Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical hydrogenated TiO2 nanotube arrays decorated with 3D cotton-like porous MnO2 enables superior supercapacitive performance†
Jiaqin Liu*acd,
Juan Xuab,
Yan Wangac,
Jiewu Cuiac,
Hark Hoe Tan d and
Yucheng Wu
d and
Yucheng Wu *ac
*ac
aSchool of Materials Science and Engineering, Hefei University of Technology, Hefei 230009, China. E-mail: jqliu@hfut.edu.cn
bSchool of Chemistry and Chemical Engineering, Hefei University of Technology, Hefei 230009, China
cKey Laboratory of Advanced Functional Materials and Devices of Anhui Province, Hefei 230009, China
dDepartment of Electronic Materials Engineering, Research School of Physics and Engineering, The Australian National University, Canberra, ACT 2601, Australia
First published on 20th June 2017
Abstract
Highly ordered TiO2 nanotube arrays (TNTAs) have shown great promise to serve as an efficient current collector as well as an outstanding support for the application of constructing high performance supercapacitor electrode materials. In this study, a novel-structured MnO2/EH-TNTAs electrode with superior supercapacitive performance was developed by galvanostatic electrodeposition of MnO2 nanoflakes onto both the outer and inner walls of electrochemically hydrogenated TNTAs (EH-TNTAs). The as-fabricated MnO2/EH-TNTAs electrode could achieve a specific capacitance of up to 650.0 F g−1 at 1.0 A g−1 with 86.9% of the initial capacitance remaining after 5000 charge/discharge cycles at 5 A g−1, outperforming other reported TNTAs-based electrodes. The prominent supercapacitive performance of MnO2/EH-TNTAs electrode could be attributed to the unique 3D cotton-like porous structure and high specific surface area of MnO2 deposit as well as the remarkably improved electrical conductivity and electrochemical performances of EH-TNTAs induced by the introduction of oxygen vacancies during the electrochemical hydrogenation process. This work offers theoretical insight and practical guidelines for TNTAs-based electrodes applied for high-performance supercapacitors as well as other energy storage devices.
Introduction
Supercapacitors (SCs), also called electrochemical capacitors, store energy using either reversible ion adsorption (electrical double layer capacitors, EDLCs) or fast surface redox reactions (pseudo-capacitors, PCs). They can fill the gap between batteries and conventional solid state and electrolytic capacitors due to their high power density, rapid charge/discharge rates and long cycling life. Compared to the EDLCs, whose capacitances are restricted by the limited charge accumulation in the electrical double layer, PCs achieved substantially much higher specific capacitances through surface redox reactions, and thus, have a potential to meet the requirements of future energy storage systems.1–4 Electrode materials are the most critical component determining the performance and the cost of the SCs. For EDLCs, carbon-based materials are commonly used as electrodes due to their high electrical conductivity, long-term electrochemical stability and moderate cost, while for PCs, conducting polymers and transition metal oxides, hydroxides and sulfides are the two main categories of pseudo-capacitive active materials. Many kinds of conducting polymers (e.g., polyaniline,5,6 polypyrrole7,8 and their derivatives) have been widely studied as pseudo-capacitive materials and have shown high gravimetric and volumetric pseudo-capacitance in various non-aqueous electrolytes. When used as bulk materials, conducting polymers generally suffered from a limited cycle stability. Recently, transition metal oxides,9–12 hydroxides13,14 and sulfides15–18 received more attentions owing to their high specific pseudo-capacitance and low-cost, but suffered from the high electrical resistance, resulting in the low power density. At present, nanostructuring redox-active materials to increase pseudo-capacitance and building hybrid systems to achieve high energy density are the two most effective strategies to develop high performance electrode materials for SCs application.MnO2 is one of the most promising pseudo-capacitive materials due to its high theoretical capacity of ∼1400 F g−1, wide operating potential window, low-cost, natural abundance and environmental compatibility.19–21 However, the actual value of specific capacitance is deviated far from the theoretical value, which is ascribed to its poor electrical conductivity and slow ion diffusion rate. To overcome this limitation, deposition of nanostructured pseudo-capacitive MnO2 onto highly ordered, high-surface-area and high conductivity nano-frameworks (acting as current collectors) such as carbon nanotubes22,23 and nanofibers,24,25 graphene,26,27 and transition metal oxides nanostructures28–31 etc., has been well demonstrated to be an effective and promising approach.
Highly ordered TiO2 nanotube arrays (TNTAs) anodized from Ti foils hold great promise as supercapacitor electrode materials owing to their high-surface-area, chemical stability, direct electron transport pathways and simple fabrication. Moreover, TNTAs may serve as a good support for nano-structured high specific capacitance materials to form hybrid systems. However, the poor electric conductivity of TNTAs that derives from the n-type semiconductor nature rendered them not the ideal current collectors for the application of constructing high performance SCs. In our previous work, annealing in H2-contained atmosphere32 and carbon layer33 modification of TiO2 nanotubes (NTs) have demonstrated electrical conductivity enhancement of dozens of times than that of the pristine TNTAs. Then Nickel oxide,32 Cu2O34 and Co3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 layer were successfully loaded onto both the inner and outer walls of TiO2 NTs for constructing various TNTAs-based electrodes with high supercapacitive performance.
35 layer were successfully loaded onto both the inner and outer walls of TiO2 NTs for constructing various TNTAs-based electrodes with high supercapacitive performance.
Herein, a novel-structured MnO2/EH-TNTAs electrode with superior supercapacitive performance was developed by galvanostatic electrodepositing MnO2 nanoflakes (NFs) onto both the outer and inner walls of electrochemically hydrogenated TNTAs (EH-TNTAs). The as-fabricated MnO2/EH-TNTAs electrode yields a specific capacitance of up to 650.0 A g−1 at 1.0 A g−1 with 86.9% of the initial capacitance remaining after 5000 charge/discharge cycles at 5 A g−1, outperforming the best performance of reported MnO2–TNTAs hybrids. Now that facile and cost-effective fabrication of high performance MnO2/EH-TNTAs electrode for SCs application could be achieved via a full electrochemical approach. More importantly, impacts of different strategies for TNTAs hydrogenation and MnO2 depositing on the electrochemical performance of the resulting MnO2/TNTAs electrodes as well as the corresponding mechanism are also proposed in detail. Our research results will provide theoretical and technical guideline for the application of TNTAs-based electrode in the SCs.
Experimental section
Preparation of TNTAs
Highly ordered and well-separated TNTAs were directly grown on a Ti foil (0.1 mm, 99.7%) using electrochemical anodization. Prior to anodization, Ti foils were ultrasonically cleaned in acetone, ethanol, and deionized water, respectively, for 20 min. Electrochemical anodization was conducted on a two-electrode setup using a DC voltage of 60 V for 6 h in ice bath. A Ti foil was used as the working electrode, a graphite foil as the cathode, a solution of 0.25 M NH4F in ethylene glycol with 8 vol% H2O as the electrolyte. After anodization, the as-fabricated amorphous TNTAs were annealed in air at 500 °C for 2 h with a heating rate of 2 °C min−1, and the corresponding sample was named as air-TNTAs.Electrochemical hydrogenation of TNTAs
Electrochemical hydrogenation of TNTAs to obtain the EH-TNTAs was conducted on a two-electrode setup using a DC voltage of 4 V for 20 min at room temperature. The as-fabricated air-TNTAs sample was used as the cathode, a graphite foil as the anode, and an aqueous solution of 0.1 M Na2SO4 as the electrolyte. For comparison, hydrogenation was also conducted by means of annealing the amorphous TNTAs in H2-contained atmosphere (10% H2 + 90% Ar), and the corresponding sample was labelled as H2-TNTAs.Preparation of MnO2/EH-TNTAs
A galvanostatic electrodeposition (GED) approach was adopted for the fabrication of MnO2/EH-TNTAs composites. In a typical synthesis, MnO2 depositing was performed on a three-electrode system at 0.5 mA cm−2 for 120 s with the EH-TNTAs, a Pt wire, and a Ag/AgCl (saturated KCl) electrode as working, counter, and reference electrodes, respectively, using a 0.01 M MnSO4 aqueous solution as electrolyte. Actually, impacts of current density, depositing time as well as the electrolyte concentration on the microstructure, morphology and pseudocapacitive performance of MnO2/EH-TNTAs were systematically studied in our research work. Owing to space limitation, we will discuss in detail elsewhere. MnO2/EH-TNTAs electrode discussed in this work was fabricated with optimized parameters. The fabrication process of electrode materials is illustrated in Fig. 1.For comparison, MnO2/air-TNTAs and MnO2/H2-TNTAs were also prepared by galvanostatic electrodepositing MnO2 onto the walls of air-TiO2 NTs and H2–TiO2 NTs respectively. Furthermore, MnO2 was also deposited onto both the bare Ti foil and the walls of EH-TiO2 NTs using our previously reported chemical bath deposition (CBD) technique,36 and the resulting samples were labelled as MnO2/Ti and EH-TNTAs@MnO2 respectively.
Material characterization
Morphologies were observed using FESEM (SU-8020, operated at 5.0 kV) and TEM (JEM-2100F, operated at 200 kV) equipped with an Oxford INCA energy dispersive X-ray (EDX) analyser. Structural features of as-prepared samples were identified by XRD (Rigaku D/Max-2500 V) and Raman spectroscopy (LabRAM HR Evolution). The chemical constituents were investigated by XPS (ESCALAB250Xi). Nitrogen adsorption isotherms were conducted at 77.15 K on a SA3100 Micromeritics analyzer after degassing samples at 373.15 K for 2 h, and the BET surface area was estimated in a relative pressure range from 0.05 to 0.2.Electrochemical evaluation
All electrochemical measurements were carried out on an electrochemical workstation (Autolab PGSTAT302N) in a three-electrode system using the tested sample, a Pt wire, an Ag/AgCl (3 M KCl) electrode as the working, counter, and reference electrodes, respectively, and using 0.5 M Na2SO4 aqueous solution as electrolyte. The electrochemical properties of the tested samples were investigated by cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) testing. Electrochemical impedance spectroscopy (EIS) measurements were conducted in the frequency ranging from 10 mHz to 100 kHz at open-circuit potential with a AC-voltage amplitude of 5 mV. The cycling stability of the electrode was evaluated by GCD at 5 A g−1 for 5000 cycles.Results and discussion
Highly ordered and well-separated TiO2 NTs were fabricated by anodization of Ti foils, and then annealed in air. The as-formed TiO2 NTs have a uniform diameter of ∼180 nm, a wall thickness of ∼25 nm (Fig. 2a) and a length of ∼10 μm (Fig. S1†). The obvious difference to the conventional TNTAs is that the TiO2 NTs are free-standing and well-separated from each other with intertube spacings ranging from 8 to 20 nm. The free space among the tubes allows for more exposed outer surface for the deposition of a secondary material as well as efficient mass transport. After electrochemical hydrogenation (EH), the obtained EH-TNTAs display no differences in tubular structure compared to air-TNTAs (Fig. 2b). Worth mentioning here is that the EH-TNTAs evidence a dark blue colour versus the grey air-TNTAs, indicating a strong absorption in the visible region due to hydrogen diffusion into TiO2 lattice during the EH process. All microstructures of EH-TNTAs enable them to serve as a good support for the capacitive active materials to form composite structures. Besides, XRD patterns of both air-TNTAs, and EH-TNTAs show almost the same diffraction patterns (Fig. 2d), and all diffraction peaks could be well indexed to the characteristic peaks of anatase TiO2 (JCPDS#21-1272) except for the Ti peaks. That means amorphous structure transforms to anatase phase upon thermal treatment, while no phase transformation occurs during the EH process. Then, depositing MnO2 onto EH-TiO2 NTs was achieved via GED approach (Fig. 2c and S1†). FESEM analysis revealed that both the outer and inner walls of the EH-TiO2 NTs were fully and uniformly coated with large amounts of tiny NFs. These NFs intersect with each other, thus constructing a 3D porous cotton-like structure. Moreover, the tube-mouths were not blocked by MnO2 deposit and the intertube spacings remained interconnected. TEM observation further revealed that compare to the smooth surface of EH-TiO2 NTs, surface of MnO2/EH-TiO2 NTs became rough and bumpy due to the full dispersion of tiny MnO2 NFs (Fig. 3a). In HRTEM image (Fig. 3b), d space of 3.50 Å corresponding to the (101) planes of anatase TiO2 could be observed clearly, while no well-defined lattice fringes for MnO2 crystal. This demonstrated the high crystallinity for TNTAs but amorphous or low crystallinity for MnO2. XRD analysis also confirmed the amorphous structure for MnO2. No identifiable peaks related to MnO2 crystal emerge in the MnO2/EH-TNTAs pattern. Energy-dispersive X-ray spectrometry (EDS) mapping analysis for a hybrid single nanotube (Fig. 3c–f) further confirmed the MnO2/EH-TNTAs hybrid with elements Ti, O, and Mn resulting from TiO2 NTs and MnO2 NFs. More dense element distribution of Ti and O than that of Mn indicates the element Mn resulting from MnO2 fully and uniformly distributed on the surface of EH-TiO2 NTs.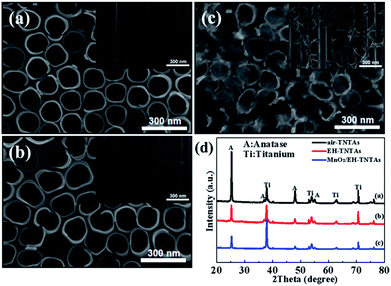 | ||
| Fig. 2 FESEM images of (a) air-TNTAs, (b) EH-TNTAs, and (c) MnO2/EH-TNTAs (insets are the corresponding side-view images), (d) XRD patterns of air-TNTAs, EH-TNTAs and MnO2/EH-TNTAs. | ||
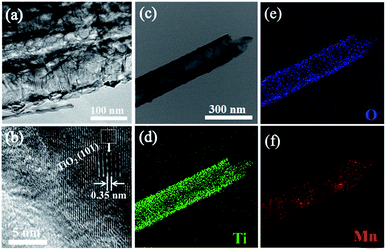 | ||
| Fig. 3 TEM (a) and HRTEM (b) images of MnO2/EH-TNTAs, TEM image of one single MnO2/EH-TiO2 NT (c) and the corresponding EDS mapping of Ti (d), O (e), Mn (f). | ||
X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy studies were conducted to reveal the effect of EH process on the chemical composition and oxidation state of TNTAs. Fig. 4a compares the normalized Ti 2p core level XPS spectra of air-TNTAs (black curve) and EH-TNTAs (red curve), together with their difference spectrum. Two peaks centered at 465.33 and 459.13 eV that correspond to the characteristic Ti 2p1/2 and Ti 2p3/2 peaks of Ti4+ are observed for both samples. In contrast to air-TNTAs, both Ti 2p1/2 and Ti 2p3/2 peaks for EH-TNTAs shift to the lower binding energy, suggesting that they have different bonding environments. By subtracting the normalized Ti 2p spectra of EH-TNTAs with air-TNTAs, two extra peaks centered at 463.78 and 458.48 eV are clearly observed. These two peaks are attributed to the characteristic Ti 2p1/2 and Ti 2p3/2 peaks of Ti3+, proving that Ti3+ sites (oxygen vacancies) are created during EH process.37–39 Same conclusion was also obtained from Raman analysis (Fig. 4b). The characteristic peaks at 145.12 cm−1 (Eg), 198.40 cm−1 (Eg), 397.32 cm−1 (B1g), 517.28 cm−1 (A1g/B1g) and 639.07 cm−1 (Eg) in the air-TNTAs are assigned to anatase TiO2. Interestingly, a slightly blue-shift of Eg peaks at 146.95 cm−1 and 199.77 cm−1 can be identified in EH-TNTAs, suggesting the increased amount of oxygen vacancies that originate from Ti4+ reduction.40–42 Moreover, O 1s XPS spectra of air-TNTAs (black curve) and EH-TNTAs (red curve) are shown in Fig. 4c. The peaks centered at 529.90 and 531.53 eV for both air-TNTAs and EH-TNTAs are attributed to Ti–O and surface Ti–OH groups.40,43,44 There is no noticeable difference in the peak at 531.53 eV between air-TNTAs and EH-TNTAs, implying that the hydrogenation process does not change the surface species of EH-TNTAs. Mn 2p XPS spectra of MnO2/EH-TNTAs reveals two peaks located at 642.3 and 654.0 eV (Fig. 4d), which are consistent with the characteristic Mn 2p3/2 and Mn 2p1/2 binding energies of MnO2 and confirm the successful MnO2 deposition.25,39,45
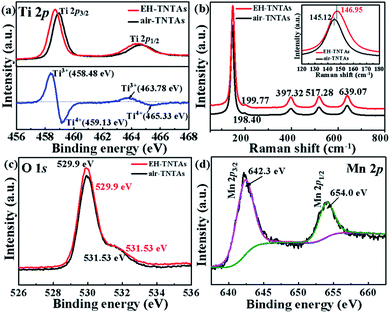 | ||
| Fig. 4 XPS spectra of (a) Ti 2p, (c) O 1s and (b) Raman spectra of air-TNTAs and EH-TNTAs, (d) XPS spectra of Mn 2p of MnO2/EH-TNTAs. | ||
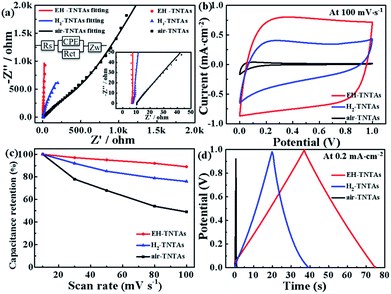
To evaluate the electrical properties of various TNTAs electrodes, EIS measurements were subsequently performed at open circuit potential with an amplitude of 5 mV. Fig. 5a shows the Nyquist plots of the air-TNTAs, H2-TNTAs and EH-TNTAs electrodes. It is obvious that EH-TNTAs electrode shows much lower impedance value than both air-TNTAs and H2-TNTAs electrodes. Moreover, note that the plot of EH-TNTAs exhibits an even steeper impedance line (nearly vertical) in comparison to H2-TNTAs, which demonstrates the superior capacitive performance for EH-TNTAs electrode. For quantitative analysis, experimental data of impedance spectra were fitted to the model depicted by the equivalent circuit (inset in Fig. 5a). In this model, Rs is the solution resistance, Rct represents the inherent resistance of the working electrode together with the charge transfer resistance through the electrolyte, the constant phase element (CPE) refers to the double layer capacitance, and Zw is the Warburg impedance. Table S1† lists the fitted parameter values for various TNTAs electrodes. The Rct for air-TNTAs is up to 1475.0 Ω, while only 279.20 and 22.38 Ω for H2-TNTAs and EH-TNTAs respectively, and the EH-TNTAs electrode has the lowest Rct. This dramatic decline in the resistance of EH-TNTAs electrode can be attributed to the greatly improved conductivity of the electrode and efficient charge carrier transport induced by the introduction of large numbers of oxygen vacancies during the EH process.
Besides, in order to investigate the effect of EH process on the electrochemical properties of various TNTAs electrodes, CV curves were first collected (from 10 to 100 mV s−1, Fig. S2†). In comparison to air-TNTAs and H2-TNTAs, EH-TNTAs electrode exhibits the best capacitive performance by delivering CV profiles with higher current density and closer to ideal rectangular shape (Fig. 5b). Areal capacitance of the electrodes as a function of scan rate were calculated and shown in Table S2.† EH-TNTAs achieves an areal capacitance up to 6.96 mF cm−2 at 100 mV s−1, which is significantly higher than that of air-TNTAs (0.18 mF cm−2) and H2-TNTAs (3.14 mF cm−2). The capacitance retentions of EH-TNTAs at different scan rates are obviously higher than that of air-TNTAs and H2-TNTAs, revealing the good rate capability (Fig. 5c). The rate capability is related to the ion diffusion rate and conductivity of the electrode. Given that the morphologies of all TNTAs electrodes are similar, they should have similar ion diffusion rate. Therefore, the improved rate capacitance in EH-TNTAs should be due to the greatly enhanced electrical conductivity. The corresponding GCD curves of different TNTAs electrodes were also collected at different current densities (Fig. S3†). Obviously that GCD curves of EH-TNTAs electrode are symmetric and substantially prolonged over the air-TNTAs and H2-TNTAs electrodes, also revealing good capacitive behavior for EH-TNTAs. Fig. 5d compares the GCD curves of these TNTAs electrodes at 0.2 mA cm−2. The areal capacitance of EH-TNTAs is calculated to be 7.5 mF cm−2, which is also substantially higher than the values obtained from the air-TNTAs (0.1 mF cm−2) and H2-TNTAs (3.8 mF cm−2). Therefore, above electrochemical analysis demonstrated that greatly enhanced electrical properties and electrochemical performance enabled EH-TNTAs to serve as an ideal current collector for the application of constructing high performance SCs.
MnO2, as one of the most promising pseudocapacitive materials with high theoretical specific capacitance was successfully loaded onto EH-TiO2 NTs using GED technique. SEM and TEM analysis show that both outer and inner surfaces of EH-TiO2 NTs are fully and uniformly coated with MnO2 NFs. In order to gain deeper insight regarding the effectiveness of TiO2 support, CV curves of different MnO2/TNTAs electrodes as well as MnO2/Ti electrode were firstly collected from 10 to 100 mV s−1, as shown in Fig. S4.† The specific capacitance were calculated and listed in Table. S3.† Obviously, the capacitances of all MnO2/TNTAs electrodes are substantially higher than the MnO2/Ti electrode, suggesting the tubular TiO2 substrate has a progressive and positive effect on the capacitive behavior of the deposited MnO2 compared to the planar Ti substrate. Futher, CV curves of different MnO2/TNTAs electrodes exhibit rectangular-like shape and good symmetry at various scan rates, revealing good capacitive behaviour. Significantly, MnO2/EH-TNTAs exhibit greatly enhanced capacitive current density compare to both MnO2/air-TNTAs and MnO2/H2-TNTAs (Fig. 6a). The specific capacitance of MnO2/EH-TNTAs achieves 558.9 F g−1 at 10 mV s−1 based on the MnO2 mass, which is approximately 1.7 and 2.5 times that of MnO2/H2-TNTAs (326.4 F g−1) and MnO2/air-TNTAs (218.8 F g−1). Furthermore, MnO2/EH-TNTAs electrode shows good rate capability with a capacitance retention of 63.52% when the scan rate increase from 10 to 100 mV s−1, while it is only 31.9% and 48.1% for MnO2/air-TNTAs and MnO2/H2-TNTAs respectively. The corresponding GCD tests were also operated at different current densities (from 1 A g−1 to 10 A g−1, Fig. S5†). GCD curves of different MnO2/TNTAs electrodes are symmetric with nearly linear slopes, indicating the high-rate capability and good reversibility. The specific capacitance of MnO2/EH-TNTAs reaches up to 650 F g−1 at 1 A g−1, which is substantially larger than the values obtained from MnO2/H2-TNTAs (388.7 F g−1), MnO2/air-TNTAs (290.7 F g−1) and MnO2/Ti (257.7 F g−1) electrodes (Fig. 6b and Table S3†). Thus, both GCD and CV results suggest that the electrochemical performance of MnO2/TNTAs composites is strongly correlated to TiO2 support. Compare to the air-TNTAs, H2-TNTAs or various reported supports for MnO2 deposition summarized in Table S4,† EH-TNTAs with superior electrical conductivity and efficient charge separation and transport was proved to be an excellent support for electrochemically active MnO2.
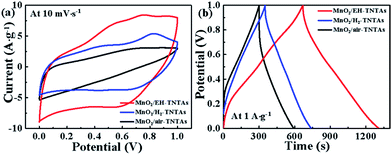 | ||
| Fig. 6 (a) CV curves at 10 mV s−1, and (b) CD curves at 1 A g−1 of the MnO2/air-TNTAs, MnO2/H2-TNTAs and MnO2/EH-TNTAs electrodes. | ||
How the MnO2 depositing technique affecting the electrochemical performance of MnO2/TNTAs composites was further explored. Compare to our reported36 EH-TNTAs@MnO2 fabricated by CBD approach, MnO2/EH-TNTAs exhibits much higher electrochemical performance, as shown in Fig. 7. The specific capacitance of MnO2/EH-TNTAs reaches up to 650 F g−1, much larger than that of EH-TNTAs@MnO2 (523.90 F g−1) at the same current density of 1 A g−1. Since both adopted the EH-TNTAs as the support, the enhanced electrochemical performance of MnO2/EH-TNTAs should be ascribed to MnO2 deposit.
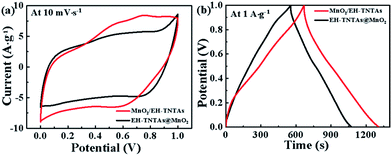 | ||
| Fig. 7 (a) CV curves at 10 mV s−1, and (b) GCD curves at 1 A g−1 of the MnO2/EH-TNTAs and EH-TNTAs@MnO2 electrodes. | ||
Lot of studies reported that how to maximize the electrochemically active sites for redox reactions is the key point to achieve high energy storage density of MnO2. Increasing specific surface area and obtaining 3D porous structures are the two most effective strategies. Microstructure characteristics of MnO2 deposited by two different techniques were firstly compared, as shown in Fig. S6.† Obviously that ultrafine MnO2 NFs in EH-TNTAs@MnO2 obtained by CBD technique are densely-packed together to form a thin layer, while MnO2 NFs in MnO2/EH-TNTAs achieved by GED technique intersect with each other, thus constructing a 3D porous cotton-like structure.
As is known to all, materials with a 3D porous structure are beneficial for reducing the ion diffusion resistance and optimizing the transport kinetics, which could ensure that porous MnO2 possesses both high energy density and power capability. Moreover, 3D porous structure should render the material a higher specific area, accordingly the relative BET surface area of the testing samples was measured and calculated via a special self-defined method (Table S5†). As expected that 3D porous structured MnO2 enabled the MnO2/EH-TNTAs the higher relative BET specific area compared to EH-TNTAs@MnO2 with densely-packed MnO2. Consequently MnO2/EH-TNTAs with larger surface area is capable to provide more electrochemically active sites to electrolyte for a fast surface redox reaction, which contributes to the high pseudocapacitance and good rate capability.21,46,47 CV curves of the MnO2/EH-TNTAs electrode show no obvious distortion from 10 to 100 mV s−1, proving the excellent ion diffusion in the electrode. Therefore, the unique 3D porous cotton-like structure of MnO2 and larger specific surface area endowed the MnO2/EH-TNTAs electrode with superior supercapacitive performance.
More importantly, the MnO2/EH-TNTAs electrode displays promising cyclability. As shown in Fig. S7,† MnO2/EH-TNTAs retains 86.9% of its original capacitance after 5000 cycles at 5 A g−1, indicating the excellent stability of the deposited 3D porous MnO2 during the charge–discharge process, which is favourable for practical applications.
Conclusions
In summary, electrochemically hydrogenation significantly improves the electrical conductivity and electrochemical capacitance of TNTAs. The as-fabricated EH-TNTAs were proved to be an efficient current collector as well as an outstanding support for constructing high-performance supercapacitor electrode materials with nano-structured high specific capacitance materials. Further, depositing MnO2 NFs onto both the outer and inner walls of EH-TiO2 NTs was successfully achieved via a facile GED technique. The MnO2/EH-TNTAs electrode delivers a specific capacitance of up to 650.0 F g−1 at 1.0 A g−1 with 86.9% of the initial capacitance remaining after 5000 charge/discharge cycles at 5 A g−1, outstripping other reported MnO2–TNTAs electrodes. Comprehensive characterization and electrochemical analysis demonstrate that the superior supercapacitive performance of MnO2/EH-TNTAs electrode mainly results from the synergistic effect of unique 3D cotton-like porous structure and high specific surface area of MnO2 as well as remarkably improved electrical conductivity and electrochemical performances of EH-TNTAs.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51402078, 51302060), the National Basic Research Program of China (973 Program, 2014CB660815), and Young Scholar Enhancement Foundation (Plan B) of Hefei University of Technology (JZ2016HGTB0711, JZ2016HGTB0719).Notes and references
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- B. C. Kim, J.-Y. Hong, G. G. Wallace and H. S. Park, Adv. Energy Mater., 2015, 1500959, 1–33 Search PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed. Engl., 2013, 53, 1488–1504 CrossRef PubMed.
- M.-M. Titirici, R. J. White, N. Brun, V. L. Budarin, D. S. Su, F. d. Monte, J. H. Clarkd and M. MacLachlan, Chem. Soc. Rev., 2014, 44, 250–290 RSC.
- Z. Shao, H. Li, M. Li, C. Li, C. Qu and B. Yang, Energy, 2015, 87, 578–585 CrossRef CAS.
- Y. Zhao, Z. Zhang, Y. Ren, W. Ran, X. Chen, J. Wu and F. Gao, J. Power Sources, 2015, 286, 1–9 CrossRef CAS.
- J. S. Lee, D. H. Shin and J. Jang, Energy Environ. Sci., 2015, 8, 3030–3039 CAS.
- Z. H. Huang, Y. Song, X. X. Xu and X. X. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 25506–25513 CAS.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L.-C. Qin, Carbon, 2011, 49, 2917–2925 CrossRef CAS.
- Z. Peng, X. Liu, H. Meng, Z. Li, B. Li, Z. Liu and S. Liu, ACS Appl. Mater. Interfaces, 2016, 9, 4577–4586 Search PubMed.
- V. Kannan, A. I. Inamdar, S. M. Pawar, H.-S. Kim, H.-C. Park, H. Kim, H. Im and Y. S. Chae, ACS Appl. Mater. Interfaces, 2016, 8, 17220–17225 CAS.
- A. García-Gómez, S. Eugénio, R. G. Duarte, T. M. Silva, M. J. Carmezim and M. F. Montemor, Appl. Surf. Sci., 2016, 382, 34–40 CrossRef.
- X. Xiong, D. Ding, D. Chen, G. Waller, Y. Bu, Z. Wang and M. Liu, Nano Energy, 2015, 11, 154–161 CrossRef CAS.
- J. Xing, S. Wu and K. Y. S. Ng, RSC Adv., 2015, 5, 88780–88786 RSC.
- X. Xiong, G. Waller, D. Ding, D. Chen, B. Rainwater, B. Zhao, Z. Wang and M. Liu, Nano Energy, 2015, 16, 71–80 CrossRef CAS.
- X. Ou, X. Xiong, F. Zheng, C. Yang, Z. Lin, R. Hu, C. Jin, Y. Chen and M. Liu, J. Power Sources, 2016, 325, 410–416 CrossRef CAS.
- A. M. Patil, V. C. Lokhande, A. C. Lokhande, N. R. Chodankar, T. Ji, J. H. Kim and C. D. Lokhande, RSC Adv., 2016, 6, 68388–68401 RSC.
- X. Xiong, G. Wang, Y. Lin, Y. Wang, X. Ou, F. Zheng, C. Yang, J.-H. Wang and M. Liu, ACS Nano, 2016, 10, 10953–10959 CrossRef CAS PubMed.
- J.-G. Wang, F. Kang and B. Wei, Prog. Mater. Sci., 2015, 74, 51–124 CrossRef CAS.
- L. Zhao, J. Yu, W. Li, S. Wang, C. Dai, J. Wu, X. Bai and C. Zhi, Nano Energy, 2014, 4, 39–48 CrossRef CAS.
- W. Wei, X. Cui, W. Chen and D. G. Ivey, Chem. Soc. Rev., 2011, 40, 1697–1721 RSC.
- H. Huang, W. Zhang, Y. Fu and X. Wang, Electrochim. Acta, 2015, 152, 480–488 CrossRef CAS.
- D. Z. W. Tan, H. Cheng, S. T. Nguyen and H. M. Duong, Mater. Technol., 2014, 29, A107–A113 CrossRef CAS.
- F. Miao, C. Shao, X. Li, N. Lu, K. Wang, X. Zhang and Y. Liu, Energy, 2016, 95, 233–241 CrossRef CAS.
- D. Zhou, H. Lin, F. Zhang, H. Niu, L. Cui, Q. Wang and F. Qu, Electrochim. Acta, 2015, 161, 427–435 CrossRef CAS.
- L. Peng, X. Peng, B. Liu, C. Wu, Y. Xie and G. Yu, Nano Lett., 2013, 13, 2151–2157 CrossRef CAS PubMed.
- Y. He, W. Chen, X. Li, Z. Zhang, J. Fu, C. Zhao and E. Xie, ACS Nano, 2013, 7, 174–182 CrossRef CAS PubMed.
- X. Lu, M. Yu, G. Wang, T. Zhai, S. Xie, Y. Ling, Y. Tong and Y. Li, Adv. Mater., 2013, 25, 267–272 CrossRef CAS PubMed.
- Z. Ma, G. Shao, Y. Fan, M. Feng, D. Shen and H. Wang, ACS Sustainable Chem. Eng., 2017, 5, 4856–4868 CrossRef CAS.
- Z. Ma, G. Shao, Y. Fan, G. Wang, J. Song and D. Shen, ACS Appl. Mater. Interfaces, 2016, 8, 9050–9058 CAS.
- X. Xia, D. Chao, Z. Fan, C. Guan, X. Cao, H. Zhang and H. J. Fan, Nano Lett., 2014, 14, 1651–1658 CrossRef CAS PubMed.
- L. H. Cui, Y. Wang, X. Shu, J. F. Zhang, C. P. Yu, J. W. Cui, H. M. Zheng, Y. Zhang and Y. C. Wu, RSC Adv., 2016, 6, 12185–12192 RSC.
- C. Yu, Y. Wang, J. Zhang, X. Shu, J. Cui, Y. Qin, H. Zheng, J. Liu, Y. Zhang and Y. Wu, New J. Chem., 2016, 40, 6881–6889 RSC.
- Y. Qin, J. Zhang, Y. Wang, X. Shu, C. Yu, J. Cui, H. Zheng, Y. Zhang and Y. Wu, RSC Adv., 2016, 6, 47669–47675 RSC.
- C. Yu, Y. Wang, H. Zheng, J. Zhang, W. Yang, X. Shu, Y. Qin, J. Cui, Y. Zhang and Y. Wu, J. Solid State Electrochem., 2017, 21, 1069–1078 CrossRef CAS.
- X. Juan, L. Jia-Qin, L. Jing-Wei, W. Yan, L. Jun and W. Yu-Cheng, Acta Phys.-Chim. Sin., 2016, 32, 2545–2554 Search PubMed.
- X. Lu, G. Wang, T. Zhai, M. Yu, J. Gan, Y. Tong and Y. Li, Nano Lett., 2012, 12, 1690–1696 CrossRef CAS PubMed.
- H. Zhou and Y. Zhang, J. Power Sources, 2013, 239, 128–131 CrossRef CAS.
- Z. Pei, M. Zhu, Y. Huang, Y. Huang, Q. Xue, H. Geng and C. Zhi, Nano Energy, 2016, 20, 254–263 CrossRef CAS.
- H. Wu, D. Li, X. Zhu, C. Yang, D. Liu, X. Chen, Y. Song and L. Lu, Electrochim. Acta, 2014, 116, 129–136 CrossRef CAS.
- H. Cui, W. Zhao, C. Yang, H. Yin, T. Lin, Y. Shan, Y. Xie, H. Gu and F. Huang, J. Mater. Chem. A, 2014, 2, 8612–8616 CAS.
- J. Huo, Y. Hu, H. Jiang and C. Li, Nanoscale, 2014, 6, 9078–9084 RSC.
- Z. Li, Y. Ding, W. Kang, C. Li, D. Lin, X. Wang, Z. Chen, M. Wu and D. Pan, Electrochim. Acta, 2014, 161, 40–47 CrossRef.
- J. Zhang, Y. Wang, J. Wu, X. Shu, C. Yu, J. Cui, Y. Qin, Y. Zhang, P. M. Ajayan and Y. Wu, Chem. Eng. J., 2017, 313, 1071–1081 CrossRef CAS.
- H. Zhou and Y. Zhang, J. Power Sources, 2014, 272, 866–879 CrossRef CAS.
- P. Trogadas, V. Ramani, P. Strasser, T. F. Fuller and M.-O. Coppens, Angew. Chem., Int. Ed., 2016, 55, 122–148 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra04883a |
| This journal is © The Royal Society of Chemistry 2017 |