentrated glycerol (2−3 mol L−1) to lactic acid than both sole monometallic Cu and Au nanoparticles, probably due to the alloying tendency between the metallic Cu and Au nanoparticles. When the reaction was carried out with the initial glycerol and NaOH concentrations of 3.0 and 3.3 mol L−1 at 200 °C for 2 h, the yields of lactic acid over the bimetallic CuAu2, CuAu3, and CuAu4 catalysts were above 83% and the formation rate of lactic acid was more than 0.17 mol gcat−1 h−1. The carbon balance values ranged from 89.2% to 92.9%. The reaction activation energies for glycerol conversion over the bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 catalysts were 64.0, 53.4, 46.8, and 36.9 kJ mol−1, respectively. The hydrothermal conversion of high-concentrated glycerol to lactic acid catalyzed by the bimetallic CuAux nanoparticles is an alternative to the conventional fermentation process starting from carbohydrate.
1. Introduction
To replace the traditional fossil energy sources by sustainable energy, biodiesel, the first-generation biofuel, is being produced on a large scale worldwide. However, glycerol as a by-product, is largely produced in the biodiesel production via the transesterification of vegetable oil or animal fat with methanol. In the transesterification process, the production capacity of glycerol is ca. 10–20% of raw material in weight.1–3 Currently, in the United States, the biodiesel industry produces ca. 7.6 billion liters of glycerol each year,1,4 which is oversupplied in the market. Although the second-generation biofuels obtained from ligno-cellulose biomass and the third-generation biofuels produced from algae-derived biomass have been paid a great attention in Europe,5 production of the first-generation biofuels still steadily increases with the production amount of 9.9 billion liters in Europe because their production is cheaper than those of the second and third generation biofuels.6,7 Glycerol production is produced in quantities of more than 1 billion liters per year in Europe. Effective conversion of the oversupplied biomass glycerol to high valued chemicals has attracted great attention of researchers.8Many valuable glycerol derivatives, such as lactic acid, 1,3-propanediol, 1,2-propanediol, and succinic acid, can be synthesized through various catalytic processes.1,2 Notably, selective dehydrogenation of glycerol to lactic acid is an attractive research topic because lactic acid has been widely used in food, pharmaceutical, leather, cosmetic, textile, and biodegradable polymer (polylactic acid) fields.2 Polylactic acid has the potential to replace conventional petroleum-based polyethylene terephthalate plastics due to its biocompatibility and biodegradability.8 It is estimated that the market demand of polylactic acid will be ca. 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons by 2017 and 400
000 tons by 2017 and 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons by 2022.9
000 tons by 2022.9
Nowadays, lactic acid is mainly produced by the fermentation technique using sugar as feedstock at a concentration of 10 wt%.10 Although the fermentation process gives a high lactic acid yield of 90%, it is facing challenges in relatively high level of the production cost, low efficiency, waste disposal issues, and worldwide food shortage. As an alternative to the conventional fermentation process, catalytic conversion of glycerol to lactic acid becomes a hot research topic.
The catalytic conversion of glycerol to lactic acid can be categorized into homogeneous and heterogeneous catalysis methods. For the homogeneous catalysis method, it was reported that when the hydrothermal conversion of glycerol (0.33 mol L−1) was catalyzed by NaOH in an aqueous solution at 300 °C, the lactic acid yield of 90% was obtained.11 It was found that alkali metal hydroxides exhibited higher catalytic activity than alkaline earth metal hydroxides. Ramírez-López et al.12 reported that the lactic acid yield of 84.5% was obtained when the hydrothermal conversion of glycerol (2.5 mol L−1) was carried at 280 °C for 1.5 h with a NaOH/glycerol mole ratio of 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. According to the above-mentioned results, NaOH as a homogeneous catalyst exhibited good catalytic activity for the hydrothermal conversion of glycerol to lactic acid. However, high reaction temperature was the drawback for the homogeneous conversion of glycerol to lactic acid.
1. According to the above-mentioned results, NaOH as a homogeneous catalyst exhibited good catalytic activity for the hydrothermal conversion of glycerol to lactic acid. However, high reaction temperature was the drawback for the homogeneous conversion of glycerol to lactic acid.
Heterogeneously catalytic conversion of glycerol to lactic acid has been investigated over various supported noble and non-noble metal catalysts. When Pt/ZrO2 was used as the catalyst for conversion of glycerol to lactic acid at 180 °C in a NaOH aqueous solution under anaerobic condition, the highest yield of lactic acid was 84%.13 When AuPt/TiO2, Au/CeO2, and PtAu/CeO2 were used as the catalysts to catalyze aerobic oxidation of glycerol at 90 °C in a NaOH aqueous solution, the maximum yields of lactic acid of 27%, 81%, and 79% were obtained, respectively.14–16 The synergistic effect between Au and Pt improved catalytic activity in glycerol oxidation to lactic acid. It was reasonable to conclude that noble metal catalysts exhibited high catalytic activities for the conversion of glycerol to lactic acid at a lower reaction temperature under aerobic or anaerobic condition. However, considering the high cost and scarcity of noble metals, exploring an efficient catalyst with a lower cost is highly necessary.
Recently, the conversion of glycerol to lactic acid over Cu-based catalysts17–19 with a lower cost and a high catalytic activity was investigated. When the hydrothermal conversion of glycerol (1.1 mol L−1) in a NaOH aqueous solution was carried out at 240 °C for 6 h, the lactic acid selectivities of 79.7%, 78.6%, and 78.1% at the glycerol conversions of 75.2%, 97.8%, and 93.6% were obtained using Cu/SiO2, CuO/Al2O3, and Cu2O as catalysts, respectively.17 In our previous work, Cu/hydroxyapatite and Cu nanoparticles were used as the catalysts for hydrothermal conversion of glycerol to lactic acid at 230 °C with the initial glycerol concentration of 1 mol L−1, the lactic acid selectivities reached 90% and 92% at the glycerol conversions of 91% and 98%, respectively.18,19 However, in the viewpoint of economy, the low glycerol concentration may lead to a risk in industrial application.
In our present work, bimetallic CuAux nanoparticles were prepared by the wetness chemical reduction method and characterized by XRD, TEM, HRTEM, XPS, and atomic absorption spectrophotometer techniques. In the bimetallic CuAux nanoparticles, the Cu and Au nanoparticles coalesced to form secondary bimetallic nanoparticles with alloy trend. The bimetallic CuAux nanoparticles effectively catalyzed the conversion of high-concentrated glycerol to lactic acid at a relative low reaction temperature in a batch reactor. The reaction kinetics for glycerol conversion over the CuAux nanoparticles were also analyzed.
2. Experimental
2.1. Materials
The chemicals, anhydrous ethanol, sodium hydroxide, copper nitrate trihydrate (Cu(NO3)2·3H2O), hexadecyl trimethyl ammonium bromide (CTAB), chloroauric acid (HAuCl4·3H2O) hydrazine hydrate (N2H4·H2O, 85%), glycerol, lactic acid, 1,2-propanediol, formic acid, acetic acid, oxalic acid, and isopropyl alcohol were of reagent grade and were purchased from Sinopharm Chemical Reagent Co., Ltd. All the chemicals were used as received without further purification. Deionized water was used through all the experiments.2.2. Preparation of catalysts
Bimetallic CuAux nanoparticle catalysts (x mole of Au to 100 mole of Cu) were prepared by the wetness chemical reduction method. One gram of CuAux nanoparticle catalyst was prepared by reducing copper nitrate trihydrate and chloroauric acid with hydrazine hydrate in anhydrous ethanol. A given amount of copper nitrate and organic modifier (CTAB) (the weight ratio of CTAB to copper nitrate, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) were dissolved in 30 mL of anhydrous ethanol by ultrasonic treatment for 30 min. In a thermostatic bath, the solution was preheated to 55 °C under stirring. An ethanol solution of NaOH (1.0 M, 20 mL) was added dropwise to adjust the pH value of the reaction solution to 8–9. After that, an ethanol solution of hydrazine hydrate (8 mL in 80 mL anhydrous ethanol) was added dropwise to the reaction solution under mild stirring for 2.5 h. After the reaction mixture was cooled down to 30 °C, 20 mL of chloroauric acid aqueous solution with given concentration was added dropwise to the reaction mixture under mild stirring for 0.5 h. After reaction, the reaction mixture was cooled down to room temperature. The as-prepared bimetallic CuAux nanoparticles were filtrated and washed with anhydrous ethanol for three times to remove the organic modifier and kept in an anhydrous ethanol solution before they were characterized and used as the catalysts for hydrothermal conversion of glycerol to lactic acid. The as-prepared bimetallic CuAux nanoparticles were donated as CuAu1, CuAu2, CuAu3, and CuAu4, respectively. x was the mole number of metallic Au to 100 mol of metallic Cu in the bimetallic CuAux nanoparticles.
10) were dissolved in 30 mL of anhydrous ethanol by ultrasonic treatment for 30 min. In a thermostatic bath, the solution was preheated to 55 °C under stirring. An ethanol solution of NaOH (1.0 M, 20 mL) was added dropwise to adjust the pH value of the reaction solution to 8–9. After that, an ethanol solution of hydrazine hydrate (8 mL in 80 mL anhydrous ethanol) was added dropwise to the reaction solution under mild stirring for 2.5 h. After the reaction mixture was cooled down to 30 °C, 20 mL of chloroauric acid aqueous solution with given concentration was added dropwise to the reaction mixture under mild stirring for 0.5 h. After reaction, the reaction mixture was cooled down to room temperature. The as-prepared bimetallic CuAux nanoparticles were filtrated and washed with anhydrous ethanol for three times to remove the organic modifier and kept in an anhydrous ethanol solution before they were characterized and used as the catalysts for hydrothermal conversion of glycerol to lactic acid. The as-prepared bimetallic CuAux nanoparticles were donated as CuAu1, CuAu2, CuAu3, and CuAu4, respectively. x was the mole number of metallic Au to 100 mol of metallic Cu in the bimetallic CuAux nanoparticles.
Monometallic Cu and Au nanoparticles were also prepared according to the above-mentioned method, correspondingly. The properties of the monometallic Cu, monometallic Au, and bimetallic CuAux nanoparticle catalysts are listed in Table 1.
| Catalysts | Cu/Au atomic ratiosa | Cu/Au atomic ratios from XPS | Particle sizes of Cu and Aub (nm) | Crystallite sizes of Cu and Auc (nm) | Binding energies (eV) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Au | Cu (1 1 1) | Au (1 1 1) | Cu2p1/2 | Cu2p3/2 | Au4f5/2 | Au4f7/2 | |||
| a Cu/Au atomic ratios were analyzed by atomic absorption spectrophotometer.b The particle sizes of Cu and Au were determined by TEM and HRTEM.c The crystallite sizes of Cu and Au were calculated by Scherrer's equation. | ||||||||||
| Cu | 13.2 | — | 17.8 | 952.4 | 932.6 | |||||
| CuAu1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.99 0.99 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.55 1.55 |
13.0 | 4.0 | 17.5 | 4.5 | 952.3 | 932.5 | 87.5 | 83.8 |
| SpentCuAu1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.99 0.99 |
17.6 | 4.6 | |||||||
| CuAu2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.98 1.98 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.46 2.46 |
13.0 | 4.0 | 15.6 | 4.8 | 952.1 | 932.3 | 87.4 | 83.9 |
| Spent CuAu2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.93 1.93 |
15.6 | 4.9 | |||||||
| CuAu3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.98 2.98 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.12 5.12 |
13.0 | 5.0 | 17.2 | 5.4 | 952.0 | 932.1 | 87.6 | 83.9 |
| Spent CuAu3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.87 2.87 |
17.3 | 5.4 | |||||||
| CuAu4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.96 3.96 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.59 9.59 |
12.0 | 6.0 | 16.9 | 6.3 | 951.7 | 931.8 | 87.6 | 83.9 |
| SpentCuAu4 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.95 3.95 |
17.1 | 6.4 | |||||||
| Au | 6.2 | 87.7 | 84.0 | |||||||
2.3. Characterization of catalyst
The powder X-ray diffraction (XRD) patterns of the monometallic Cu, monometallic Au, and bimetallic CuAux samples were recorded on a diffractometer (D8 super speed Bruke-AEX Company, Germany) using Cu Kα radiation (λ = 1.54056 Å) with Ni filter, scanning from 10° to 85° (2θ). The crystallite sizes of Cu0 (1 1 1) and Au0 (1 1 1) in the bimetallic CuAux nanoparticle catalysts were calculated according to the Scherrer's equation, D = Kλ/(B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ). The value of K was taken 1.0 and B was the full width of the diffraction line at half of the maximum of XRD peak of metallic Cu0 (1 1 1) or Au0 (1 1 1). The crystallite sizes are listed in Table 1.
θ). The value of K was taken 1.0 and B was the full width of the diffraction line at half of the maximum of XRD peak of metallic Cu0 (1 1 1) or Au0 (1 1 1). The crystallite sizes are listed in Table 1.
The X-ray photoelectron spectra (XPS) of the monometallic Cu, monometallic Au, and bimetallic CuAux samples were recorded on an ESCALAB 250 spectrometer (PHI5000VersaProbe, UlVAC-PHI Company, Japan) using Al Kα radiation at 1486.6 eV. The binding energies of Cu and Au of the samples were calculated with respect to C1s peak at 284.6 eV.
The transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were obtained on a microscope (JEM-2100) operated at an acceleration voltage of 200 kV to investigate the microstructures and the crystal structures of the monometallic Cu, monometallic Au, and bimetallic CuAux samples. To prepare TEM specimens, the sample was dispersed in an anhydrous ethanol via ultrasonic equipment for 10 min, then a drop of the ethanol suspension was placed onto a copper grid coated with a layer of amorphous carbon. The particle sizes of the Cu and Au nanoparticles were measured from the TEM and HRTEM images by counting at least 150 individual particles. The average particle sizes of the Cu and Au nanoparticles were calculated by a weighted-average method according to the individual particle sizes of all the counted particles.19,20
The atomic ratios of Cu/Au of the fresh and spent bimetallic CuAux samples were analyzed on an atomic absorption spectrophotometer (TAS-986). The results are listed in Table 1.
2.4. Catalytic test
To investigate the catalytic activities of the monometallic Cu, monometallic Au, and bimetallic CuAux nanoparticles, a 100 mL of aqueous solution of glycerol and NaOH and an appointed amount of nanoparticle sample were charged into a 300 mL stainless steel autoclave with a mechanical stirrer. And the autoclave was flushed with N2 to replace air inside for 10 min. The stirring speed was set at 600 rpm. The reaction time was counted without temperature rising time period and the reaction mixture was heated to the desired temperature in ca. 0.5 h. After reacting for an appointed time period, the reaction mixture was cooled down to room temperature with cooling water through a coil in the reactor.The reaction mixture was filtered before analyzing the concentrations of reactant and products. The filtrate was acidified with hydrochloric acid (37%) to the pH value of 2−3 and diluted with deionized water for HPLC analysis. A gas-phase chromatograph (SP-6800A) equipped with a PEG-20 M packed capillary column (0.25 mm × 30 m) and a FID was used to analyze the concentrations of the unreacted glycerol and produced 1,2-propanediol using isopropyl alcohol as the internal standard. The products, lactic acid, acetic acid, oxalic acid, and formic acid, were analyzed on an Agilent HPLC system equipped with a tunable absorbance UV detector and a reverse-phase column (Innoval ASB C18, 5 μm, 100 Å, 4.6 × 250 mm) at 30 °C. The mobile phase was a methanol aqueous solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) with a flow rate of 0.8 mL min−1. The pH value of the mobile phase was 2.3, which was adjusted with phosphate buffer. The detection wavelength was set at 210 nm. The product selectivity was calculated by carbon balance. The catalytic test for each catalyst was repeated for at least twice in order to ensure the accuracy of the data. The following equation was used to calculate the product selectivity.
90, v/v) with a flow rate of 0.8 mL min−1. The pH value of the mobile phase was 2.3, which was adjusted with phosphate buffer. The detection wavelength was set at 210 nm. The product selectivity was calculated by carbon balance. The catalytic test for each catalyst was repeated for at least twice in order to ensure the accuracy of the data. The following equation was used to calculate the product selectivity.
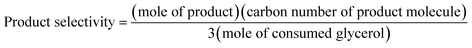 | (1) |
3. Results and discussion
3.1. XRD analysis
The XRD patterns of the monometallic Cu, monometallic Au, and fresh and spent bimetallic CuAux samples with different Au contents prepared by the wet chemical reduction method are shown in Fig. 1. The XRD peaks of the monometallic Cu sample appearing at 2θ = 43.3, 50.4, and 74.1° were indexed as the (1 1 1), (2 0 0), and (2 2 0) planes of face centered-cubic (fcc) copper (JCPDS 04-0836), respectively. The XRD peaks of the metallic Au sample appearing at 2θ = 38.2, 44.3 64.7, and 77.6° were indexed as the (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes of face centered-cubic (fcc) gold (JCPDS 46-1043). The XRD peaks of Cu present in the fresh bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 samples appeared at 2θ = 43.5–43.4, 50.7–50.4, and 74.4–74.1°, respectively. And no diffraction peaks of copper oxides or copper hydroxides were detected, indicating that metallic Cu nanoparticles were formed in the metallic CuAux nanoparticles. The XRD peaks of Cu present in the fresh CuAux catalysts slightly shifted to low values with the increase in Au contents. It is reasonable to conclude that there was an interaction between Cu and Au nanoparticles. The XRD peaks of Au present in the fresh bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 samples appeared at 2θ = 38.4, 44.5 (shoulder), 64.9, and 77.8°, which were higher than those of the monometallic Au sample by 0.2°. As compared to the XRD peaks of monometallic Au sample, the XRD peak shifts of Au in the bimetallic CuAux nanoparticle catalysts indicated that there probably existed an alloy trend between Cu and Au. The alloy trend between Cu and Au means that there was an interaction between Cu nanoparticle and Au nanoparticle. TEM analysis also shows that Au nanoparticles anchored at the surfaces of Cu nanoparticles, which was discussed in the following section.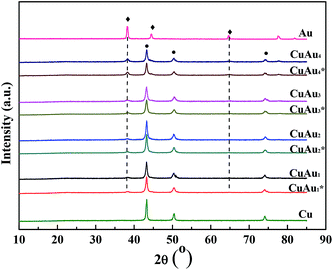 | ||
| Fig. 1 XRD patterns of the monometallic Cu, monometallic Au, and fresh and spent bimetallic CuAu catalysts. ●, Cu0; ◆, Au0; *, spent catalysts. | ||
The crystallite sizes of the monometallic Cu, monometallic Au, and fresh bimetallic CuAux nanoparticle samples were estimated by the Scherrer's equation (Table 1). The crystallite sizes of Cu (1 1 1) and Au (1 1 1) of these samples were around 17 and 5 nm, respectively. The results revealed that small-sized metallic Cu and Au nanoparticles were formed in our present samples.
After taking part in the reaction, the XRD patterns of the spent CuAux nanoparticle catalysts and the crystallite sizes of Cu (1 1 1) and Au (1 1 1) calculated by the Scherrer's equation were close to those of the fresh ones, indicating that the chemical structures of the CuAux nanoparticle catalysts did not change under our present reaction conditions.
3.2. XPS analysis
To determine the chemical states of CuAux nanoparticles, the XPS measurement was employed. The XPS of Cu2p and Au4f regions are shown in Fig. 2 and the binding energies are listed in Table 1.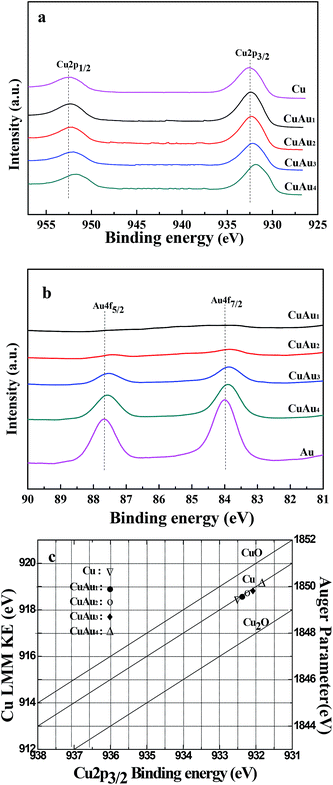 | ||
| Fig. 2 XPS spectra of (a) Cu2p, (b) Au4f, and (c) Wagner plots of the monometallic Cu, monometallic Au, and bimetallic CuAu nanoparticles. | ||
For the bimetallic CuAu1, CuAu2, CuAu3 and CuAu4 samples, the binding energies of Cu2p1/2 and Cu2p3/2 were 952.3, 932.5 eV; 952.1, 932.3 eV; 952.0, 932.1 eV; and 951.7, 931.8 eV, respectively, which were lower than those (952.4 eV for Cu2p1/2, 932.6 eV for Cu2p3/2) of monometallic Cu sample. The binding energies of Cu2p1/2 and Cu2p3/2 of the bimetallic CuAux samples decreased with increasing the Au content (Fig. 2a). The binding energies of Au4f5/2 and Au4f7/2 of the monometallic Au sample were 87.7 and 84.0 eV, respectively. The binding energies of Au4f5/2 and Au4f7/2 of the bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 samples were 87.5, 83.8; 87.4, 83.9; 87.6, 83.9; and 87.6, 83.9 eV, respectively (Fig. 2b). The Au4f binding energies of the bimetallic CuAux samples were decreased by 0.1 eV as compared to those of the monometallic Au sample. As compared to the monometallic Cu and Au nanoparticles, the binding energy shifts of Cu2p and Au4f of the bimetallic CuAux nanoparticles were probably due to the alloy trend between Cu and Au nanoparticles, which was consistent with the conclusion obtained by XRD analysis. The Cu/Au atomic ratios of the CuAux samples obtained by XPS analysis were obviously lower than those obtained by atomic absorption spectrophotometer, respectively, indicating that the Au nanoparticles coated on the surfaces of Cu nanoparticles (Table 1).
The binding energy values of metallic Cu and Cu+ are almost the same as each other, the metallic Cu and Cu+ species cannot be distinguished directly according to their binding energy values. To ascertain the chemical states of the copper species present in the monometallic Cu and bimetallic CuAux nanoparticles, the Wagner plots are drawn and shown in Fig. 2c. The kinetic energy (KE) of the Auger transition and the Cu2p3/2 binding energy of the photoemission were on Y and X axes, respectively. The data for Cu, Cu2O, and CuO reference samples were taken from the ref. 21 and 22. According to Fig. 2c, the Auger parameters of the monometallic Cu and bimetallic CuAux nanoparticles fell on the line of metallic copper, which indicated that the copper species in the nanoparticles were in metallic state, being consistent with the conclusion obtained by XRD analysis.
3.3. TEM and HRTEM analyses
The TEM and HRTEM images (or SAED pattern) of the monometallic Cu, monometallic Au, and bimetallic CuAux nanoparticles are shown in Fig. 3. Fig. 3a1 and a2 show the TEM image and SAED pattern of the monometallic Cu nanoparticles. The TEM image shows that the Cu nanoparticles were spherical with the average particle size of 13.2 nm and the particle size distribution of 3–48 nm (Table 1). According to the SAED analysis (Fig. 3a2), the diffraction fringes were examined to be close to the {1 1 1} (0.209 nm), {2 0 0} (0.181 nm), {2 2 0} (0.128 nm), and {3 1 1} (0.111 nm) lattice spacings of fcc copper, respectively. The as-prepared monometallic Cu nanoparticles had a poly-crystalline structure.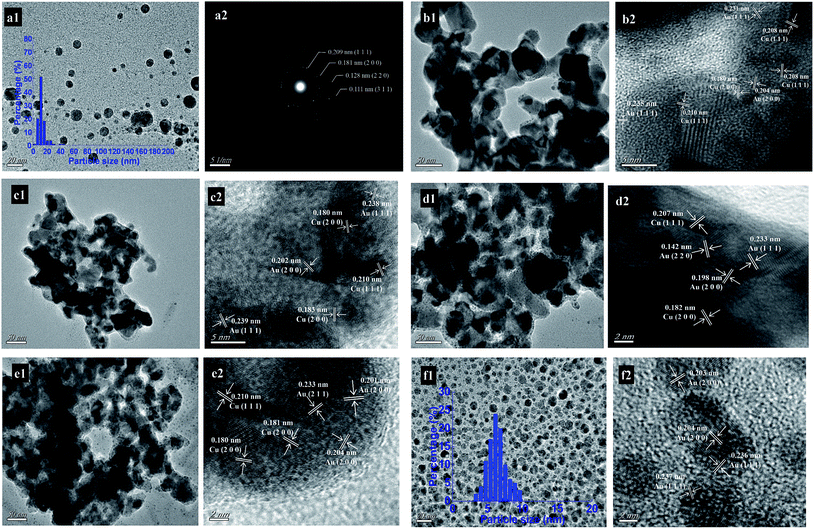 | ||
| Fig. 3 TEM and HRTEM images of (b1–e1, b2–e2) bimetallic CuAu nanoparticles, TEM and SAED images of (a1 and a2) monometallic Cu, and TEM and HRTEM images of (f1 and f2) monometallic Au. | ||
The TEM and HRTEM images of the monometallic Au sample shows that the spherical Au nanoparticles were formed with the average particle size of 6.2 nm and the particle sizes ranging from 3 to 10 nm (Fig. 3f1 and f2). The lattice fringes of the Au nanoparticles were examined to be 0.203 (0.204) and 0.236 (0.237) nm, being close to the {2 0 0}and {1 1 1} lattice spacings of fcc metallic gold.
Fig. 3b1–e1 and b2–e2 show the TEM and HRTEM images of the bimetallic CuAux samples. It was observed that the irregular particulates were constructed by the coalescence of Cu and Au nanoparticles. The average particle sizes of Cu and Au nanoparticles in the bimetallic CuAux samples were around 13 and 5 nm, respectively.
For the bimetallic CuAu1 sample, the lattice fringes of 0.180 and 0.208 (0.210) nm were detected, which were close to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic copper. The lattice fringes of 0.204 and 0.231 (0.235) nm were close to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic gold.
The lattice fringes of 0.180 (0.183) and 0.210 nm for the bimetallic CuAu2 sample were close to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic copper while the lattice fringes of 0.202 and 0.238 (0.239) nm were close to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic gold.
For the CuAu3 sample, the lattice fringes of 0.182 and 0.207 nm were close to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic copper while the lattice fringes of 0.142, 0.198, and 0.233 nm were close to the{2 2 0}, {2 0 0}, and {1 1 1} lattice spacings of fcc metallic gold.
For the CuAu4 sample, the lattice fringes of 0.180 (0.181) and 0.210 nm were corresponding to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic copper while the lattice fringes of 0.201 (0.204) and 0.233 nm were corresponding to the {2 0 0} and {1 1 1} lattice spacings of fcc metallic gold.
The TEM and HRTEM images show that both metallic Cu and Au nanoparticles were formed in the bimetallic CuAux nanoparticles. The bimetallic CuAux nanoparticles were formed by the coalescence of Cu and Au nanoparticles, indicating that there was probably an interaction between Cu and Au nanoparticles.
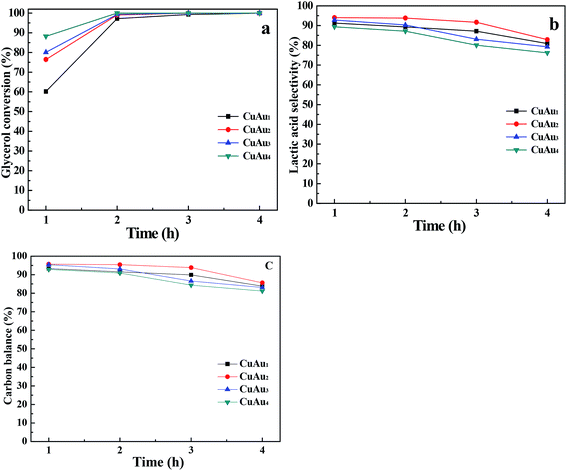
3.4. Hydrothermal conversion of glycerol to lactic acid
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 0.736 g of catalyst at 200 °C for 2 h. The experimental results are listed in Table 2. Lactic acid was formed as the main product and small amount of oxalic acid, formic acid, acetic acid, and 1,2-propanediol as by-products was detected. H2 was formed in gas phase.
1 and 0.736 g of catalyst at 200 °C for 2 h. The experimental results are listed in Table 2. Lactic acid was formed as the main product and small amount of oxalic acid, formic acid, acetic acid, and 1,2-propanediol as by-products was detected. H2 was formed in gas phase.
| Catalysts | Conversionsa (%) | Selectivities (%) | Activities for glycerol consumptionb (mol molcat−1 h−1) | Activities for lactic acid formationc (mol molcat−1 h−1) | Carbon balancesd (%) | Residual carbone (wt%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lactic acid | Oxalic acid | Formic acid | Acetic acid | 1,2-Propanediol | ||||||
a Reaction conditions: glycerol aqueous solution, 2.0 mol L−1, 100 mL; NaOH/glycerol molar ratio 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; catalyst, 0.736 g; reaction temperature 200 °C; reaction time, 2.0 h. The catalyst compositions are listed in Table 1.b Glycerol conversion activities normalized per metal atom.c Lactic acid formation activities normalized per metal atom.d Carbon balances were calculated according to both detected products and reacted glycerol.e Residual carbon was analyzed by the FLASH1112A element analyzer.f The amount of monometallic Au catalyst was equal to the amount of Au in the CuAu4 catalyst. 1; catalyst, 0.736 g; reaction temperature 200 °C; reaction time, 2.0 h. The catalyst compositions are listed in Table 1.b Glycerol conversion activities normalized per metal atom.c Lactic acid formation activities normalized per metal atom.d Carbon balances were calculated according to both detected products and reacted glycerol.e Residual carbon was analyzed by the FLASH1112A element analyzer.f The amount of monometallic Au catalyst was equal to the amount of Au in the CuAu4 catalyst. |
||||||||||
| Cu | 67.2 | 80.3 | 0.3 | 1.1 | 0.8 | 1.5 | 5.8 | 4.7 | 84.0 | |
| CuAu1 | 97.2 | 89.3 | 0.5 | 0.8 | 0.7 | 0.2 | 8.6 | 7.7 | 91.5 | 2.4 |
| CuAu2 | 99.0 | 93.8 | 0.3 | 0.6 | 0.4 | 0.3 | 9.0 | 8.4 | 95.4 | 0.3 |
| CuAu3 | 99.3 | 90.3 | 0.5 | 1.0 | 0.9 | 0.4 | 9.2 | 8.3 | 93.1 | 3.2 |
| CuAu4 | 100 | 87.2 | 0.7 | 1.3 | 1.1 | 0.6 | 9.4 | 8.2 | 90.9 | 0.4 |
| Auf | 18.3 | 66.4 | 1.0 | 1.7 | 1.2 | 0.8 | 9.8 | 6.5 | 71.1 | |
When NaOH and the bimetallic CuAux catalyst were added in the reaction solution together, the conversions of glycerol were more than 97% with the lactic acid selectivities of above 87% (Table 2). However, when sole NaOH or sole bimetallic CuAu2 catalyst was added in the reaction solution, only trace amount of glycerol was converted (Table S1†). It was reasonable to conclude that NaOH and bimetallic CuAux catalyst synergistically catalyzed the conversion of glycerol to lactic acid. The synergistic effect of NaOH and metallic catalyst on the glycerol conversion to lactic acid was also observed when metallic Cu was used as the catalyst in a NaOH aqueous solution.18,19
When the monometallic Cu nanoparticles and monometallic Au nanoparticles (the amount of Au equal to that in the CuAu4 catalyst) were used as catalysts, the conversions of glycerol and selectivities of lactic acid were 67.2%, 80.3%; 18.3%, 66.4%, respectively. The conversions of glycerol and selectivities of lactic acid over the bimetallic CuAux catalysts were higher than those over the monometallic Cu and Au nanoparticles.
The catalytic activities of the bimetallic CuAux catalysts for glycerol conversion were 1.48–1.62 times that of the monometallic Cu nanoparticle catalyst (Table 2). The catalytic activities of the bimetallic CuAux catalysts for glycerol conversion increased with the increase in Au contents. And the catalytic activity of the CuAu4 catalyst for glycerol conversion was comparable to that of the monometallic Au catalyst with the same Au loading.
The catalytic activities of the bimetallic CuAux catalysts for lactic acid formation were 1.63–1.79 times that of the monometallic Cu nanoparticle catalyst and were 1.18–1.29 times that of the monometallic Au nanoparticle catalyst, respectively (Table 2). The bimetallic CuAux catalysts exhibited higher catalytic activity for the conversion of glycerol to lactic acid than both monometallic Cu and Au nanoparticle catalysts. It is reasonable to suggest that the interaction between Cu and Au nanoparticles in the bimetallic CuAux catalysts played an important role for the conversion of glycerol to lactic acid.
The carbon balance values over the bimetallic CuAux catalysts were above 90% while over the monometallic Cu and Au catalysts, the values were 84% and 71.1%, respectively. The results revealed that the bimetallic CuAux catalysts favored the formation of the useful chemicals and decreased the formation of polymer-like chemicals and the decomposition of resultant products. The residual carbon on the bimetallic CuAux catalysts was less than 3.3%, indicating that only a small amount of polymer-like chemicals was deposited on the catalyst surfaces.
Considering that after reacting for 2 h, high yield of lactic acid was obtained over the bimetallic CuAux catalysts, the reaction time period was fixed at 2 h to investigate the effect of other reaction parameters on the catalytic conversion of glycerol to lactic acid.
| Catalysts | Glycerol concentrations (mol L−1) | Glycerol conversions (%) | Selectivities (%) | Activities for glycerol consumptionb (mol molcat−1 h−1) | Carbon balancesc (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic acid | Oxalic acid | Formic acid | Acetic acid | 1,2-Propanediol | |||||
a Reaction conditions: glycerol aqueous solution, 100 mL; NaOH/glycerol mole ratio,1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; catalyst loading, 0.736 g; reaction temperature, 200 °C; reaction time, 2.0 h.b Glycerol conversion activities normalized per metal atom.c Carbon balances were calculated according to both detected products and reacted glycerol. 1; catalyst loading, 0.736 g; reaction temperature, 200 °C; reaction time, 2.0 h.b Glycerol conversion activities normalized per metal atom.c Carbon balances were calculated according to both detected products and reacted glycerol. |
|||||||||
| CuAu1 | 1.5 | 99.2 | 91.8 | 0.7 | 0.9 | 0.9 | 0.2 | 6.6 | 94.5 |
| 2.0 | 97.2 | 89.3 | 0.5 | 0.8 | 0.7 | 0.2 | 8.6 | 91.5 | |
| 2.5 | 94.7 | 86.9 | 0.3 | 0.6 | 0.7 | 0.4 | 10.5 | 88.9 | |
| 3.0 | 90.1 | 80.7 | 0.3 | 0.6 | 0.4 | 1.0 | 12.0 | 83.0 | |
| CuAu2 | 1.5 | 100 | 94.0 | 0.5 | 0.7 | 0.6 | 0.3 | 6.8 | 96.1 |
| 2.0 | 99.0 | 93.8 | 0.3 | 0.6 | 0.4 | 0.3 | 9.0 | 95.4 | |
| 2.5 | 97.5 | 92.3 | 0.3 | 0.5 | 0.4 | 0.6 | 11.0 | 94.1 | |
| 3.0 | 95.8 | 90.9 | 0.2 | 0.4 | 0.3 | 1.1 | 13.0 | 92.9 | |
| CuAu3 | 1.5 | 100 | 92.5 | 0.8 | 1.2 | 1.3 | 0.3 | 6.9 | 96.1 |
| 2.0 | 99.3 | 90.3 | 0.5 | 1.0 | 0.9 | 0.4 | 9.2 | 93.1 | |
| 2.5 | 98.7 | 89.0 | 0.4 | 0.7 | 0.7 | 0.7 | 11.4 | 91.5 | |
| 3.0 | 96.0 | 88.4 | 0.3 | 0.7 | 0.6 | 1.0 | 13.3 | 91.0 | |
| CuAu4 | 1.5 | 100 | 91.9 | 1.0 | 1.4 | 1.4 | 0.4 | 7.0 | 96.1 |
| 2.0 | 100 | 87.2 | 0.7 | 1.3 | 1.1 | 0.6 | 9.4 | 90.9 | |
| 2.5 | 99.3 | 87.0 | 0.6 | 1.0 | 1.1 | 0.9 | 11.6 | 90.6 | |
| 3.0 | 97.4 | 85.7 | 0.5 | 0.8 | 0.9 | 1.3 | 13.7 | 89.2 | |
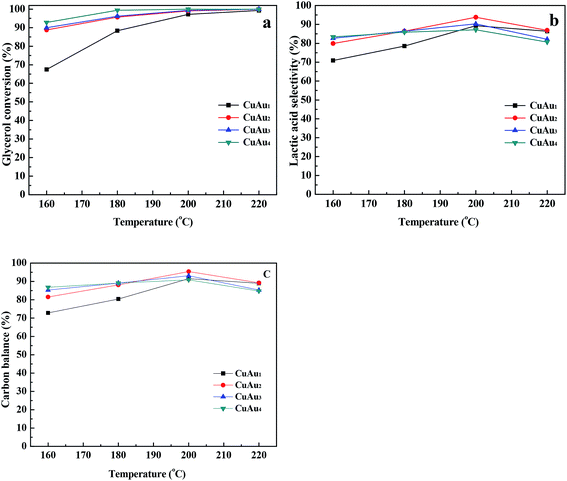
The catalytic activities for glycerol conversion over the bimetallic CuAux catalysts increased upon increasing the glycerol concentration. The bimetallic CuAu2, CuAu3, and CuAu4 catalysts exhibited similar catalytic activities. However, these catalysts exhibited higher catalytic activities than the CuAu1 catalyst. An appropriate Au content was necessary for improving the catalytic activity of bimetallic CuAux catalyst.
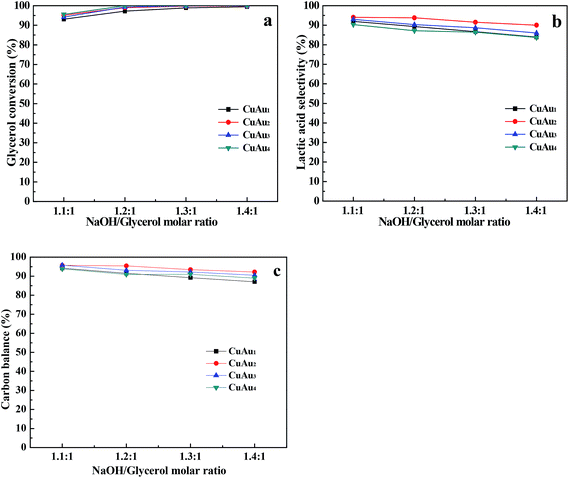
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1.4
1 to 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the glycerol conversions increased from 93.1% to 99.5%, 95.2% to 99.8%, 94.2% to 100%, and 95.6% to 100%, respectively (Fig. 6a). And the selectivities of lactic acid decreased from 92.0% to 83.9%, 94.0% to 90.0%, 93.0% to 86%, and 90.3% to 83.7%, respectively (Fig. 6b). The selectivities of all the measured by-products were less than 1.9% (Fig. S3†). The carbon balances over these catalysts decreased from ca. 95% to 90% upon increasing NaOH/glycerol mole ratio from 1.1
1, the glycerol conversions increased from 93.1% to 99.5%, 95.2% to 99.8%, 94.2% to 100%, and 95.6% to 100%, respectively (Fig. 6a). And the selectivities of lactic acid decreased from 92.0% to 83.9%, 94.0% to 90.0%, 93.0% to 86%, and 90.3% to 83.7%, respectively (Fig. 6b). The selectivities of all the measured by-products were less than 1.9% (Fig. S3†). The carbon balances over these catalysts decreased from ca. 95% to 90% upon increasing NaOH/glycerol mole ratio from 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1.4
1 to 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6c). High NaOH concentration probably caused the decomposition of resultant products to carbonate.12 The results revealed that high NaOH/glycerol mole ratio favored the hydrothermal conversion of glycerol. But excessive NaOH decreased the selectivity of lactic acid.
1 (Fig. 6c). High NaOH concentration probably caused the decomposition of resultant products to carbonate.12 The results revealed that high NaOH/glycerol mole ratio favored the hydrothermal conversion of glycerol. But excessive NaOH decreased the selectivity of lactic acid.
| Catalysts | Catalyst loadings (g) | Glycerol conversions (%) | Selectivities (%) | Activities for glycerol consumptionb (mol molcat−1 h−1) | Carbon balancesc (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic acid | Oxalic acid | Formic acid | Acetic acid | 1,2-Propanediol | |||||
a Reaction conditions: glycerol aqueous solution, 2.0 mol L−1, 100 mL; NaOH/glycerol mole ratio, 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; reaction temperature, 200 °C; reaction time, 2.0 h.b Glycerol conversion activities normalized per metal atom.c Carbon balances were calculated according to both detected products and reacted glycerol. 1; reaction temperature, 200 °C; reaction time, 2.0 h.b Glycerol conversion activities normalized per metal atom.c Carbon balances were calculated according to both detected products and reacted glycerol. |
|||||||||
| CuAu1 | 0.368 | 87.8 | 75.6 | 0.3 | 0.4 | 0.2 | 0.5 | 15.6 | 77.0 |
| 0.552 | 91.5 | 86.3 | 0.5 | 0.7 | 0.4 | 0.5 | 10.8 | 88.4 | |
| 0.736 | 97.2 | 89.3 | 0.5 | 0.8 | 0.7 | 0.2 | 8.6 | 91.5 | |
| 0.920 | 99.6 | 90.6 | 0.7 | 1.0 | 0.8 | 0.2 | 7.1 | 93.3 | |
| CuAu2 | 0.368 | 90.5 | 84.2 | 0.1 | 0.4 | 0.3 | 0.7 | 16.4 | 85.7 |
| 0.552 | 95.5 | 92.9 | 0.3 | 0.5 | 0.4 | 0.3 | 11.5 | 94.4 | |
| 0.736 | 99.0 | 93.8 | 0.3 | 0.6 | 0.4 | 0.3 | 9.0 | 95.4 | |
| 0.920 | 100.0 | 83.6 | 0.5 | 0.8 | 0.7 | 0.2 | 7.2 | 85.8 | |
| CuAu3 | 0.368 | 93.2 | 88.5 | 0.3 | 0.7 | 0.4 | 0.9 | 17.2 | 90.8 |
| 0.552 | 97.4 | 91.4 | 0.5 | 0.9 | 0.6 | 0.6 | 12.0 | 94.0 | |
| 0.736 | 99.3 | 90.3 | 0.5 | 1.0 | 0.9 | 0.4 | 9.2 | 93.1 | |
| 0.920 | 100.0 | 79.6 | 0.8 | 1.3 | 1.0 | 0.3 | 7.4 | 83.0 | |
| CuAu4 | 0.368 | 93.9 | 90.0 | 0.5 | 0.9 | 0.6 | 1.2 | 17.6 | 93.2 |
| 0.552 | 98.6 | 87.9 | 0.6 | 1.1 | 0.7 | 0.7 | 12.3 | 91.0 | |
| 0.736 | 100.0 | 87.2 | 0.7 | 1.3 | 1.1 | 0.6 | 9.4 | 90.9 | |
| 0.920 | 100.0 | 77.4 | 1.2 | 1.6 | 1.4 | 0.5 | 7.5 | 82.1 | |
The catalytic activities for glycerol conversion over the bimetallic CuAux catalysts increased with the increase in Au contents. The presence of Au in the bimetallic CuAux catalyst enhanced its catalytic activity.
The typical results of the present work and the previously reported results are summarized in Table 5. The presence of CuAux catalysts decreased the reaction temperature by 80 °C as compared to the hydrothermal method.11,12 The CuAux catalysts gave higher lactic acid yield at a higher glycerol concentration than the noble metal catalysts.14,16 The bimetallic CuAux catalysts favored the catalytic conversion of glycerol to lactic acid as compared to the supported and nanosized metallic copper catalysts.17–19 The interaction between Au and Cu played an important role for the formation of lactic acid from glycerol.
| Catalysts | Initial concentrations (mol·L−1) | Reaction temperatures (°C) | Reaction time (h) | Glycerol conversions (%) | Lactic acid yields (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| glycerol | NaOH | ||||||
| — | 0.33 | 1.25 | 300 | 1.5 | — | 90 | 11 |
| — | 2.5 | 2.75 | 280 | 2.5 | ∼98 | 84.5 | 12 |
| Pt/ZrO2 | 0.54 | 1.0 | 180 | 24 | 94.0 | 84 | 13 |
| Au/TiO2 | 0.22 | 0.88 | 90 | — | ∼30 | 22.1 | 14 |
| Pt/TiO2 | 0.22 | 0.88 | 90 | — | ∼30 | 25.4 | 14 |
| AuPt/TiO2 | 0.22 | 0.88 | 90 | — | ∼30 | 25.7 | 14 |
| AuPt/CeO2 | 0.17 | 0.68 | 100 | 0.5 | 99.0 | 79.2 | 16 |
| Cu/SiO2 | 1.1 | 1.21 | 240 | 6 | 75.2 | 59.9 | 17 |
| Cu/HAP | 1.0 | 1.1 | 230 | 2 | 91.0 | 81.9 | 18 |
| Nanosized Cu | 2.5 | 2.75 | 230 | 2 | 77.5 | 59.3 | 19 |
| CuAu2 | 3.0 | 3.3 | 200 | 2 | 95.8 | 87.1 | This work |
| CuAu3 | 3.0 | 3.3 | 200 | 2 | 96.0 | 84.9 | This work |
| CuAu4 | 3.0 | 3.3 | 200 | 2 | 97.4 | 83.5 | This work |
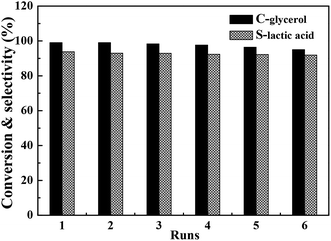
3.5. Recycling performance of bimetallic CuAu2 nanoparticle catalyst
Considering the prominent performance of the bimetallic CuAu2 catalyst, it was selected as the model catalyst to investigate its stability and recycling performance. The results are shown in Fig. 7. After reacting at 200 °C for 2 h, the used catalyst was separated from reaction solution by centrifugation at 8000 rpm and dried at 60 °C in a vacuum oven for 4 h before next recycling. For the fresh CuAu2 nanoparticle catalyst, the glycerol conversion and lactic acid selectivity were 99.0% and 93.8%, respectively. And after recycling for six times, the glycerol conversion and lactic acid selectivity were 95.0% and 91.9%, respectively. According to the recycling experimental results, it was found that the CuAu2 catalyst exhibited good recycling performance.After recycling for six times, the Cu/Au atomic ratios in the spent CuAu2 catalyst was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.93, indicating that the bimetallic CuAu2 catalyst was stable during the catalytic reaction.
1.93, indicating that the bimetallic CuAu2 catalyst was stable during the catalytic reaction.
3.6. Reaction kinetics
In order to eliminate the effect of diffusion, the bimetallic CuAux catalysts with different loadings in a range of 0.092–0.328 g were used for the catalytic conversion of glycerol at 140 °C and a stirring speed of 600 rpm with the initial glycerol and NaOH concentrations of 1.0 and 1.1 mol L−1, respectively. A linear correlation between the catalyst loading and the glycerol conversion was obtained (Fig. S4†), which indicating the reaction was controlled by chemical reaction rather than mass diffusion.23,24
Raising the autoclave temperature to the prescribed reaction temperature needed ca. 0.5 h without stirring. And glycerol was almost not converted during the time period for raising reaction temperature. Moreover, when the reaction was carried out over the bimetallic CuAux catalysts under stirring at 600 rpm or 1000 rpm for 1 h, the glycerol conversions were close to each other, indicating that the diffusion effect was eliminated under stirring at 600 rpm.
The power-function type reaction kinetic equation is expressed as follows.
| −rA = −dnA/(mcatdt) = kCAaCBb | (2) |
The rate constant k follows the Arrhenius equation.
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/(RT)) exp(−Ea/(RT))
| (3) |
ln(−rA) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k + a k + a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA + b CA + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB CB
| (4) |
To calculate the reaction orders of a and b according to eqn (4), the initial conversion rates of glycerol were calculated according to the data shown in Table 6, which shows the glycerol conversions at 140 °C under different initial concentrations of glycerol and NaOH. The initial conversion rates of glycerol at 140 °C under different initial concentrations of glycerol and NaOH were calculated at the first hour.
| Reaction conditions | Glycerol conversions (%) | |||||
|---|---|---|---|---|---|---|
| Glycerol concentrations (mol L−1) | NaOH/glycerol molar ratios | Reaction temperatures (°C) | CuAu1 | CuAu2 | CuAu3 | CuAu4 |
| a Reaction conditions: glycerol aqueous solution, 100 mL; catalyst, 0.276 g; reaction time, 1.0 h. | ||||||
| 0.5 | 1.1 | 140 | 18.6 | 22.7 | 27.2 | 34.6 |
| 1.0 | 1.1 | 140 | 15.4 | 19.3 | 21.6 | 29.7 |
| 1.5 | 1.1 | 140 | 13.4 | 15.5 | 18.6 | 22.7 |
| 2.0 | 1.1 | 140 | 10.5 | 12.4 | 14.4 | 17.6 |
| 1.0 | 0.5 | 140 | 7.8 | 13.6 | 15.4 | 20.1 |
| 1.0 | 1.0 | 140 | 9.2 | 16.7 | 17.4 | 26.1 |
| 1.0 | 1.1 | 140 | 15.4 | 19.3 | 21.6 | 29.7 |
| 1.0 | 1.5 | 140 | 18.6 | 23.2 | 27.2 | 33.1 |
| 1.0 | 1.1 | 110 | 3.6 | 6.8 | 7.5 | 12 |
| 1.0 | 1.1 | 120 | 5.6 | 8.8 | 11.9 | 17.2 |
| 1.0 | 1.1 | 130 | 11.2 | 15.4 | 17.3 | 21.6 |
| 1.0 | 1.1 | 140 | 15.4 | 19.3 | 21.6 | 29.7 |
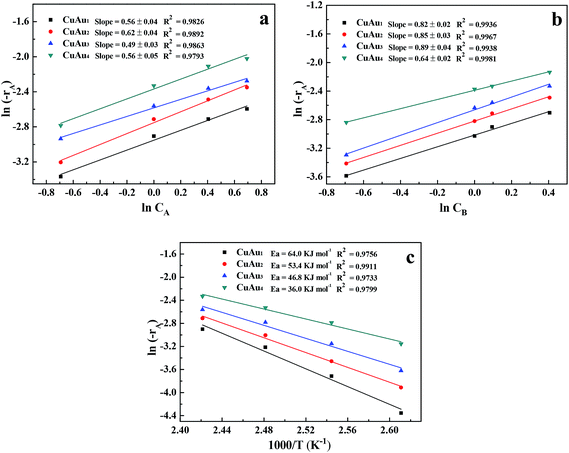
Fig. 8a shows the lines by plotting ln(−rA) vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA over the bimetallic CuAux nanoparticle catalysts. The data used in Fig. 8a were calculated according to those shown in Table 6, straight lines were obtained while plotting ln(−rA) vs. ln
CA over the bimetallic CuAux nanoparticle catalysts. The data used in Fig. 8a were calculated according to those shown in Table 6, straight lines were obtained while plotting ln(−rA) vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA. The straight lines over the CuAu1, CuAu2, CuAu3, and CuAu4 catalysts gave good linear correlations of 0.9826, 0.9892, 0.9863 and 0.9793, respectively. The reaction orders of glycerol over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts are 0.56, 0.62, 0.49, and 0.56, respectively (Table 7).
CA. The straight lines over the CuAu1, CuAu2, CuAu3, and CuAu4 catalysts gave good linear correlations of 0.9826, 0.9892, 0.9863 and 0.9793, respectively. The reaction orders of glycerol over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts are 0.56, 0.62, 0.49, and 0.56, respectively (Table 7).
 | ||
| Fig. 8 Estimation of (a and b) the reaction orders and (c) the reaction activation energies for the power-function type reaction kinetics over the bimetallic CuAux nanoparticle catalysts. | ||
| Catalysts | Initial concentrations of glycerol and NaOH (mol L−1) | a | R2 | b | R2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.5, 1.1 | 1.0, 1.1 | ||||||||||
| −rA (mol gcat−1 h−1) | 1.5, 1.1 | 2.0, 1.1 | 1.0, 0.5 | 1.0, 1.0 | 1.0, 1.5 | ||||||
| a a and b are the reaction orders for glycerol and NaOH, respectively. | |||||||||||
| CuAu1 | 0.0344 | 0.0549 | 0.0664 | 0.0746 | 0.0277 | 0.0484 | 0.0670 | 0.56 ± 0.04 | 0.9826 | 0.82 ± 0.02 | 0.9936 |
| CuAu2 | 0.0405 | 0.0664 | 0.0830 | 0.0954 | 0.0329 | 0.0595 | 0.0827 | 0.62 ± 0.04 | 0.9892 | 0.85 ± 0.03 | 0.9967 |
| CuAu3 | 0.0530 | 0.0771 | 0.0943 | 0.1025 | 0.0371 | 0.0718 | 0.0973 | 0.49 ± 0.03 | 0.9863 | 0.89 ± 0.04 | 0.9938 |
| CuAu4 | 0.0618 | 0.0971 | 0.1214 | 0.1325 | 0.0585 | 0.0932 | 0.1182 | 0.56 ± 0.05 | 0.9793 | 0.64 ± 0.02 | 0.9981 |
Fig. 8b shows the lines by plotting ln(−rA) vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB over the bimetallic CuAux nanoparticle catalysts. The data used in Fig. 8b were calculated according to those shown in Table 6, straight lines were obtained while plotting ln(−rA) vs. ln
CB over the bimetallic CuAux nanoparticle catalysts. The data used in Fig. 8b were calculated according to those shown in Table 6, straight lines were obtained while plotting ln(−rA) vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CB. The straight lines over the CuAu1, CuAu2, CuAu3, and CuAu4 catalysts gave good linear correlations of more than 0.9936, respectively. The reaction orders of NaOH over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts are 0.82, 0.85, 0.89, and 0.64, respectively (Table 7).
CB. The straight lines over the CuAu1, CuAu2, CuAu3, and CuAu4 catalysts gave good linear correlations of more than 0.9936, respectively. The reaction orders of NaOH over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts are 0.82, 0.85, 0.89, and 0.64, respectively (Table 7).
The power-function type reaction kinetic eqn (5)−(8) over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts can be written as follows.
| −rA = −dnA/(msdt) = kCA0.56CB0.82 | (5) |
| −rA = −dnA/(msdt) = kCA0.62CB0.85 | (6) |
| −rA = −dnA/(msdt) = kCA0.49CB0.89 | (7) |
| −rA = −dnA/(msdt) = kCA0.56CB0.64 | (8) |
−rA = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/(RT))CA0.56CB0.82 exp(−Ea/(RT))CA0.56CB0.82
| (9) |
−rA = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/(RT))CA0.62CB0.85 exp(−Ea/(RT))CA0.62CB0.85
| (10) |
−rA = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/(RT))CA0.49CB0.89 exp(−Ea/(RT))CA0.49CB0.89
| (11) |
−rA = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/(RT))CA0.56CB0.64 exp(−Ea/(RT))CA0.56CB0.64
| (12) |
By taking the natural logarithm of both sides of eqn (9)−(12), the equations can be rearranged as follows.
| ln(−rA) = ln(ACA0.56CB0.82) − Ea/(RT) | (13) |
| ln(−rA) = ln(ACA0.62CB0.85) − Ea/(RT) | (14) |
| ln(−rA) = ln(ACA0.49CB0.89) − Ea/(RT) | (15) |
| ln(−rA) = ln(ACA0.56CB0.64) − Ea/(RT) | (16) |
The data listed in the Table 6 were used to calculate the initial reaction rates, −rA, at different reaction temperatures of 110–140 °C over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts, respectively. According to the eqn (13)−(16), four straight lines with good linear correlations of more than 0.9733, respectively, were obtained while plotting ln(−rA) vs. 1/T (Fig. 8c). From the slopes and intercepts of the straight lines, the activation energies (Ea) and frequency factors (A) were obtained, which are listed in Table 8. Over the CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts, the reaction kinetic equations are listed as follows, respectively.
−rA = −dnA/(mcatdt) = 7.50 × 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−64.0/(RT))CA0.56CB0.82 exp(−64.0/(RT))CA0.56CB0.82
| (17) |
−rA = −dnA/(mcatdt) = 3.88 × 105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−53.4/(RT))CA0.62CB0.85 exp(−53.4/(RT))CA0.62CB0.85
| (18) |
−rA = −dnA/(mcatdt) = 6.75 × 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−46.8/(RT))CA0.49CB0.89 exp(−46.8/(RT))CA0.49CB0.89
| (19) |
−rA = −dnA/(mcatdt) = 3.57 × 103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−36.9/(RT))CA0.56CB0.64 exp(−36.9/(RT))CA0.56CB0.64
| (20) |
| Catalysts | −rA (mol g−1 h−1) | A (mol1−(a+b) gcat(a+b)−1 h−1) | Ea (kJ mol−1) | R2 | |||
|---|---|---|---|---|---|---|---|
| 110 °C | 120 °C | 130 °C | 140 °C | ||||
| a A is the frequency factor, and Ea is the reaction activation energy. | |||||||
| CuAu1 | 0.0129 | 0.0244 | 0.0401 | 0.0549 | 7.50 × 106 | 64.0 | 0.9756 |
| CuAu2 | 0.0200 | 0.0315 | 0.0496 | 0.0664 | 3.88 × 105 | 53.4 | 0.9911 |
| CuAu3 | 0.0268 | 0.0430 | 0.0619 | 0.0771 | 6.75 × 104 | 46.8 | 0.9733 |
| CuAu4 | 0.0426 | 0.0615 | 0.0797 | 0.0971 | 3.57 × 103 | 36.9 | 0.9799 |
The activation energies for the bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts were 64.0, 53.4, 46.8, and 36.9 kJ mol−1, respectively. The activation energies and frequency factors decreased with increasing the Au content in the bimetallic CuAux nanoparticle catalysts. The results indicated that the Au contents in the bimetallic catalysts affected their activation energies and reaction orders.
3.7. Reaction routes
Based on the analysis in the paragraph 3.4.1, the co-presence of the bimetallic CuAux catalyst and NaOH effectively catalyzed the conversion of high-concentrated glycerol to lactic acid. According to the findings in our present work and the reported reaction pathways for the catalytically hydrothermal conversion of glycerol to lactic acid in an aqueous solution,11,12,25 the reaction routes over the bimetallic CuAux nanoparticle catalysts in an alkaline solution are discussed as follows.It was speculated that the catalytic dehydrogenation of glycerol to glyceraldehyde was the first step,13,26 which was a crucial step for the whole reaction. The bimetallic CuAux nanoparticle catalysts play an important role in the catalytic dehydrogenation of terminal hydroxyl group of glycerol to glyceraldehyde. Then in a basic environment, the 2-hydroxypropenal was formed via the intramolecular dehydration of glyceraldehyde.18,25 Pyruvaldehyde was conveniently formed from 2-hydroxypropenal via the keto–enol tautomerization.26 The resultant pyruvaldehyde was converted to lactate via the Cannizzaro reaction.12,15,16,18
In addition, the by-products, such as 1,2-propanediol, formic acid, acetic acid, oxalic acid, existed in our present experiments, which probably formed in the following routes. There were three routes for the formation of 1,2-propanediol, the catalytic hydrogenation of glycerol with resultant H2 could produce 1,2-propanediol, and both 2-hydroxypropenal and pyruvaldehyde could also be hydrogenated to 1,2-propanediol over the bimetallic CuAux nanoparticle catalysts. The acetate and formate anions were formed by the decomposition of lactate anions in an alkaline solution, accompanied with the formation of carbonate anions.9 Oxalate anions were probably from the oxidative cleavage of glyceraldehyde in an alkaline solution.27
Some intermediates, such as pyruvaldehyde, glyceraldehyde, and 2-hydroxypropenal, were not detected under our present experimental conditions at the reaction temperature of above 200 °C, indicating that these intermediates could be rapidly converted to subsequent chemicals and finally to lactate.
4. Conclusions
The bimetallic CuAux nanoparticles were prepared by the wetness chemical reduction method successfully. Metallic Cu and Au nanoparticles with the average particle sizes of ca. 13 and 5 nm were formed in the bimetallic CuAux nanoparticles. Based on the XRD and XPS analyses, there existed an alloy trend between Cu and Au nanoparticles.The bimetallic CuAux nanoparticles effectively catalyzed the hydrothermal conversion of high-concentrated glycerol to lactic acid at a relative low reaction temperature of 200 °C. The catalytic activities of the bimetallic CuAux nanoparticles were higher than those of the monometallic Cu and Au nanoparticles. When the catalytic hydrothermal conversion of glycerol (2 mol L−1) over the bimetallic CuAu2 nanoparticles in an alkaline solution was carried out at 200 °C for 2 h, the selectivity of lactic acid was 93.8% at the conversion of glycerol of 99.0%. The CuAu2 nanoparticle catalyst exhibited good recycling performance and stability. Over the bimetallic CuAu1, CuAu2, CuAu3, and CuAu4 nanoparticle catalysts, the power-function type reaction kinetic model well fitted the experimental data, and the reaction activation energies were 64.0, 53.4, 46.8, and 36.9 kJ mol−1, respectively.
Acknowledgements
The work was financially supported by the funds from National Natural Science Foundation of China (21506078 and 21506082), China Postdoctoral Science Foundation (2016M601739) and Science and Technology Department of Jiangsu Province of China (BY2014123-10).References
- Z. Lu, I. Demianets, R. Hamze, N. J. Terrile and T. J. Williams, ACS Catal., 2016, 6, 2014–2017 CrossRef CAS.
- M. R. A. Arcanjo, I. J. S. Júnior, E. Rríguez-Castellón, A. Infantes-Molina and R. S. Vieira, Catal. Today, 2017, 279, 317–326 CrossRef CAS.
- S. Adhikari, S. D. Fernando and A. Haryanto, Renew. Energ., 2008, 33, 1097–1100 CrossRef CAS.
- Global Glycerol Market Size, Market Share, Application Analysis, Regional Outlook, Growth, Trends, Competitive Scenario and Forecasts, 2012 to 2020, http://www.hexaresearch.com/research-report/glycerol-industry.
- European Commission, The impact of biofuels on transport and the environment, and their connection with agricultural development in Europe, Directorate General for the Internal Policies Policy Department B: Structural and Cohesion Policies-Transport and Tourism, Brussells, 2015, http://www.europarl.europa.eu/RegData/etudes/STUD/2015/513991/IPOL_STU(2015)513991_EN.pdf Search PubMed.
- D. Gambelli, F. Alberti, F. Solfanelli, D. Vairo and R. Zanoli, Energy Policy, 2017, 103, 165–178 CrossRef.
- L. E. Hombach, C. Cambero, T. Sowlati and G. Walther, J. Cleaner Prod., 2016, 133, 565–575 CrossRef.
- C. Zhang, T. Wang, X. Liu and Y. J. Ding, Chin. J. Catal., 2016, 37, 502–509 CrossRef CAS.
- U.S. Department of Agriculture, Final Report: Renewable Chemicals & Materials Opportunity Assessment, 2014, E-mail: http://www.usda.gov/oce/reports/energy/USDA_RenewChems_Jan2014.pdf Search PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, Biotechnol. Adv., 2013, 31, 877–902 CrossRef CAS PubMed.
- H. Kishida, F. Jin, Z. Zhou, T. Mory and H. Enomoto, Chem. Lett., 2005, 34, 1560–1561 CrossRef CAS.
- C. A. Ramírez-López, J. R. Ochoa-Gómez, M. Fernández-Santos, O. Goméz-Jiménez-Aberasturi, A. Alonso-Vicario and J. Torrecilla-Soria, Ind. Eng. Chem. Res., 2010, 49, 6270–6278 CrossRef.
- J. Ftouni, N. Villandier, F. Auneau, M. Besson, L. Djakovitch and C. Pinel, Catal. Today, 2015, 257, 267–273 CrossRef CAS.
- Y. H. Shen, S. H. Zhang, H. J. Li, Y. Ren and H. C. Liu, Chem.–Eur. J., 2010, 16, 7368–7371 CrossRef CAS PubMed.
- P. Lakshmanan, P. P. Upare, N. T. Le, Y. K. Hwang, D. W. Hwang, U. H. Lee, H. R. Kim and J. S. Chang, Appl. Catal., A, 2013, 468, 260–268 CrossRef CAS.
- R. K. P. Purushothaman, J. V. Haveren, D. S. V. Es, I. Melián-Cabrera, J. D. Meeldijk and H. J. Heeres, Appl. Catal., B, 2014, 147, 92–100 CrossRef CAS.
- D. Roy, B. Subramaniam and R. V. Chaudhari, ACS Catal., 2011, 1, 548–551 CrossRef CAS.
- H. Yin, C. Zhang, H. Yin, D. Gao, L. Shen and A. Wang, Chem. Eng. J., 2016, 288, 332–343 CrossRef CAS.
- H. Yin, H. Yin, A. Wang, L. Shen, Y. Liu and Y. Zheng, J. Nanosci. Nanotechnol., 2017, 17, 1255–1266 CrossRef.
- H. Yin, Y. Wada, T. Kitamura, S. Kambe, S. Murasawa, H. Mori, T. Sakata and S. Yanagida, J. Mater. Chem., 2001, 11, 1694–1703 RSC.
- S. Poulston, P. M. Parlett, P. Stone and M. Bowker, Surf. Interface Anal., 1996, 24, 811–820 CrossRef CAS.
- D. Zhang, H. Yin, J. Xue, C. Ge, T. Jiang, L. Yu and Y. Shen, Ind. Eng. Chem. Res., 2009, 48, 11220–11224 CrossRef CAS.
- Y. H. Feng, H. B. Yin, D. Z. Gao, A. L. Wang, L. Q. Shen and M. J. Meng, J. Catal., 2014, 316, 67–77 CrossRef CAS.
- X. Huang, X. Wang, X. Wang, X. Wang, M. Tan, W. Ding and X. Lu, J. Catal., 2013, 301, 217–226 CrossRef CAS.
- L. Chen, S. J. Ren and X. P. Ye, Fuel Process. Technol., 2014, 120, 40–47 CrossRef CAS.
- F. Auneau, C. Michel, F. Delbecq, C. Pinel and P. Sautet, Chem.–Eur. J., 2011, 17, 14288–14293 CrossRef CAS PubMed.
- F. Auneau, L. S. Arani, M. Besson, L. Djakovitch, C. Michel, F. Delbecq, P. Sautet and C. Pinel, Top. Catal., 2012, 55, 474–479 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04415a |
| This journal is © The Royal Society of Chemistry 2017 |