Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced performance of a flexible supercapacitor due to a combination of the pseudocapacitances of both a PANI/MWCNT composite electrode and a gel polymer redox electrolyte
G. P. Shumakovich a,
O. V. Morozova
a,
O. V. Morozova a,
M. E. Khlupova
a,
M. E. Khlupova a,
I. S. Vasil'eva
a,
I. S. Vasil'eva a,
E. A. Zaitseva
a,
E. A. Zaitseva b and
A. I. Yaropolov
b and
A. I. Yaropolov *a
*a
aBach Institute of Biochemistry, Research Center of Biotechnology of the Russian Academy of Sciences, Leninsky Ave. 33, bld. 2, 119071 Moscow, Russia. E-mail: yaropolov@inbi.ras.ru; Fax: +7 495 954 2732; Tel: +7 495 954 4477
bDepartment of Chemistry, Lomonosov Moscow State University, Leninskie Gory 1/3, 119991 Moscow, Russia
First published on 6th July 2017
Abstract
In this paper, a combination of the pseudocapacitances of both an enzymatically synthesized polyaniline/multi-walled carbon nanotube (PANI/MWCNT) composite with a core shell structure and a gel polymer redox electrolyte was used to improve the specific characteristics of a supercapacitor (SC). The gel of polyvinyl alcohol in sulfuric acid containing sodium 1,2-naphthoquinone-4-sulfonate (PVA/H2SO4/NQS) was used as a gel polymer redox electrolyte. Electrochemical studies have shown that the redox behavior of NQS is a diffusion-controlled and quasi-reversible process. The PANI/MWCNT composite in 13 mM NQS solution had a high specific capacitance of ca. 1100 F g−1 at a scan rate of 5 mV s−1 (three electrode cell configuration). The symmetrical flexible SC device based on the PANI/MWCNT composite and the PVA/NQS gel redox electrolyte had a power density of 1.8 kW kg−1 and an energy density of 23.3 W h kg−1. After 3000 cycles of potential scanning, the specific capacitance of the SC device decreased by less than 7%.
1. Introduction
Energy accumulation and storage is a problem fundamental to the further development of various technical devices, such as hybrid electric vehicles, mobile electronic devices, solar panels, memory backup systems, medical appliances, etc.Supercapacitors (SC) are currently the most promising devices for this purpose. A classic SC is an electrochemical double electric layer capacitor in which carbon materials with a high specific surface area are used as electrodes.1–3 Such SCs have high power density, whereas their energy density is much lower than that of Li-ion batteries. Certain metal oxides or conducting polymers which ensure the faradaic reaction were added to carbon materials to enhance the specific capacitance and energy density of SC devices.4–8 The combination of the double electric layer capacitance and the faradaic reaction can significantly improve the specific characteristics of SC devices. However, such devices based on conducting polymers show an insufficient stability in charge/discharge cycles. Therefore, it is essential to increase the stability of SC and improve its characteristics. The cycling stability of the composite can be enhanced by forming a thin polyaniline film on the surface of carbon nanomaterials.9
Another way of increasing the energy density and the device stability is to add redox active compounds into the electrolyte.10–18 The method originally developed for carbon-based double layer supercapacitors with electrochemically inert electrodes14,15 has recently been used to enhance the capacitance of polyaniline-based supercapacitors.16–18 The introduction of hydroquinone into the conventional electrolyte H2SO4 reported in16 resulted in a 2-fold increase in the specific capacitance of polyaniline supercapacitors and in a dramatic enhancement of their cycling stability, which in the authors's opinion can be explained by: firstly, the additional pseudocapacitance of hydroquinone–quinone couples; secondary, the improvement of the ionic conductivity of the electrolyte, and finally, the reactions proceeding between the redox electrolyte components and electrochemically active material of the composite electrode. A similar effect was observed with the benzoquinone-hydroquinone redox couple.17 High pseudocapacitance of this polyaniline–benzoquinone–hydroquinone supercapacitor is attributed to the fast and reversible charge-transfer process between the polyaniline-modified electrodes and the quinones. Simultaneously, the enhancement of the long-term stability can be explained by a lower extend of unfavorable redox processes in polyaniline. Thus, quinones show promise as redox-additives to electrolytes.
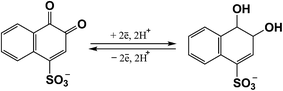
To improve the characteristics of a flexible supercapacitor we used sodium 1,2-naphthoquinone-4-sulfonate (NQS) as a redox-active additive.
2. Experimental section
2.1 Materials
Aniline (Labtech, Russia) was distilled under reduced pressure before use. Poly(vinyl alcohol) (hydrolyzed 99+%, Mw 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–98
000–98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), N-phenyl-p-phenylendiamine, ABTS, sodium 1,2-naphthoquinone-4-sulfonate (Sigma-Aldrich), p-toluenesulfonic acid monohydrate (Acros Organics), H2SO4, HNO3 (Chimmed, Russia) were used without further purification. Flexible graphite foil (thickness 0.2 mm) was purchased from Unichimtek (Russia). Multi-walled carbon nanotubes “Taunit M” (NanoTechCentre Ltd, Tambov, Russia) were used after treatment with hot 70% nitric acid (MWCNT). PANI/MWCNT composite was obtained by in situ enzymatic aniline polymerization. The laccase from the fungus Trametes hirsuta (Wulfen) Pilát CF-28 was purified to homogeneity as described previously.19 The specific activity of the enzyme was ca. 160 U mg−1 of protein using ABTS as a chromogenic substrate.20
000), N-phenyl-p-phenylendiamine, ABTS, sodium 1,2-naphthoquinone-4-sulfonate (Sigma-Aldrich), p-toluenesulfonic acid monohydrate (Acros Organics), H2SO4, HNO3 (Chimmed, Russia) were used without further purification. Flexible graphite foil (thickness 0.2 mm) was purchased from Unichimtek (Russia). Multi-walled carbon nanotubes “Taunit M” (NanoTechCentre Ltd, Tambov, Russia) were used after treatment with hot 70% nitric acid (MWCNT). PANI/MWCNT composite was obtained by in situ enzymatic aniline polymerization. The laccase from the fungus Trametes hirsuta (Wulfen) Pilát CF-28 was purified to homogeneity as described previously.19 The specific activity of the enzyme was ca. 160 U mg−1 of protein using ABTS as a chromogenic substrate.20
2.2 Preparation of PANI/MWCNT composite
PANI/MWCNT composite was obtained by in situ enzymatic aniline polymerization with atmospheric oxygen as terminal oxidant. The fungal laccase catalyzed aniline polymerization in the presence of aniline dimer as a redox enhancer of enzymatic polymerization. The reaction of aniline polymerization was carried out under conditions described in our previous work21 using toluenesulfonic acid as a dopant.2.3 Preparation of a flexible supercapacitor
The supercapacitor was fabricated from two identical thin flexible electrodes coated with enzymatically synthesized PANI/MWCNT composite as an electroactive material. The PVA/H2SO4/NQS gel polymer redox electrolyte was employed as both electrolyte and separator.2.4 Characterization
The morphology of PANI/MWCNT composite was characterized by scanning electron microscopy (SEM, Supra 40 VP, Carl Zeiss) and transmission electron microscopy (TEM, LEO 912 AB OMEGA, Carl Zeiss). FTIR spectra were recorded using KBr pellets on a FTIR spectrometer (IRPrestige Fourier, Shimadzu, Japan). Four-point conductivity measurements were carried out with a Loresta GP (Mitsubishi, Japan).The characterization of the PANI/MWCNT composite in the redox electrolyte (1 M H2SO4/13 mM NQS) was performed by cyclic voltammetry (CV) using a BAS CV-50 W voltammetric analyzer (Bioanalytical System, USA). An electrochemical cell with a commercial Ag/AgCl electrode (Bioanalytical System, USA) as a reference electrode and platinum sheet as a counter electrode were used for electrochemical studies. Either a glassy carbon electrode (Bioanalytical System, USA) or flexible graphite foil coated with PANI/MWCNT composite served as a working electrode. The specific capacitance (Cs) of the composite was calculated using the formula 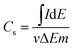 where I is the current, ΔE is the potential range, v is the potential scan rate, m is the mass of electroactive composite.
where I is the current, ΔE is the potential range, v is the potential scan rate, m is the mass of electroactive composite.
Electrochemical characteristics of the SC device were recorded using a galvanostatic charge/discharge test (MetrohmAutolab, Holland) and calculated according to the formulas described in ref. 22.
3. Results and discussion
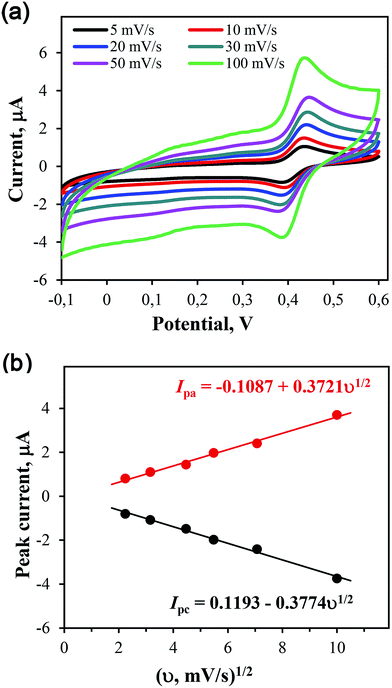
To increase the specific capacitance and cyclic stability of the SC device, we used a combination of two redox systems, i.e. an enzymatically synthesized PANI/MWCNT composite with improved morphology was combined with the redox-active compound NQS, whose formula is shown in Fig. 1.NQS was studied in detail in 3-electrode experiments by cyclic voltammetry using a glassy carbon electrode. Cyclic voltammograms recorded at different scan rates in 1 M of H2SO4, containing 0.1 mM NQS, are shown in Fig. 2a. At a potential scan rate of 5 mV s−1, the middle point potential and ΔE were 415 mV and ca. 33 mV, respectively. Therefore, the redox behavior of NQS on the glassy carbon electrode was almost reversible. Both the oxidation and reduction peak currents of NQS showed a linear relationship with the square root of the scan rate (Fig. 2b), which is characteristic of a diffusion controlled process.
The number of transferred electrons in the course of the NQS electroreduction reaction was calculated by the Randles-Sevčik equation23 using the diffusion coefficient of 0.58 × 10−5 cm2 s−1 (ref. 17) was ca. 1.9. The peak currents of NQS did not change within the potential range from −0.1 to +0.6 V after 100 cycles of the potential scanning with a scan rate of 100 mV s−1, which indicates a high electrochemical stability of NQS.
PANI-based materials have high electrochemical capacitance and high electrical conductivity, but the poor stability in charge/discharge cycles hampers their use as electrode material for SC devices. The stability of these materials depends on the morphological homogeneity of PANI/MWCNT composite and can be improved by forming a uniform thin layer of the conducting polymer on carbon nanomaterials. The amount of the functional polymer not bound to the MWCNTs, should be minimal, which is difficult to achieve using traditional chemical synthesis, since the monomer polymerization occurs not only on the MWCNTs but also in the reaction medium. The enzymatic approach reported in the given paper enabled us to improve the morphology of the composite.
We performed in situ laccase-catalyzed aniline polymerization using aniline dimer (AD, N-phenyl-p-phenylendiamine) as an enhancer of aniline enzymatic polymerization24 in order to obtain a uniform thin PANI layer on the surface of MWCNTs. The composite was synthesized as follows. AD was pre-adsorbed on MWCNTs by incubating 5 mg of acid-treated MWCNTs in 5 ml of 1 mM AD solution for 1 h. The required amount of MWCNTs with the adsorbed AD was dispersed in a solution (pH 2.8) containing equimolar concentrations of aniline monomer and toluenesulfonic acid (65 mM). Aniline polymerization was initiated by adding the laccase stock solution. The specific activity of the enzyme in the reaction mixture was 4.0 U ml−1. The reaction was carried out at room temperature for 24 h with magnetic stirring under aerobic conditions.
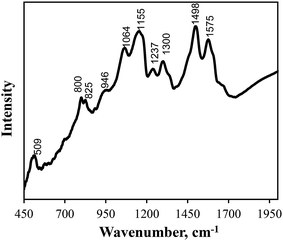
The PANI/MWCNT composite was separated by centrifugation, washed with ethanol and dried to a constant weight. The content of PANI in the composite was ca. 46 wt%. The FTIR spectrum of PANI/MWCNT (Fig. 3) exhibits characteristic absorption bands arising from the vibration mode of both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the quinoid diimine unit near 1575 cm−1, while the band near 1498 cm−1 is attributed to C
C stretching of the quinoid diimine unit near 1575 cm−1, while the band near 1498 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring stretching of the benzenoid diamine unit.25 Absorption bands near 1300 cm−1 and 1155 cm−1 belong to N–H and –N = stretching, respectively.26 The 1,4-disubstituted aromatic rings vibrate at 825 cm−1 and 800 cm−1.27
C aromatic ring stretching of the benzenoid diamine unit.25 Absorption bands near 1300 cm−1 and 1155 cm−1 belong to N–H and –N = stretching, respectively.26 The 1,4-disubstituted aromatic rings vibrate at 825 cm−1 and 800 cm−1.27
The morphology of the PANI/MWCNT composite was studied by transmission electron microscopy (TEM). As is seen in the TEM (Fig. 4) a core–shell structure composite was formed on MWCNTs on laccase-catalyzed aniline polymerization, i.e. a uniform thin, rough layer of PANI coated the surface of MWCNTs. Besides, the composite had no PANI not bound to the surface of MWCNTs. The conductivity of the composite measured by the four-point probe method at room temperature was 11.2 ± 1.2 S cm−1.
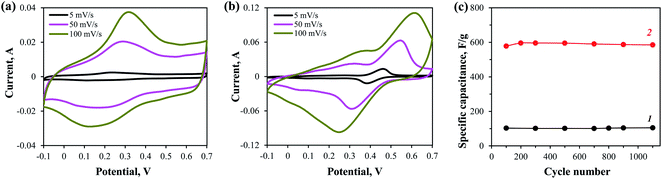
Electrochemical characteristics of the PANI/MWCNT composite were examined in 1 M H2SO4 by cyclic voltammetry using a three electrode cell configuration at various scan rates (Fig. 5a). The specific capacitance of the composite was ca. 420 F g−1 (5 mV s−1) and after 1100 scanning cycles at a potential scan rate of 100 mV s−1 decreased by about 3% (Fig. 5c, curve 1). Cyclic voltammograms of the PANI/MWCNT composite electrode in 1 M H2SO4/13 mM NQS are shown in Fig. 5b. The cyclic voltammograms demonstrate current peaks corresponding to the redox behavior of both PANI and NQS. The specific capacitance of PANI/MWCNT electrode in 1 M H2SO4/13 mM NQS redox electrolyte was ca. 1100 F g−1 at a potential scan rate of 5 mV s−1. Fig. 5c presents the stability of PANI/MWCNT composite electrode in 1 M H2SO4 (curve 1) and 1 M H2SO4/13 mM NQS (curve 2). It is seen that the electrode stability hardly varies in both solution. However, the specific capacitance of the PANI/MWCNT electrode in H2SO4/NQS redox electrolyte is ∼6 times as high.
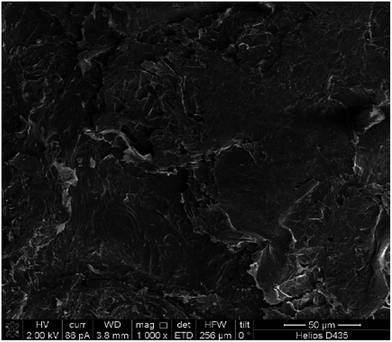
A flexible supercapacitor of symmetric architecture was fabricated from two identical thin flexible electrodes with the enzymatically synthesized PANI/MWCNT composite as an electroactive material. First, a current collector was formed as follows. Some regular Scotch tape was stuck to the graphite foil and then peeled off. As a result, a thin graphite layer was formed on the sticky side. Sheet resistance of the carbon layer was 5–8 Ω □−1. The SEM image of the graphite layer is shown in Fig. 6. Then, an alcohol dispersion of PANI/MWCNT composite was deposited on the graphite current collector. The gel polymer redox electrolyte (10 wt% PVA/1 M H2SO4/13 mM NQS) was “sandwiched” between the electrodes to fabricate a thin flexible supercapacitor.
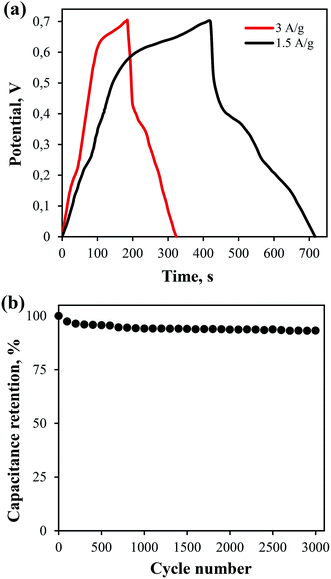
Electrochemical studies of the flexible symmetric SC were performed by the galvanostatic method in a two electrode configuration. Fig. 7a shows the dependence of the SC voltage change on time with a given current density. The discharge curve at 1.5 A g−1 was used to calculate the power and energy densities, which were 1.8 kW kg−1 and 23.3 W h kg−1, respectively. The loss of the SC capacitance after 3000 cycles was less than 7% of the initial value (Fig. 7b).
4. Conclusions
In the present study two redox systems (polyaniline as an electrochemically active electrode material and sodium 1,2-naphthoquinone-4-sulfonate as a redox additive to the electrolyte) were combined to improve the supercapacitor performance. An enzymatic approach enabled us to obtain a PANI/MWCNT composite with a core–shell structure and minimize the formation of the functional polymer not bound to the surface of the carbon nanomaterial. The resulting composite electrode showed a better long cycling stability.Electrochemical studies have shown that the redox behavior of NQS used as a redox-active additive to the electrolyte is a diffusion controlled and reversible process involving the transfer of two electrons via the electrode/electrolyte interface. A thin flexible SC device with high specific characteristics was constructed on the basis of the PANI/MWCNT composite and the PVA/H2SO4/NQS gel polymer redox electrolyte.
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (project no. 15-08-00142-a).Notes and references
- B. E. Conway, Electrochemical supercapacitors: Scientific fundamentals and technological applications, Plenum Press, New York, 1999 Search PubMed.
- R. Kötz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- E. Frackowiak, V. Khomenko, K. Jurewicz, K. Lota and F. Beguin, J. Power Sources, 2006, 153, 413–418 CrossRef CAS.
- T.-Y. Wei, C.-H. Chen, H.-C. Chien, S.-Y. Lu and C.-C. Hu, Adv. Mater., 2010, 22, 347–351 CrossRef CAS PubMed.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- L. Yu and G. Z. Chen, J. Power Sources, 2016, 326, 604–612 CrossRef CAS.
- A. Eftekhari, L. Li and Y. Yang, J. Power Sources, 2017, 347, 86–107 CrossRef CAS.
- Y. Meng, K. Wang, Y. Zhang and Z. Wei, Adv. Mater., 2013, 25, 6985–6990 CrossRef CAS PubMed.
- D. Vonlanthen, P. Lazarev, K. A. See, F. Wudl and A. J. Heeger, Adv. Mater., 2014, 26, 5095–5100 CrossRef CAS PubMed.
- W. Han, L.-B. Kong, M.-C. Liu, D. Wang, J.-J. Li and L. Kang, Electrochim. Acta, 2015, 186, 478–485 CrossRef CAS.
- Q. Wang, Y. F. Nie, X. Y. Chen, Z. H. Xiao and Z. J. Zhang, J. Power Sources, 2016, 323, 8–16 CrossRef CAS.
- Y. Tian, M. Liu, R. Che, R. Xue and L. Huang, J. Power Sources, 2016, 324, 334–341 CrossRef CAS.
- S. Roldán, Z. González, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Electrochim. Acta, 2011, 56, 3401–3405 CrossRef.
- S. Roldán, M. Granda, R. Menéndez, R. Santamaría and C. Blanco, Electrochim. Acta, 2012, 83, 241–246 CrossRef.
- W. Chen, R. B. Rakhi and H. N. Alshareef, Nanoscale, 2013, 5, 4134–4138 RSC.
- D. Vonlanthen, P. Lazarev, K. A. See, F. Wudl and A. J. Heeger, Adv. Mater., 2014, 26, 5095–5100 CrossRef CAS PubMed.
- H. Xie, Y. Zhu, Y. Wu, Z. Wu and E. Liu, Mater. Res. Bull., 2014, 50, 303–306 CrossRef CAS.
- E. S. Gorshina, T. V. Rusinova, V. V. Biryukov, O. V. Morozova, S. V. Shleev and A. I. Yaropolov, Appl. Biochem. Microbiol., 2006, 42, 558–563 CrossRef CAS.
- M. D. Cannatellia and A. J. Ragauskas, J. Mol. Catal. B: Enzym., 2015, 119, 85–89 CrossRef.
- G. V. Otrokhov, G. P. Shumakovich, M. E. Khlupova, I. S. Vasil'eva, I. B. Kaplan, B. T. Zaitchik, E. A. Zaitseva, O. V. Morozova and A. I. Yaropolov, RSC Adv., 2016, 6, 60372–60375 RSC.
- C. Meng, C. Liu, L. Chen, C. Hu and S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Willey and Sons, New York, 1980, pp. 218–231 Search PubMed.
- G. P. Shumakovich, S. V. Shleev, O. V. Morozova, P. S. Khohlov, I. G. Gazaryan and A. I. Yaropolov, Bioelectrochemistry, 2006, 69, 16–24 CrossRef CAS PubMed.
- G. Louarn, M. Lapkowski, S. Quillard, A. Pron, J. P. Buisson and S. Lefrant, J. Phys. Chem., 1996, 100, 6998–7006 CrossRef CAS.
- S. Tao, B. Hong and Z. Kerong, Spectrochim. Acta, Part A, 2007, 66, 1364–1368 CrossRef PubMed.
- C. H. Lim and Y. J. Yoo, Process Biochem., 2000, 36, 233–241 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |