Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modulating photo-luminescence of Au2Cu6 nanoclusters via ligand-engineering†
Xi Kang ,
Xiaowu Li*,
Haizhu Yu,
Ying Lv,
Guodong Sun,
Yangfeng Li,
Shuxin Wang and
Manzhou Zhu
,
Xiaowu Li*,
Haizhu Yu,
Ying Lv,
Guodong Sun,
Yangfeng Li,
Shuxin Wang and
Manzhou Zhu *
*
Department of Chemistry, Center for Atomic Engineering of Advanced Materials, Anhui Province Key Laboratory of Chemistry for Inorganic/Organic Hybrid Functionalized Materials, Anhui University, Hefei, Anhui 230601, China. E-mail: xiaowuli2006@hotmail.com; zmz@ahu.edu.cn
First published on 31st May 2017
Abstract
In this work, the luminescence of Au2Cu6 nanoclusters was controlled by tailoring the ligand to metal charge transfer via engineering the phosphine ligands with electron-donating or -withdrawing substituents. The fluorescence intensity was significantly enhanced from the Au2Cu6 nanocluster with P(Ph–F)3 ligands (quantum yield QY = 5.7%) to that with P(Ph–OMe)3 ligands (QY = 17.7%). In addition, the fluorescence of Au2Cu6 protected by P(Ph–OMe)3 slightly red-shifts compared to that of Au2Cu6 protected by P(Ph–F)3, which is similar to the trends of UV-vis spectra tendency.
Metal nanoclusters (NCs) with precise atomic number and well-defined composition (structure) have attracted intensive research interest owing to their highly promising applications in optics, catalysis and electrochemistry.1–11 In this context, photo-luminescence (PL) represents one of the most attractive properties of the metal NCs.1,4,6,8,12 Thanks to the advantages such as low toxicity, great photo-stability and high biocompatibility, the fluorescent NCs have recently become highly promising nanomaterials in phototherapy, cell labeling and biosensing.6,8,13,14 Thus far, several fluorescent NCs have been successfully synthesized.13–23 Unfortunately, their practical applications have been limited due to the significantly lower quantum yield (QY) compared to the typical fluorescent nanomaterials (e.g., quantum dots).1,15 To this end, an efficient strategy to enhance the QY of weakly fluorescent NCs is highly desirable.
In the past decades, two main strategies (i.e., foreign-metal-doping15,18,19 and ligand-engineering strategies15–17,22) have been developed to enhance the fluorescence of NCs. For instance, Bakr et al. observed a 26-fold PL QY enhancement on Ag29 NC when doped with the central Au atom.19b In addition, Wu and coworkers reported that the fluorescence intensity of Au24 NC increased with an increase in the electron-donating ability of the ligand.14 In recent years, the ligand-engineering strategy has attracted increasing interest.15–17,22 As the electronic structures of the organic ligands are essentially different from those of the metal atoms, the mechanistic understanding on the structure (composition)–fluorescence correlation could be more achievable.14,17c Additionally, with the structure–fluorescence relationships in hand, the target NCs with stronger PL could be easily prepared due to the synthetic similarities of NCs protected by different organic ligands.
In our recent study, the Au2Cu6(S-Adm)6(PPh2Py)2 (where S-Adm = 1-adamantanethiol) NC with high fluorescence (QY = 11.7%) was synthesized via the aggregation-induced-emission method.18c The DFT calculations indicate that the fluorescence corresponds to the LUMO–HOMO transition, and is mainly caused by the charge transfer between the aromatic groups on the phosphine ligand and copper atoms. In other words, the luminescence originated from ligand to metal charge transfer (LMCT).18c Inspired by these conclusions, we tried to tailor the PL of Au2Cu6 NCs by engineering the phosphine ligands via functionalizing the aromatic group with electron-donating groups (EDGs) or electron-withdrawing substituents (EWGs). As summarized in Scheme 1, the EDG will hopefully strengthen the LMCT process to enhance the fluorescence. On the contrary, the LMCT and fluorescence could be significantly weakened when the phosphine ligand is relatively electron-deficient (in the presence of EWG).
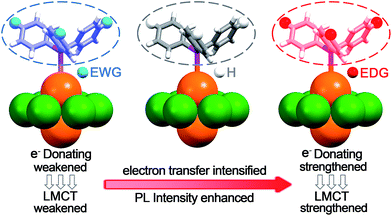 | ||
| Scheme 1 The illustrations of decreased LMCT and fluorescence induced by EWG; contrastive LMCT (PPh3); enhanced LMCT and fluorescence induced by EDG. | ||
Herein, we reported the PL modulation of Au2Cu6 NC systems via engineering the phosphine ligands with the electronic effect of the substituents. The structures and compositions of the Au2Cu6(S-Adm)6(PR3)2 (R = Ph–OMe for Au2Cu6-1; R = Ph for Au2Cu6-2; and R = Ph–F for Au2Cu6-3) NCs were verified by single crystal X-ray diffraction (SC-XRD), thermogravimetric analysis (TGA), inductively coupled plasma (ICP) and X-ray photoelectric spectroscopy (XPS) measurements. According to these characterizations, all the NCs share the same framework with the previously reported Au2Cu6(S-Adm)6(PPh2Py)2 NC. Compared with Au2Cu6-2 (QY = 12.2%), Au2Cu6-1 with EDGs exhibits enhanced PL (QY = 17.7%), while Au2Cu6-3 with EWGs shows weaker luminescence (QY = 5.7%). In addition, the emission peak on the PL spectra slightly red-shifts from Au2Cu6-1 to Au2Cu6-2 and Au2Cu6-3, consistent with the variation tendency in UV-vis spectra of different Au2Cu6 systems.
The aforementioned three Au2Cu6 NCs were prepared by the similar procedures with our previous study18c (see ESI† for details). Specifically, CuCl was dissolved in the mixture of acetonitrile and methanol, and AdmSH dissolved in toluene was then added to the solution. The overall solution was vigorously stirred for 15 min. Then, Au(PR3)Cl (R = Ph–OMe, Ph or Ph–F) in toluene and NaBH4 in ice-cold water were added dropwise to the flask simultaneously under vigorous stirring. The reaction was aged for 60 h under N2 atmosphere. Afterwards, the products were centrifuged to obtain the solid, which was then washed several times with toluene for further characterizations.
According to the SC-XRD characterization, the composition and total structures of the as-prepared Au2Cu6-2 and Au2Cu6-3 are similar with the overall framework of previously reported Au2Cu6(S-Adm)6(PPh2Py)2 (Fig. S1 and S2†). However, the crystal structure of Au2Cu6-1 failed to be obtained because of the relatively weak stability. Thus, TGA, XPS and ICP measurements were performed to ascertain the composition of Au2Cu6-1. The weight loss of 69.15% (Fig. S3–S5†) is consistent with the theoretical value (cal. 68.79%) of AdmSH and (Ph–OMe)3P ligands in Au2Cu6-1. In addition, the XPS and ICP results suggest that the metallic ratio of Au and Cu in Au2Cu6-1 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. S6–S8 and Table S3†). Combining the TGA, XPS and ICP results with the almost identical UV-vis spectra (vide infra), we conclude that the structure of Au2Cu6-1 is similar to those of the other Au2Cu6 NCs. In other words, all these Au2Cu6 structures follow the same framework (i.e., Au2(PR3)2 axis surrounded by six Cu(S-Adm) complexes on the equatorial plane) no matter whether the electron-donating –OMe or electron-withdrawing –F substituents are introduced.
3 (Fig. S6–S8 and Table S3†). Combining the TGA, XPS and ICP results with the almost identical UV-vis spectra (vide infra), we conclude that the structure of Au2Cu6-1 is similar to those of the other Au2Cu6 NCs. In other words, all these Au2Cu6 structures follow the same framework (i.e., Au2(PR3)2 axis surrounded by six Cu(S-Adm) complexes on the equatorial plane) no matter whether the electron-donating –OMe or electron-withdrawing –F substituents are introduced.
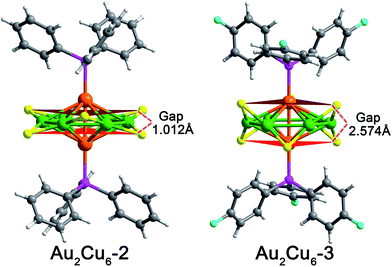
Although the point groups of Au2Cu6-2 and Au2Cu6-3 NCs are both D3d, the crystal system in space group of Au2Cu6-2 is trigonal, unlike the triclinic arrangement of Au2Cu6-3 (see Tables S1 and S2 for more details, ESI†). In addition, Au2Cu6-2 and Au2Cu6-3 exhibit some distinct structural parameters. As shown in Fig. 1, depending on the relative location around the equatorial plane, the S atoms in each NC could be categorized into two groups. The Au–Cu–S angles in Au2Cu6-3 are 85° (with upward S atoms) and 115° (with downward S atoms). This related angles in Au2Cu6-2 are significantly larger (103° or 117°). Meanwhile, the gap between the two planes constituted by the two groups of S atoms (in red planes) is 1.102 Å in Au2Cu6-2, while the gap is remarkably larger in Au2Cu6-3 (2.574 Å). The lower steric hindrance between the different thiol groups in Au2Cu6-3 results in a more regular thiolate ligands configuration (Fig. S9†). In view of the electronic effect, the weaker electron donating ability of P(Ph–F)3 results in inferior electron transfer from phosphine ligands to metallic core in Au2Cu6-3 compared to Au2Cu6-2. Accordingly, the interaction between Au and P is relatively weaker in Au2Cu6-3, and thus the Au–P bond distances Au2Cu6-3 (2.336 Å in average) are slightly longer compared with those in Au2Cu6-2 (2.325 Å in average).
The UV-vis spectra of these three Au2Cu6 NCs were compared to illustrate the ligand effect on the optical adsorption. As shown in Fig. 2, no obvious shifts occur on the front three peaks (i.e., 325, 420 and 515 nm). In contrary, the final characteristic absorption peak slightly red-shifts from 585 nm of Au2Cu6-1 to 600 nm of Au2Cu6-3 (cal. 15 nm). Therefore, the almost maintained spectra validate the similar framework in these Au2Cu6 NCs, and the similar HOMO–LUMO gap suggests that the similar transition occurs in all these Au2Cu6 NCs.18c Nonetheless, the alternation of the substituents with different electronic effect induces the slight difference in the optical property. The EDGs tend to elevate both the HOMO and LUMO energies, and the HOMO–LUMO gap is enlarged because LUMO is more sensitive to the electronic effect of the substituents.24,25 By contrast, both HOMO and LUMO energies of the NCs reduce when the EWGs are introduced, and the significantly lowered LUMO energy (compared to that of HOMO energy) results in a reduced HOMO–LUMO gap.24,25
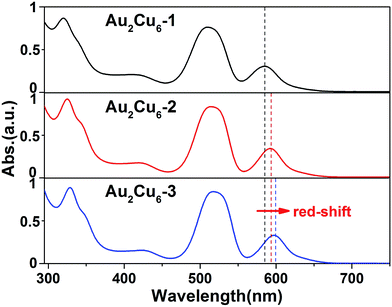 | ||
| Fig. 2 UV-vis spectra of Au2Cu6-1, Au2Cu6-2 and Au2Cu6-3 NCs. The final characteristic absorption peak was red-shifted from 585 nm to 594 nm and 600 nm. | ||
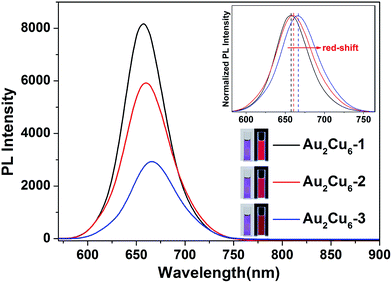
The PL spectra of Au2Cu6 NCs was characterized to verify the aforementioned inference of electronic effect. Comparing the different fluorescent spectra of Au2Cu6 NCs in Fig. 3, we find that Au2Cu6-1 shows the strongest fluorescence and Au2Cu6-3 shows the weakest one. Specifically, the QY of Au2Cu6-2 was 12.2%. The QY was enhanced to 17.7% when the more electron donating P(Ph–OMe)3 ligands (i.e., Au2Cu6-1) were used. By contrast, the Au2Cu6-3 protected by the less electron donating P(Ph–F)3 ligands shows a lower QY (5.7%). Consequently, the Au2Cu6-1 exhibits a brighter fluorescent response under UV light irradiation compared with the other Au2Cu6 NCs (Fig. 3, insets). In addition, the PL peak of Au2Cu6-1 centered at 656 nm slightly red-shifts to 660 nm in Au2Cu6-2 and 667 nm in Au2Cu6-3. The relatively smaller LUMO–HOMO gap induced by the electronic effect of the substituents accounts for the luminescence red-shift. This observation also correlates well with the similar red-shift tendency in the UV-vis spectra.
The relaxation dynamics of the three Au2Cu6 NCs were analysed (Fig. S10†). The time constant of the Au2Cu6-1 NC excited-state decay (about 8.6 μs) due to the LMCT process.18,19,26,27 When the phosphine ligands were altered to PPh3 or P(Ph–F)3, the relaxation time constant was found to decrease to 6.4 and 5.4 μs, respectively. It has been discussed above that the replacement of P(Ph–OMe)3 ligands to PPh3 and P(Ph–F)3 ligands reduced the HOMO–LUMO gap and weakened the LMCT process, which might be the reason of shorter relaxation time constants of Au2Cu6-2 and Au2Cu6-3 NCs.
In summary, the fluorescent intensity of Au2Cu6 NCs was controllable tailored by engineering the phosphine ligands with electron-donating (i.e., –OMe) or electron-withdrawing (i.e., –F) substituents. The distinct electronic effect was successfully used to modulate the LMCT process, and then control the fluorescent intensity. When protected by the electron-rich P(Ph–OMe)3 ligand, the Au2Cu6 NC exhibits enhanced fluorescence with QY = 17.7% compared with that protected by PPh3 (QY = 12.2%). On the contrary, the QY was decreased when the Au2Cu6 NC is protected by relatively electron-deficient ligands (i.e., P(Ph–F)3). In addition, the PL spectrum of Au2Cu6 protected by P(Ph–F)3 displays a slight red-shift compared with other Au2Cu6 NCs, which is similar to the observations in UV-vis spectra. This study presents a controllable strategy to enhance the PL of noble metal NCs, and also sheds lights on synthesizing new class of highly fluorescent NCs.
Acknowledgements
We acknowledges financial support by NSFC (21372006, U1532141 & 21631001), the Ministry of Education, the Education Department of Anhui Province, 211 Project of Anhui University.Notes and references
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- P. Liu, R. Qin, G. Fu and N. Zheng, J. Am. Chem. Soc., 2017, 139, 2122–2131 CrossRef CAS PubMed.
- C. P. Joshi, M. S. Bootharaju and O. M. Bakr, J. Phys. Chem. Lett., 2015, 6, 3023–3035 CrossRef CAS PubMed.
- N. Goswami, Q. Yao, Z. Luo, J. Li, T. Chen and J. Xie, J. Phys. Chem. Lett., 2016, 7, 962–975 CrossRef CAS PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, J.-Q. Wang and Q.-M. Wang, Dalton Trans., 2017, 46, 3427–3434 RSC.
- A. Mathew and T. Pradeep, Part. Part. Syst. Charact., 2014, 31, 1017–1053 CrossRef CAS.
- W. Kurashige, Y. Niihori, S. Sharma and Y. Negishi, J. Phys. Chem. Lett., 2014, 5, 4134–4142 CrossRef CAS PubMed.
- Y. Tao, M. Li, J. Ren and X. Qu, Chem. Soc. Rev., 2015, 44, 8636–8663 RSC.
- S. Yamazoe, S. Takano, W. Kurashige, T. Yokoyama, K. Nitta, Y. Negishi and T. Tsukuda, Nat. Commun., 2016, 7, 10414 CrossRef CAS PubMed.
- T. M. Carducci, R. E. Blackwell and R. W. Murray, J. Am. Chem. Soc., 2014, 136, 11182–11187 CrossRef CAS PubMed.
- A. Dass, S. Theivendran, P. R. Nimmala, C. Kumara, V. R. Jupally, A. Fortunelli, L. Sementa, G. Barcaro, X. Zuo and B. C. Noll, J. Am. Chem. Soc., 2015, 137, 4610–4613 CrossRef CAS PubMed.
- K. L. D. M. Weerawardene and C. M. Aikens, J. Am. Chem. Soc., 2016, 138, 11202–11210 CrossRef CAS PubMed.
- X.-D. Zhang, Z. Luo, J. Chen, X. Shen, S. Song, Y. Sun, S. Fan, F. Fan, D. T. Leong and J. Xie, Adv. Mater., 2014, 26, 4565–4568 CrossRef CAS PubMed.
- Z. Gan, Y. Lin, L. Luo, G. Han, W. Liu, Z. Liu, C. Yao, L. Weng, L. Liao, J. Chen, X. Liu, Y. Luo, C. Wang, S. Wei and Z. Wu, Angew. Chem., Int. Ed., 2016, 55, 11567–11571 CrossRef CAS PubMed.
- S. Wang, X. Meng, A. Das, T. Li, Y. Song, T. Cao, X. Zhu, M. Zhu and R. Jin, Angew. Chem., Int. Ed., 2014, 53, 2376–2380 CrossRef CAS PubMed.
- R.-W. Huang, Y.-S. Wei, X.-Y. Dong, X.-H. Wu, C.-X. Du, S.-Q. Zang and T. C. W. Mak, Nat. Chem., 2017 DOI:10.1038/nchem.2718.
- (a) G. Li, Z. Lei and Q.-M. Wang, J. Am. Chem. Soc., 2010, 132, 17678–17679 CrossRef CAS PubMed; (b) Z. Lei, X.-L. Pei, Z.-G. Jiang and Q.-M. Wang, Angew. Chem., Int. Ed., 2014, 53, 12771–12775 CrossRef CAS PubMed; (c) X.-Y. Liu, Y. Yang, Z. Lei, Z.-J. Guan and Q.-M. Wang, Chem. Commun., 2016, 52, 8022–8025 RSC.
- (a) X. Kang, M. Zhou, S. Wang, S. Jin, G. Sun, M. Zhu and R. Jin, Chem. Sci., 2017, 8, 2581–2587 RSC; (b) X. Kang, L. Xiong, S. Wang, H. Yu, S. Jin, Y. Song, T. Chen, L. Zheng, C. Pan, Y. Pei and M. Zhu, Chem.–Eur. J., 2016, 22, 17145–17150 CrossRef CAS PubMed; (c) X. Kang, S. Wang, Y. Song, S. Jin, G. Sun, H. Yu and M. Zhu, Angew. Chem., Int. Ed., 2016, 55, 3611–3614 CrossRef CAS PubMed.
- (a) M. S. Bootharaju, C. P. Joshi, M. R. Parida, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 922–926 CrossRef CAS PubMed; (b) G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A.-H. Emwas, O. F. Mohammed and O. M. Bakr, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- (a) Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CrossRef CAS PubMed; (b) K. Zheng, X. Yuan, K. Kuah, Z. Luo, Q. Yao, Q. Zhang and J. Xie, Chem. Commun., 2015, 51, 15165–15168 RSC; (c) Y. Yu, Z. Luo, D. M. Chevrier, D. Tai Leong, P. Zhang, D.-e. Jiang and J. Xie, J. Am. Chem. Soc., 2014, 136, 1246–1249 CrossRef CAS PubMed.
- Y. Wang, H. Su, L. Ren, S. Malola, S. Lin, B. K. Teo, H. Häkkinen and N. Zheng, Angew. Chem., Int. Ed., 2016, 55, 15152–15156 CrossRef CAS PubMed.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed.
- T. Udayabhaskararao, Y. Sun, N. Goswami, S. K. Pal, K. Balasubramanian and T. Pradeep, Angew. Chem., Int. Ed., 2012, 51, 2155–2159 CrossRef CAS PubMed.
- L. Sementa, G. Barcaro, O. Baseggio, M. D. Vetta, A. Dass, E. Apra, M. Stener and A. Fortunelli, J. Phys. Chem. C, 2017, 121, 10832–10842 CAS.
- G. Lugo, V. Schwanen, B. Fresch and F. Remacle, J. Phys. Chem. C, 2015, 119, 10969–10980 CAS.
- S. H. Yau, O. Varnavski and T. Goodson, Acc. Chem. Res., 2013, 46, 1506–1516 CrossRef CAS PubMed.
- M. Pelton, Y. Tang, O. M. Bakr and F. Stellacci, J. Am. Chem. Soc., 2012, 134, 11856–11859 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization details and X-ray crystallographic (CIF) data. CCDC 1539336 & 1539376. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra04743f |
| This journal is © The Royal Society of Chemistry 2017 |