Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Durable, self-healing, superhydrophobic fabrics from fluorine-free, waterborne, polydopamine/alkyl silane coatings†
Hongxia Wang*a,
Hua Zhoua,
Shuai Liub,
Hao Shaoa,
Sida Fua,
Gregory C. Rutledgec and
Tong Lin *a
*a
aInstitute for Frontier Materials, Deakin University, Geelong, VIC3216, Australia. E-mail: tong.lin@deakin.edu.au; Hong.wang@deakin.edu.au
bSchool of Mechanical and Electric Engineering, Soochow University, 215000, China
cDepartment of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
First published on 5th July 2017
Abstract
Superhydrophobic fabrics have diverse applications in both textile and non-textile fields. Most of the waterborne materials for superhydrophobic treatment of fabrics use fluoro-containing substances, which have potential issues with bio-accumulation in both the human body and animals. Fluorine-free waterborne coatings are highly desirable for superhydrophobic treatment. In this study, we have prepared a fully waterborne coating solution through dispersion of an alkyl silane (hexadecyl trimethoxysilane) in an aqueous dopamine solution. After applying to fabrics through a wet-chemical process, the coating made the fabrics have a superhydrophobic surface with a water contact angle and a sliding angle of 163° and 8.6°, respectively. The treated fabrics are durable enough to withstand multiple washing. The coating is self-healable against acid/base etching and plasma damage. Such a fluorine-free, durable coating may be useful for the development of various superhydrophobic fabric products.
1. Introduction
Superhydrophobic fabrics have potential applications in a number of areas including personal protection, anti-contamination, oil–water separation, anti-ice, anti-adhesion and functional sportswear.1–18 Many methods have been developed to prepare superhydrophobic fabrics. Most of the superhydrophobic fabrics are prepared using fluorine containing substances through a coating process.19–24 However, some fluorine-containing substances used for superhydrophobic treatment have issues with bio-accumulation. For example, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) of carbon chains greater than six are difficult to be degraded within the human body and thus could cause health issues. They have been restricted for applications in fabric finishing. Therefore, fluorine-free materials are more desirable.Fluorine-free superhydrophobic treatments have been reported by several groups.25 For examples, one-step coating treatments were reported by using different solution systems such as polystyrene solution containing hydrophobic SiO2 nanoparticles and mercaptopropyltrimethoxy silane,26 polydimethylsiloxane (PDMS) solution containing onion-like carbon microspheres,27 and PDMS containing graphene oxide.28 Two-step treatment methods were also reported, such as forming a rough surface using ormosil29 or silica nanoparticles,30 followed by lowering the surface free energy using PDMS or stearic acid/perfluorodecyltrichlorosilane, respectively. However, most of the works involved solutions in organic solvent, which not only causes issues to the environment but also increases the cost. Superhydrophobic fabrics prepared by a fluorine-free waterborne solution or not using any solvent are highly desirable. Recently, superhydrophobic fabrics prepared by multicycle self-assembly or multi-step spraying using aqueous solutions have been reported.31,32
Dopamine (DA) is a biological neurotransmitter widely found in living organisms.33 In aqueous solution, dopamine can polymerize spontaneously into polydopamine (PDA), and the polymerization products show good adhesion on solid substances.34,35 Recently, using DA to prepare superhydrophobic surfaces has been reported. However, most of the methods involved a multiple step process, to deposit PDA on a solid substrate in an alkaline condition followed by hydrophobization treatment with a fluorine-containing chemical.36–40 Some fabric materials, such as wool and polyester, tend to hydrolyze in an alkaline condition,41 and the hydrolysis of the fiber surface could result in poor bonding between the overlay coating and the fiber substrate, hence lowering the coating durability.
In this study, we show that co-deposition of dopamine with an alkyl silane, hexadecyl trimethoxysilane (HDTMS), in neutral aqueous solution renders fabrics with a durable superhydrophobic surface. The treated fabrics have a water contact angle as high as 163° and sliding angle as low as 8.6°. It has high washing durability that can withstand over 20 cycles of AATCC standard washing without losing the superhydrophobicity. The coating is self-healable against chemical damages such as plasma treatment and etching in acid (pH = 1) or alkali (pH = 14) solution. Such a novel fluorine-free coating may be useful for development of safe, durable superhydrophobic fabric products.
2. Experimental section
2.1 Materials
Dopamine hydrochloride (3-hydroxytyramine hydrochloride, C8H11NO2·HCl) and hexadecyl trimethoxysilane (HDTMS, Dynasylan 9116) were obtained from Aldrich. Commercial cotton fabric (plain weave, 165 g m−2, thickness = 460 μm), polyester fabric (plain weave, 168 g m−2, thickness = 520 μm) and wool fabric (plain weave, 190 g m−2, thickness = 540 μm) were purchased from the local supermarket. The fabrics were pre-cleaned with isopropyl alcohol to remove grease and possible dusts from the fabric substrates, without desizing, scouring and bleaching. The treatment did not influence the chemical and physical properties of the fabrics.2.2 Synthesis of coating solution
Dopamine hydrochloride (0.1 g) was dissolved in deionized water (10 mL) to get a homogeneous solution. HDTMS (0.18 g, molar ratio of dopamine/HDTMS is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was then added to the DA solution. The mixture was stirred at 50 °C until the HDTMS completely dispersed in the solution (about 12 h). The final brown solution was ready for coating treatment. The fabrics used in this work may have had some kind of finishes and they may not have been removed by the isopropyl alcohol treatment.
1) was then added to the DA solution. The mixture was stirred at 50 °C until the HDTMS completely dispersed in the solution (about 12 h). The final brown solution was ready for coating treatment. The fabrics used in this work may have had some kind of finishes and they may not have been removed by the isopropyl alcohol treatment.
2.3 Coating treatment
A dip coating technique was employed to apply the coating solution onto fabrics. In brief, a fabric sample was immersed in the coating solution for 15 min at 50 °C. After drying at 140 °C for 1 hour, the coated fabric was rinsed twice with water to remove the side products and finally dried at ambient condition. After coating, the fabric turned from white to tint brown.2.4 Characterizations
Electron microscopic images were taken on a scanning electron microscope (SEM; Leo 1530, Gemini/Zeiss, Oberkochen).Atomic force microscopy (AFM; Cypher, Asylum Research) was used to measure surface roughness. Fourier Transform Infrared (FTIR) spectra were obtained on an FTIR spectrophotometer (Bruker Optics, Ettlingen). X-ray photoelectron spectroscopy (XPS) was measured on a VG ESCALAB 220-iXL spectrometer with a monochromated Al Kα source (1486.6 eV) using samples of approximately 3 mm2 in size. The X-ray beam incidence angle was 0° with respect to the surface normal, which corresponds to a sampling depth of ∼10 nm. The obtained XPS spectra were analyzed by the XPSPEAK41 software (Shanghai, China). Contact angles were measured using a commercial contact angle meter (KSV CAM101 Instruments Ltd). Water droplets for the contact angle measurement were 3 μL in volume. The size of dopamine/HDTMS particles was tested by using dynamic light scattering (DLS) (Zetasizer, Nano ZS, 90, Malvern). Color difference was measured using a Datacolor 600™ spectrophotometer (Datacolor company, USA). The test conditions were: illuminant D65, 10° standard observer, and specular component included.2.5 Durability test
Washing durability was evaluated in reference of the washing method specified in the AATCC (American Association of Textile Chemists and Colorists) Test Method 61-2006 test no. 2A. The test was performed using a standard laundering machine (MODEL H-240, no. 4361, RAPID LABORTEX CO., LTD. Taiwan) equipped with 500 mL (75 mm × 75 mm) stainless-steel lever-lock canisters. The fabric sample (size, 50 mm × 150 mm) was laundered in a 150 mL aqueous solution containing 0.15% (w/w) AATCC standard reference detergent WOB and 50 stainless steel balls. During laundering, the temperature was controlled at 49 °C, and the stirring speed was 40 ± 2 rpm. After 45 minutes of laundering, the laundered sample was rinsed with tap water, and then dried at room temperature without spinning. The contact angle and sliding angle were then measured. The abrasion resistance was tested using the Martindale method, according to Australian Standard AS 2001.2.25-2006 (similar to ASTM D4966). The test was performed under a commercial Martindale abrasion tester (I. D. M Instrument Design & Maintenance). The fabric sample was mounted on the dynamic disk which tightly contacted with the abrandant underneath. The abrandant was mounted on the motionless disk. Pressure was applied by adding weight of standard mass onto the upper shaft. During testing, the dynamic disk rotated, and in the meanwhile the whole dynamic disk moved across the different area of the abradant surface.2.6 Self-healing tests
Three methods were employed to deliberately damage the coating on the fabrics: air plasma treatment, immersing in an aqueous H2SO4 solution (pH = 1), and immersing in an aqueous KOH solution (pH = 14). For plasma treatment, the treated fabrics were subjected to a vacuum plasma treatment (gas source: air) for 30 seconds. Such plasma treatment can make the surface completely hydrophilic (contact angle 0°). For acid/base immersion, the treated fabric was immersed in the acid/base solution for 10 min at room temperature, and then rinsed with deionized water, and dried naturally at room temperature. The fabric after this treatment turned hydrophilic. After those damages, the treated fabrics were heated at 140 °C for 10 minutes. The one that can restore the superhydrophobicity, which is confirmed by contact angle, has a self-healing property.3. Results and discussion
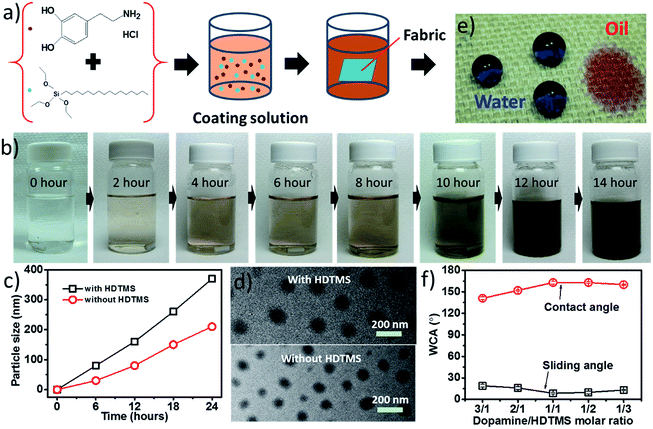
Fig. 1a shows the chemical structure of the coating materials and the procedure for coating treatment. The coating solution was prepared by dispersing HDTMS in an aqueous dopamine (DA) solution in neutral condition. At the initial stage of mixing, the HDTMS suspended on the top of the aqueous solution to form a separate layer due to the insoluble nature in water. During mechanical stirring, the HDTMS dispersed gradually in the solution with time, and meanwhile the solution turned brown. After 12 hours of stirring, HDTMS dispersed completely in the solution and the solution became dark brown (Fig. 1b). During the process of mixing and dispersion, the solution maintained the pH at 7.0.It is known that HDTMS is insoluble in water and it has a very low hydrolysis rate in neutral aqueous solution. The presence of dopamine in the solution improved the dispersing ability of HDTMS. This is because dopamine self-polymerizes to form PDA particles, which functioned as a carrier to adsorb HDTMS. The size of PDA/HDTMS particles was measured using a dynamic light scattering (DLS) method (see measurement result in the ESI†). Fig. 1c shows the average size of the particles formed in the aqueous solution. The average particle size increased with increasing the mixing time. After 12 hours, the average particle size was 160 nm. Under TEM, the PDA/HDTMS particles showed a round shape (Fig. 1d). In contrast, the PDA formed in the same condition without HDTMS was 80 nm. Apparently, dopamine in the solution improves the dispersing ability of HDTMS in neural aqueous solution. PDA forms nanoparticles in water, which have large surface energy. HDTMS can be adsorbed by PDA particles during the particle formation. Further polymerization of DA could also immobilize the HDTMS through the reaction between dopamine and hydrolyzed HDTMS through hydroxyl group. The coating solution was stable at room temperature for up to one week. Longer storage time (e.g. two weeks) led to participation of the black particles.
When the coating solution was applied onto cotton fabric, the fabric turned superhydrophobic. Fig. 1e shows a photo of blue-dyed water on the coated cotton fabric. The coated fabric had a water contact angle (CA) as high as 163° and a sliding angle (SA) of 8.6°. Water drop stayed stability on the fabric surface for long time. The fabric was oleophilic with a CA of 0° to cooking oil (surface tension = 32 mN m−1). The coated fabric is repellent to coffee, milk, cherry juice and red wine (see the ESI†).
For comparison, we also treated cotton fabric (with CA of 0° for untreated fabrics) with an aqueous solution containing either dopamine or HDTMS. In the same concentration and stirring condition, the fabric treated by the dopamine-containing solution without HDTMS had a hydrophilic surface with a CA of zero degree to water (see the ESI†). When fabric was treated by a HDTMS–water suspension without DA, a hydrophilic surface still resulted with CA of 0° (see ESI†), which is different from previous reports where solid surfaces after treatment with HDTMS–ethanol become hydrophobic.42,43 The unusual result from our experiment was attributable to the solvent water, which phase-separates from HDTMS and prevents the HDTMS molecules from spreading on the fiber substrate. To verify this explanation, we did a similar experiment but used ethanol as solvent. As expected, the fabric after treatment with HDTMS–ethanol mixture showed a hydrophobic surface.
It was noted that the DA/HDTMS molar ratio in the coating solution affected the hydrophobicity of the coated fabrics. Fig. 1f shows the effect of DA/HDTMS molar ratio on water CA and SA of the coated fabrics. When DA/HDTMS was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol mol−1), the CA was below 150° and SA above 20°. When the molar ratio was changed to 2
1 (mol mol−1), the CA was below 150° and SA above 20°. When the molar ratio was changed to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol mol−1), the CA increased to 152°, and SA reduced to 15°. The best repellency result (CA = 163°, and SA = 8.6°) was found on the fabric treated by the solution of DA/HDTMS of 1
1 (mol mol−1), the CA increased to 152°, and SA reduced to 15°. The best repellency result (CA = 163°, and SA = 8.6°) was found on the fabric treated by the solution of DA/HDTMS of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol mol−1). However, when the DA/HDTMS ratio was 1
1 (mol mol−1). However, when the DA/HDTMS ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (mol mol−1), the repellency was slightly decreased. These results can be explained by the effect of the two components on the surface energy and roughness. It is known that the superhydrophobicity is affect by surface energy and roughness. PDA is hydrophilic whereas HDTMS can form a hydrophobic surface. Changing the molecular ratio of DA/HDTMS could lead to change of these two factors. Increasing the HDTMS portion would reduce the surface energy. However, the roughness could be reduced if the HDTMS portion is high enough, because the extra silane fills the inter-particle gap. A similar result was also reported on other coating systems.44 Therefore, in the later experiments, we chose the DA/HDTMS ratio of 1
3 (mol mol−1), the repellency was slightly decreased. These results can be explained by the effect of the two components on the surface energy and roughness. It is known that the superhydrophobicity is affect by surface energy and roughness. PDA is hydrophilic whereas HDTMS can form a hydrophobic surface. Changing the molecular ratio of DA/HDTMS could lead to change of these two factors. Increasing the HDTMS portion would reduce the surface energy. However, the roughness could be reduced if the HDTMS portion is high enough, because the extra silane fills the inter-particle gap. A similar result was also reported on other coating systems.44 Therefore, in the later experiments, we chose the DA/HDTMS ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mol mol−1) for all coating treatments.
1 (mol mol−1) for all coating treatments.
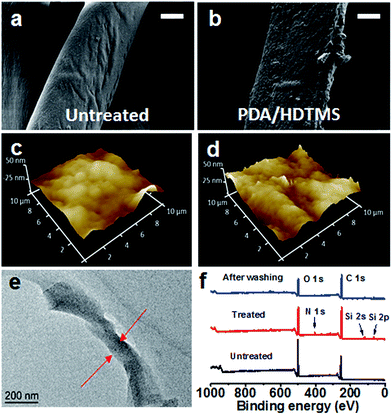
Fig. 2a and b show SEM images of the cotton fabric before and after coating treatment with dopamine/HDTMS. The coated fiber was slightly increased in roughness. AFM imaging confirmed the surface roughness increased after treatment, with the root mean square (RMS) roughness increasing from 9.0 nm to 17.3 nm (Fig. 2c and d). In some fiber surfaces, the small particles aggregated to larger particles which look like loosely adhered to the fiber surface, this does not have a large influence on the superhydrophobicity. The coating thickness was measured by cross-sectional TEM imaging, being approximately 150 nm (Fig. 2e).
The chemical composition of the cotton fabrics before and after DA/HDTMS coating treatment was examined by FTIR and XPS spectra. After dopamine/HDTMS coating, the weak peaks at 1614 cm−1 and 1560 cm−1 appeared in the FTIR spectra (ESI†), which were ascribed to the overlap of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C resonance vibrations in the aromatic rings and in-plane vibration mode of the N–H and C–N bonds, respectively.45–48 The occurrence of vibration bands at 810 cm−1, 1430 cm−1, 2855 cm−1 and 2929 cm−1 was assigned to the Si–C stretching, –CH2– bending, –CH2– stretching and CH3– stretching of HDTMS.49,50 The region of 1028 cm−1 was attributed to the asymmetric stretching vibration of Si–O–Si bonds.51 Fig. 2f shows the XPS survey spectra of the cotton fabrics. The presence of a weak N 1s peak, Si 1s peak and Si 2p peak confirmed that PDA and HDTMS were successfully coated on the fiber surface.52
C resonance vibrations in the aromatic rings and in-plane vibration mode of the N–H and C–N bonds, respectively.45–48 The occurrence of vibration bands at 810 cm−1, 1430 cm−1, 2855 cm−1 and 2929 cm−1 was assigned to the Si–C stretching, –CH2– bending, –CH2– stretching and CH3– stretching of HDTMS.49,50 The region of 1028 cm−1 was attributed to the asymmetric stretching vibration of Si–O–Si bonds.51 Fig. 2f shows the XPS survey spectra of the cotton fabrics. The presence of a weak N 1s peak, Si 1s peak and Si 2p peak confirmed that PDA and HDTMS were successfully coated on the fiber surface.52
Durability is an important requirement for superhydrophobic fabrics. The washing durability of the treated fabric was examined by reference of the washing method specified in the AATCC (American Association of Textile Chemists and Colorists) Test Method 61-2006 test no. 2A. For the PDA/HDTMS coated fabrics, the superhydrophobicity maintained even after 20 cycles of AATCC standard washing. The fabric still maintained superhydrophobicity with a water CA of 150° and a sliding angle of 15.6°. Such 20 cycles of accelerated ageing test was equivalent of 100 cycles of home laundries.21 The SEM image confirmed that the coated fabric after multiple cycles of laundering had little change in surface morphology, though led to the loss of certain particles from the fiber surface (see the ESI†). The fabric shows high hydrophobicity as long as the fabric surface was covered with PDA/HDTMS. After repeated washing, the XPS spectra (Fig. 2f) show that the intensity of C, O, and Si reduced, confirming the partial loss of the coating materials from the fiber surface.
The abrasion durability was evaluated by the Martindale method using untreated fabric to simulate actual damage. On cotton fabric, the coating can withstand 500 abrasion cycles without losing its superhydrophobicity. Further increasing the abrasion cycles resulted in decrease of water repellency. However, after 4000 abrasion cycles, the fabric was still hydrophobic with water CA above 90° (see the ESI†).
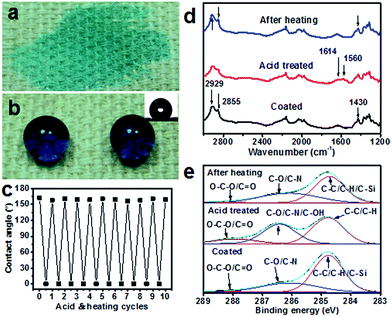
It was interesting to note that after being coated with PDA/HDTMS the fabric showed a self-healing ability to recover superhydrophobicity after being damaged by acid/alkali etching or oxygen plasma. When the PDA/HDTMS coated fabric was immersed in an acidic solution (e.g. pH < 4) for 10 minutes, rinsed with deionized water, and then dried at room temperature, it turned hydrophilic (CA = 0°) (Fig. 3a). After heating the sample at 140 °C for 10 min, however, the acid etched fabric restored the water-repellency with a CA increasing to 154° (Fig. 3b). Such a self-healing property was repeatable and can work for at least 30 cycles.
To find out the source of the CA change during acid etching and heating treatment, we examined the surface topology and chemistry. The acid etching and heating showed little effect on the surface morphology of the coated fibers (see the SEM images in ESI†). After etched in the acid solution, the peaks at 1430 cm−1, 2929 cm−1 and 2855 cm−1 in the FTIR spectra decreased in intensity (Fig. 3d). After heating treatment, the peaks at 1430 cm−1, 2928 and 2855 cm−1 re-emerged. These results indicate that the acid treatment leads to increase of the PDA composition on the coating surface. The XPS high resolution C 1s spectra showed that the C–O/C–OH and O–C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks increased, whereas the C–C/C–H/C–Si peak decreased after acid treatment (Fig. 3e), confirming the loss of HDTMS on the coating surface. After heating treatment, an opposite trend appeared for the C–C/C–H/C–Si, C–O/C–N/C–OH and O–C–O/C
O peaks increased, whereas the C–C/C–H/C–Si peak decreased after acid treatment (Fig. 3e), confirming the loss of HDTMS on the coating surface. After heating treatment, an opposite trend appeared for the C–C/C–H/C–Si, C–O/C–N/C–OH and O–C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks, the reduced carbonyl group was assumedly due to the migration of HDTMS to the surface as indicated by the increase in Si content (see Table S2 in the ESI†). The increased HDTMS content on the coating surface would lead to reduction in surface energy, which could be the main reason leading to restoring the superhydrophobicity.
O peaks, the reduced carbonyl group was assumedly due to the migration of HDTMS to the surface as indicated by the increase in Si content (see Table S2 in the ESI†). The increased HDTMS content on the coating surface would lead to reduction in surface energy, which could be the main reason leading to restoring the superhydrophobicity.
Apart from self-healing against acid etching, the coated fabric also showed a similar self-healing feature against etching in an alkaline solution (e.g. pH > 12) and air plasma treatment (see ESI†). The alkali treatment showed almost no effect on the surface morphology of the coated fibers, and its effect on surface chemistry in a similar way to the acid etching (see the ESI†). For plasma treatment, the treated surface still maintained a rough surface, except that oxygen-containing polar groups were introduced into the coating surface layer, and the heating treatment led to disappearance of these groups (see the ESI†).
In our previous papers,22 we have already reported some liquid repellent fabrics with self-healing property against plasma treatment. The self-healing was explained by the lowering the surface energy due to the rotation or migration of low surface energy substrates onto the surface where the surface free energy was increased due to the introduction of oxygen-containing high polar groups. However, superhydrophobic surface with self-healing ability against acid/alkali etching has not been reported in research literature. As far as we know, our PDA/HDTMS coated self-healing superhydrophobic fabrics represent the first of their type.
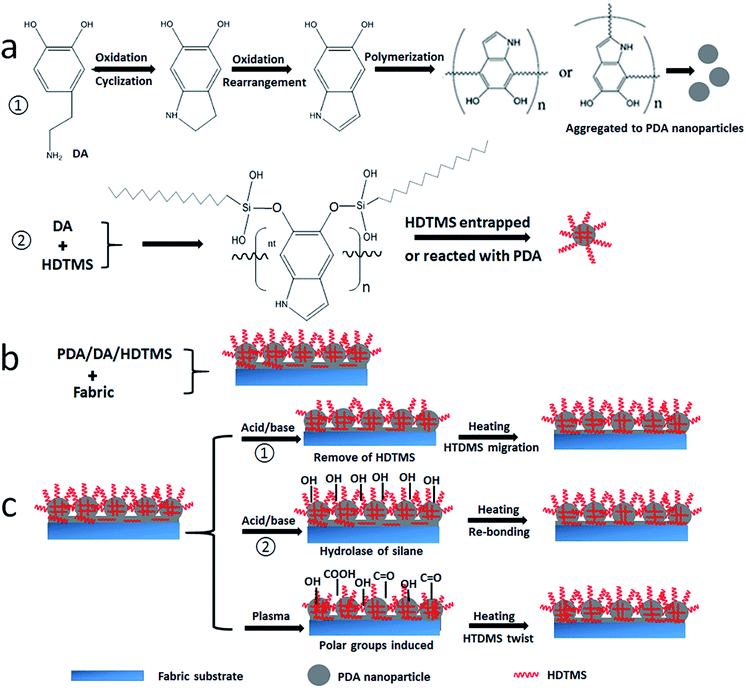
According to our experiment results, we proposed the mechanism of the coating formation and self-healing against acid/base/plasma etching, as illustrated in Fig. 4. Dopamine molecules oxidated to form dopamine quinone, and they then formed 5, 6-dihydroxyindole and 5, 6-indolequinone; 5, 6-dihydroxyindole and 5, 6-indolequinone could undergo reactions at the positions of 2, 3, 4, and 7, leading to multiple isomers and even oligomers (Fig. 4a).53 When HDTMS is added to the solution, its hydrolysis led to reaction of silanol with dopamine through hydroxyl groups. The unhydrolyzed HDTMS can be adsorbed on the PDA particles. As a result, the HDTMS molecules are trapped in the PDA and also adsorbed on the particle surface.
When fabric substrate is immersed in the solution, the above reactions also take place on the fiber surfaces. It is known that polydopamine can bond to almost all the solid surface through hydrogen bond, chelate action, ionic bond, covalent bond and physical interactions.54 The formation of a thin layer of polydopamine increases the affinity to polydopamine particles. When PDA particles deposit on the PDA coated fiber substrate, further reaction with dopamine facilitates to bridge the PDA particles with the PDA coating layer (see the illustration in Fig. 4b), therefore enhancing the adhesion.
Here, it should be pointed out that the deposition of PDA particles on fiber surface leads to increase in surface roughness. PDA is hydrophilic. PDA only cannot form a hydrophobic coating. When HDTMS is added to PDA, the long alkyl chain brought by the HDTMS molecule largely reduces the surface free energy, leading to superhydrophobic surfaces.
When the coated fabric was subjected to an oxygen plasma, high polarity groups (e.g. hydroxyl groups, carbonyl groups and carboxyl groups) were introduced on to the coating surface, which largely reduced the surface free energy (Fig. 4c). When being heated, the molecules in the coating layers increase the mobility. The chain twist and migration allow the low surface energy chains to expose to the surface, which reduces the surface free energy, hence restoring the superhydrophobicity. For the acid and base treatment, in one option, the silane molecules could be removed from the coating surface. This leads to increase in the PDA, turning the surface hydrophilic. Upon heating, however, the HDTMS molecules trapped in the PDA migrate to surface, reducing the surface free energy. The acid and base treatment could also lead to breakage of crosslinked silanes, forming hydroxyl groups and making the fabric hydrophilic. When the fabric is heated at 140 °C, the hydrolysed molecules are re-bonded, making the fabric hydrophobic. Therefore, the self-healing against acid/base etching follows a slightly different mechanism for the plasma treatment.
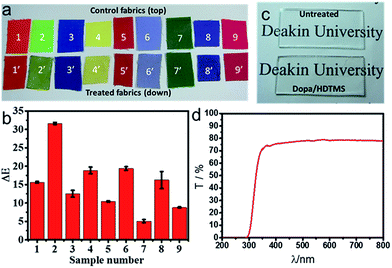
Coatings prepared from polydopamine are known to have a dark appearance. The polydopamine coating was brown in color (see the ESI†). To examine the effect of the PDA/HDTMS coating on fabric appearance, we used cotton fabric of different colors as substrates. Fig. 5a shows the photo of the fabrics before and after the DA/HDTMS treatment. When applied on dark color fabrics, the coating has a small effect on the fabric color. Therefore, for practical applications, the polydopamine coatings are more suitable for treatment of dark colored fabrics, such as brown, black, dark blue and dark green. Fig. 5b shows the color difference (ΔE) of the fabrics before and after treatment. ΔE can be expressed by the formula: ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2]1/2, where L*, a*, b* are lightness/darkness, red or green chroma, and yellow/blue chroma, respectively. The samples no 5, 7 and 9 showed smaller ΔE than the other samples, suggesting that the DA/HDTMS coating showed less effect on deep red, deep green and deep pink than other background colors (see the ESI†). Fig. 5c shows PDA/HDTMS coating on glass surface, which looks transparent. The transmittance spectrum, shown in Fig. 5d, indicates that the coating has a nearly 80% transmittance in the near UV and VIS region (wavelength 350–800 nm).
The DA/HDTMS coating treatment was also suitable for other types of fabrics, including wool and polyester fabrics. When commercial wool and polyester fabrics were treated by the dopamine/HDTMS solution, they both showed superhydrophobicity regardless of the original surface wettability, and the coatings had high washing durability as well (see the CA and washing results in the Table S2 in ESI†).
In the previous reports, the PDA was synthesized in an aqueous solution with pH typically at 8.5.41 We also prepared a coating solution at the alkaline condition (pH = 8.5), and used the solution for treatment of fabrics. In comparison with the DA/HDTMS solution synthesized in neutral condition (12 hour), the one prepared at pH = 8.5 looked darker, and the PDA/HDTMS particles formed showed a dual dispersity (size 220 nm and 780 nm), and after coating treatment, the treated fabric became uneven in color (see the ESI†). These issues were attributed to the fast polymerization of dopamine at an alkaline condition, and aggregation of the PDA particles in solution.
4. Conclusions
We have prepared superhydrophobic fabrics by co-deposition of dopamine with an alkyl silane in neutral aqueous solution. The treated fabric has a water contact angle as high as 163°. The fabrics show high washing durability that can withstand over 20 cycles of AATCC standard washing without losing the superhydrophobicity. The coating is self-healable, in that the superhydrophobicity can be restored by heat treatment after acid/base etching or plasma damage. The coating has a reasonably small effect on fabric color. Such fluorine-free durable coatings may be useful for development of various superhydrophobic fabric products.Acknowledgements
Funding support from Australian Research Council through a discovery project (ARC DP150100406) is acknowledged.Notes and references
- X. Deng, L. Mammen, H. J. Butt and D. Vollmer, Science, 2011, 335, 67–70 CrossRef PubMed.
- A. Tuteja, W. Choi, M. Ma, J. M. Mabry, S. A. Mazzella, G. C. Rutledge, G. H. McKinley and R. E. Cohen, Science, 2007, 318, 1618–1622 CrossRef CAS PubMed.
- X. M. Li, D. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 1350–1368 RSC.
- F. Zhang, L. Zhao, H. Chen, S. Xu, D. G. Evans and X. Duan, Angew. Chem., Int. Ed., 2008, 47, 2466–2469 CrossRef CAS PubMed.
- X. Yao, Y. Song and L. Jiang, Adv. Mater., 2011, 23, 719–734 CrossRef CAS PubMed.
- L. Mishchenko, B. Hatton, V. Bahadur, J. A. Taylor, T. Krupenkin and J. Aizenberg, ACS Nano, 2010, 4, 7699–7707 CrossRef CAS PubMed.
- Y. Wang, C. E. Sims, P. Marc, M. Bachman, G. Li and N. L. Allbritton, Langmuir, 2006, 22, 8257–8262 CrossRef CAS PubMed.
- E. Galopin, G. Piret, S. Szunerits, Y. Lequette, C. Faille and R. Boukherroub, Langmuir, 2009, 26, 3479–3484 CrossRef PubMed.
- D. Qúeŕe, Rep. Prog. Phys., 2005, 68, 2495–2532 CrossRef.
- L. Feng, Z. Zhang, Z. Mai, Y. Ma, B. Liu, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 2012–2014 CrossRef CAS PubMed.
- H. Wang, H. Zhou, H. Niu, J. Zhang, Y. Du and T. Lin, Adv. Mater. Interfaces, 2015, 2, 1400506 CrossRef.
- A. Scardino, R. De Nys, O. Ison, W. O'Connor and P. Steinberg, Biofouling, 2003, 19, 221–230 CrossRef PubMed.
- L. D. Chambers, K. R. Stokes, F. C. Walsh and R. J. Wood, Surf. Coat. Technol., 2006, 201, 3642–3652 CrossRef CAS.
- H. J. Lee, J. Mater. Sci., 2012, 47, 5114–5120 CrossRef CAS.
- B. Mahltig and H. Böttcher, J. Sol-Gel Sci. Technol., 2003, 27, 43–52 CrossRef CAS.
- T. Bahners, T. Textor, K. Opwis and E. Schollmeyer, J. Adhes. Sci. Technol., 2008, 22, 285–309 CrossRef CAS.
- Q. Truong and E. Wilusz, Smart Textiles for Protection, 2012 Search PubMed.
- P. Gibson, Text. Res. J., 1993, 63, 749–764 CrossRef CAS.
- Y. Li, L. Li and J. Sun, Angew. Chem., 2010, 122, 6265 (Angew. Chem., Int. Ed., 2010, 49, 6129–6133) CrossRef.
- J. D. Samuel, S. Jeyaprakash and J. Ruehe, Langmuir, 2004, 20, 10080–10085 CrossRef CAS PubMed.
- H. Wang, Y. Xue, J. Ding, L. Feng, X. Wang and T. Lin, Angew. Chem., Int. Ed., 2011, 50, 11433–11436 CrossRef CAS PubMed.
- H. Zhou, H. Wang, H. Niu, A. Gestos and T. Lin, Adv. Funct. Mater., 2013, 23, 1664–1670 CrossRef CAS.
- H. Wang, H. Zhou, A. Gestos, J. Fang and T. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 10221–10226 CAS.
- H. Zhou, H. Wang, H. Niu, J. Fang, Y. Zhao and T. Lin, Adv. Mater. Interfaces, 2015, 2, 1400559 CrossRef.
- Y. Liu, Z. Liu, Y. Liu, H. Hu, Y. Li, P. Yan, B. Yu and F. Zhou, Small, 2015, 11, 426–431 CrossRef CAS PubMed.
- X. Zhang, T. Geng, Y. Guo, Z. Zhang and P. Zhang, Chem. Eng. J., 2013, 231, 414–419 CrossRef CAS.
- H. Hu, L. Gao, C. Chen and Q. Chen, Environ. Sci. Technol., 2014, 48, 2928–2933 CrossRef CAS PubMed.
- H. Yan, H. Zhou, Q. Ye, X. Wang, C. M. Cho, A. Y. X. Tan and J. Xu, RSC Adv., 2016, 6, 66834–66840 RSC.
- C. Cao, M. Ge, J. Huang, S. Li, S. Deng, S. Zhang, Z. Chen, K. Zhang and S. Salem, J. Mater. Chem. A, 2016, 4, 12179–12187 CAS.
- C. Xue, S. Jia, J. Zhang, L. Tian, H. Z. Chen and M. Wang, Sci. Technol. Adv. Mater., 2008, 9, 035008 CrossRef PubMed.
- M. Wu, N. An, Y. Li and J. Sun, Langmuir, 2016, 32, 12361–12369 CrossRef CAS PubMed.
- Q. Zeng, C. Ding, Q. Li, W. Yuan, Y. Peng, J. Hu and K. Zhang, RSC Adv., 2017, 7, 8443–8452 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- B. P. Lee, J. L. Dalsin and P. B. Messersmith, Biomacromolecules, 2002, 3, 1038–1047 CrossRef CAS PubMed.
- B. P. Lee, C.-Y. Chao, F. N. Nunalee, E. Motan, K. R. Shull and P. B. Messersmith, Macromolecules, 2006, 39, 1740–1748 CrossRef CAS.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- E. Herlinger, R. F. Jameson and W. Linert, J. Chem. Soc., 1995, 2, 259–263 Search PubMed.
- L. A. Burzio and J. H. Waite, Biochemistry, 2000, 39, 11147–11153 CrossRef CAS PubMed.
- Y. Li, M. Liu, C. Xiang, Q. Xie and S. Yao, Thin Solid Films, 2006, 497, 270–278 CrossRef CAS.
- F. Bernsmann, V. Ball, F. Addiego, A. Ponche, M. Michel, J. J. D. A. Gracio, V. Toniazzo and D. Ruch, Langmuir, 2011, 27, 2819–2825 CrossRef CAS PubMed.
- Y. Liu, Y. Liu, H. Hu, Z. Liu, X. Pei, B. Yu, P. Yan and F. Zhou, J. Phys. Chem. C, 2015, 119, 7109–7114 CAS.
- J. Liu, W. Huang, Y. Xing, R. Li and J. Dai, J. Sol-Gel Sci. Technol., 2011, 58, 18–23 CrossRef CAS.
- M. Shateri-Khalilabad and M. Yazdanshenas, Cellulose, 2013, 20, 3039–3051 CrossRef CAS.
- C. Zeng, H. Wang, H. Zhou and T. Lin, RSC Adv., 2015, 5, 61044–61050 RSC.
- B. Prasad, A. Srivastava and M. Tiwari, J. Colloid Interface Sci., 2013, 396, 234–241 CrossRef CAS PubMed.
- M. Martin, P. Salazar, R. Villalonga, S. Campuzano, J. Pingarron and J. Gonzalez-Mora, J. Phys. Chem. B, 2014, 2, 739 CAS.
- J. Qiu, W. Zhou, J. Guo, R. Wang and R. Liang, Anal. Biochem., 2009, 385, 264–269 CrossRef CAS PubMed.
- S. Pande, S. Jana, A. Sinha, S. Sarkar, M. Basu, M. Pradhan, A. Pal, J. Chowdhury and T. Pal, J. Phys. Chem. C, 2009, 113, 6989–7002 CAS.
- J. H. Yim, V. Rodriguez, A. A. Williams and J. K. Hirvonen, Surf. Coat. Technol., 2013, 234, 21–32 CrossRef CAS.
- I. Woodward, W. C. E. Schofield, V. Roucoules and J. P. S. Badyal, Langmuir, 2003, 19, 3432–3438 CrossRef CAS.
- X. Wang, S. Xu, Y. Tan, J. Du and J. Wang, Carbohydr. Polym., 2016, 140, 188–194 CrossRef CAS PubMed.
- Y. Li, Q. Chen, M. Yi, X. Zhou, X. Wang, Q. Cai and X. Yang, Appl. Surf. Sci., 2013, 274, 248–254 CrossRef CAS.
- F. Bernsmann, V. Ball, F. Addiego, A. Ponche, M. Michel, J. J. D. A. Gracio, V. Toniazzo and D. Ruch, Langmuir, 2011, 27, 2819–2825 CrossRef CAS PubMed.
- D. Chai, Z. Xie, Y. Wang, L. Liu and Y. J. Yum, ACS Appl. Mater. Interfaces, 2014, 6, 17974–17984 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Photos, figures, SEM, FTIR, data colour, XPS. See DOI: 10.1039/c7ra04863g |
| This journal is © The Royal Society of Chemistry 2017 |