Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Larvicidal, super hydrophobic and antibacterial properties of herbal nanoparticles from Acalypha indica for biomedical applications†
Karthik S. a,
Suriyaprabha R.
a,
Suriyaprabha R. a,
Vinoth M.a,
Srither S. R.a,
Manivasakan P.a,
Rajendran V.
a,
Vinoth M.a,
Srither S. R.a,
Manivasakan P.a,
Rajendran V. *a and
Suresh Valiyaveettil
*a and
Suresh Valiyaveettil b
b
aCentre for Nano Science and Technology, K. S. Rangasamy College of Technology, Tiruchengode-637215, Tamil Nadu, India. E-mail: veerajendran@gmail.com; Fax: +91-4288-274880; Tel: +91-4288-274741-4
bDepartment of Chemistry, National University of Singapore, Singapore 117543, Singapore
First published on 25th August 2017
Abstract
The present study is aimed at developing a biocompatible nanomaterial with excellent medicinal properties using herbs. The herbal nanoparticles were prepared from shade dried leaves of Acalypha indica using the ball-milling technique. The prepared nanoparticles were characterized using X-ray diffraction, Fourier transform infrared spectroscopy, ultraviolet-visible spectroscopy, particle size analysis, scanning electron microscopy, X-ray fluorescence spectroscopy and transmission electron microscopy. The amorphous herbal AINPs posses an average particle size distribution of 54 ± 3 nm and a UV-absorption maximum at 434 nm, and are superhydrophobic (151°) in nature. The prepared herbal AINPs were tested for their antimicrobial properties against Staphylococcus aureus and Escherichia coli. Mosquito repellent properties were investigated against three disease vectors, namely, Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus, and showed significant larvicidal activity due to the existence of phytochemical compounds in the herbal nanoparticles. The acute toxicity of the herbal nanoparticles was tested with an in vivo animal model, zebrafish (Danio rerio), to ensure biocompatibility. The observed results confirmed that herbal AINPs play a dominant role in enhancing the medicinal properties for different biomedical applications.
Introduction
Everyday human life is affected by skin infections and mosquito related diseases such as boils, impetigo, cellulitis, malaria, dengue, lymphatic filariasis and yellow fever.1 Recent developments in nanotechnology demonstrate the practical applications of nanoparticles from plant extracts, due to their enhanced antibacterial, antifungal, hydrophobic, mosquito repellent and UV protection properties.2 Many natural products are widely used to develop newer medicines with potent biological and pharmacological activities. Medicinal plant based drugs have been extensively used in skin allograft, cornea allograft, intestinal allograft, cardiac allograft, and in the treatment of various diseases such as malaria, symptomatic endometriosis, uterine adenomyosis, leiomyoma, etc.3–5 The herbal nanoparticle treated materials are known in the field of biomedical applications for their exotic properties, e.g., non-toxic, mosquito repellent, hydrophobic, ultraviolet protection (UV) and antimicrobial action.6–9Acalypha indica (A. indica) is a common weed that belongs to the Euphorbiaceae family. It grows in common farmlands, gardens and uncultivated lands. All parts of A. indica (leaves, root, stalk and flower), which include constituents like acalyphine, triacetoneamine, cyanogenic glucosides, and alkaloids, are highly valuable for medicinal applications, due to their anti-inflammatory and antimicrobial properties.10 The earlier studies on the extracts of Acalypha indica confirm its antimicrobial properties against pathogenic bacteria such as Escherichia coli (E. coli), Salmonella typhi (S. typhi), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S. aureus) and Bacillus subtilis (B. subtilis).11,12
As reported by Suresh et al.,13 a GCMS study revealed that the A. indica leaf consists of 2,5-pyrrolidinedione, 1-methyl-3-o-methyl-D-glucose, tetradecanoic acid, 3,7,11,15-tetramethyl-2-hexadecen-1-ol, n-hexadecanoic acid, phytol, 9,12,15-octadecatrienoic acid, (Z,Z,Z), oleic acid, 1,2-benzenedicarboxylic acid diisooctyl ester, and squalene. Along with the aforementioned compounds, Chandra Mohan et al.14 reported that 1H-pyrrole-2,5-dione,1-ethenyl-, 3,8-nonadien-2-one, (E)-, 3,4-didehydro-proline, 4-amino-3-methoxypyrazolo[3,4-d]pyrimidine, propanenitrile, and 3-(5-diethylamino-1-methoxy-3-pentynyloxy)-compounds are also present in A. indica leaves.
Mosquito vectors are mainly responsible for endemic and pandemic diseases. The Ae. aegypti vector causes dengue fever in tropical and sub-tropical regions.1 An. strephensi is the primary vector that causes malarial infection.15 Filariasis diseases are caused by the Cx. quinquefasciatus vector in tropical and sub-tropical areas.16 Attempts are being made to eradicate mosquito vector borne diseases.17,18 Bio prospecting of the larvicidal properties is one of the approaches to solving the above issues. Even though different nanocomposites and chemicals provide excellent medicinal values, the properties such as non-biodegradability, bioaccumulation, bio control action, and toxicity keep them from being used for medical applications.2,19 Among the different herbal plants, A. indica shows good antibacterial, antimalarial, parasiticide, protisticide, plasmodicide, pesticide, antimutagenic, cancer preventive properties, and hence finds wide applications as a diuretic, antifungal, purgative, antihelmintic, anti-inflammatory and in the treatment of insect bites.20–22
The medicinal applications of herbal nanoparticles with exotic textural characteristics are validated by coating these nanoparticles onto cotton fabrics. The different antimicrobial, hydrophobic and wound healing properties are explored in nanoparticles coated fabrics.23,24 The recent studies on the self-cleaning, water repellent, high durability, antibacterial and UV protection properties, etc., of the herbal nanoparticles coated textiles strongly suggest their application in medicine.25,26
In the present study, we focus on the development of bio medically important herbal nanoparticles using the green synthesis method. The herbal nanoparticles are prepared from A. indica leaves using a ball mill without the addition of chemicals for aggregation and template shaping. In addition to the toxicity, antimicrobial and hydrophobic properties, the larvicidal properties of herbal nanoparticles are explored against three mosquito vectors, Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus.
Materials and methods
Collection of samples and preparation of herbal nanoparticles
The middle leaves of A. indica were collected and thoroughly washed several times using deionized (D.D.) water to remove dust on the leaf surface. The leaves were then shade dried for 2 weeks. The dried leaves were ground using ball milling for 15 h at 300 rpm. Zirconium balls of 10 mm diameter were used for milling. The milling with a ball ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was carried out for 10 g of leaves in a grinding jar with a zirconium protective jacket. After milling, the nanoparticles were taken for biological studies, followed by different characterization studies as reported in our previous investigations.27–29 The protocol for the preparation of herbal nanoparticles from A. indica leaves is shown in Fig. 1.
1 was carried out for 10 g of leaves in a grinding jar with a zirconium protective jacket. After milling, the nanoparticles were taken for biological studies, followed by different characterization studies as reported in our previous investigations.27–29 The protocol for the preparation of herbal nanoparticles from A. indica leaves is shown in Fig. 1.
The X-ray diffraction patterns of the prepared A. indica nanoparticles (AINPs) were obtained using a powder X-ray diffractometer (XRD; X'Pert PRO, PANalytical, Almelo, the Netherlands) operated with long fine focus of the Cu anode at 40 kV and 30 mA in Bragg–Brentano geometry. The XRD pattern was obtained in the 2θ range from 10° to 80° in a step-scan mode with a step size of 0.02°. Fourier transform infrared (FTIR) spectra of the nanoparticles were recorded using an FTIR spectrophotometer (Spectrum 100; PerkinElmer, USA) in the range of 400–4000 cm−1 using KBr (90 wt% IR Grade KBr) matrix for making transparent disks. The green synthesized leaf nanoparticles were monitored periodically using a UV-visible (UV-vis) spectrophotometer (Agilent Cary 8454, Singapore) operated in a wide range from 180–800 nm using a step size of 5 Å at different time intervals. A particle size analyzer (Nanophox, Sympatec, Germany) was used to determine the particle size distribution based on the dynamic light scattering (DLS) technique with a sub micrometer at a scattering angle of 90°. SEM, coupled with energy-dispersive X-ray (SEM-EDX, JSM 6360 JEOL, Japan) analysis, was used to identify the morphology, microstructure, and elemental composition of the prepared nanoparticles, fabrics coated with herbal nanoparticles and un-coated fabrics. Grain size and surface morphology of AINPs were examined through transmission electron microscopy (TEM, CM200; Philips, Eindhoven, The Netherlands) operated at a potential of 120 kV.
Larvicidal activity/repellent property
Larvae of mosquito vectors, namely, Ae. Aegypti, An. stephensi, and Cx. quinquefasciatus, were collected from Namakkal, Tamil Nadu, India. Mosquitoes were cultured in the laboratory and were maintained continuously in laboratory conditions as reported by WHO.30 The second and fourth instar larvae were exposed to the treatment of herbal nanoparticles with different concentrations such as 100, 200, 300, 400 and 500 mg L−1. Each treatment was carried out in triplicate, each comprising of 25 larvae. The hatching of mosquitoes under different doses of herbal nanoparticle treatments was closely monitored from 24 to 48 h, and the LC50 value and mortality percentage were calculated using the relation, (T–C/100 − C) × 100, where T is the total number of treated larvae and C the total hatchability of larvae. A standard larvicidal compound, prallethrin, was used to compare the larvicidal properties of the prepared herbal nanoparticles.In vivo toxicity
The invertebrate animal model, with zebrafish (Danio rerio) embryos, was used to assess the acute toxicity of the herbal nanoparticles of biomedical importance. Experiments were carried out with 15 zebrafish for each dose (12.5, 25, 50, 75 and 100 mg L−1) along with a control as per OECD-203 guidelines.31 All the animal model experiments were approved by the procedure of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), as instructed by Institutional Animal Ethics Committee (IAEC) guidelines,32 K. S. Rangasamy College of Technology (Reg. no. 1826/PO/EReBi/S/15/CPCSEA), Tiruchengode, Tamil Nadu, India. The hatching rate, mortality and developmental defects were determined for each tested dose in fish embryos, as reported in our earlier studies.33,34Coating of herbal nanoparticles
Bleached cotton fabric (100%, mass 138.84 g m−2, 116 ends per inch, 84 picks per inch) was used as a substrate for coating the herbal nanoparticles through a padding mangle at a rate of 35 rpm for 5 min to collect the uniformly coated fabrics35,36 in order to explore the hydrophobicity and UV-protection properties. Then, the coated fabrics were studied for their enhanced physico–mechanical and antimicrobial properties such as coating thickness, tensile and tear strength, crease recovery angle, air permeability and bacterial growth reduction; these data are given in detail in the ESI file (S1).†Hydrophobicity and UV-protection properties
Hydrophobicity and UV-protection properties of herbal nanoparticles were examined for self-cleaning and UV radiation protection applications, respectively. The hydrophobic nature of the herbal nanoparticle treated and untreated fabrics was ascertained based on the water contact angle (VCA Optima, ACT Product Inc., Japan). Digital photographic analysis of water droplets placed on the fabric surface was conducted before washing and after the 10th wash. Similarly, UV resistant properties of herbal nanoparticles were also studied on the nanoparticle coated fabrics using UV transmission spectra (Lambda 35; PerkinElmer, USA), in the wavelength range of 280–400 nm as per the ASTM D6603 standard.Antimicrobial studies
Two bacterial cultures, namely, Gram-positive S. aureus (ATCC 6538P) and Gram-negative E. coli (ATCC 9677), were obtained from the National Collection of Industrial Microorganisms (NCIM) (National Chemical Laboratory, Pune, India). The obtained bacterial cultures were further sub-cultured several times at 37 °C for 24 h. Inoculation of a loopful of test organisms into nutrient broth was carried out to prepare fresh bacterial inoculums, and then incubated at 37 °C for 5–8 h till a fair turbidity was obtained. Fresh cultures were swabbed on a nutrient agar plate and then herbal particles of different concentrations (25, 50 and 100 mg mL−1) were loaded onto the well punctured in the nutrient agar plate. After 24 h of incubation, zones of inhibition around the herbal nanoparticles loaded onto the agar well were measured using a millimeter ruler.Results and discussion
Characterization
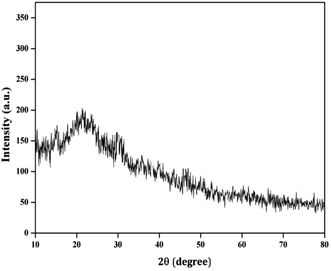
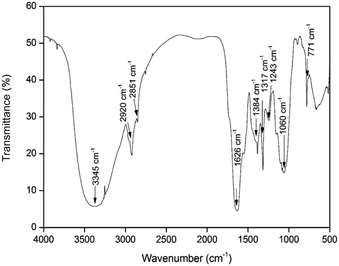
Herbal nanoparticles of AINPs were synthesized and comprehensively characterized for evaluating the influence of nanoscale particles on the medicinal, antimicrobial and functional properties. The XRD pattern of AINPs is shown in Fig. 2, which confirms the absence of diffraction peaks i.e., amorphous nature, except for the observed broad band at 2θ values (20–30°). Generally, the amorphous nature of the particles is non-toxic to living organisms and hence, amorphous herbal nanoparticles enhance the biocompatibility for clinical applications.The FTIR spectrum obtained from AINPs is shown in Fig. 3. The peaks observed between 3200 cm−1 and 3500 cm−1 are assigned to the presence of superficially absorbed water and the stretching mode of the OH/NH group, respectively. The bands observed at 2920 cm−1 and 2858 cm−1 correspond to the stretching vibrations of aliphatic and aromatic C–H bonds in the nanoparticles.37 The peak at 1626 cm−1 is identified as the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching vibration in the amide linkage of the plant protein.38 The band at 1384 cm−1 is assigned to the primary amine (N–H) bending mode, which is the appropriate characteristic peak of flavanones and terpenoids present in A. indica plant leaves.39 The peaks at 1317 cm−1 and 1243 cm−1 signify the carboxylic acid (C–O) group40 and the obtained peak at 1060 cm−1 discloses the C–N stretching vibrations of aliphatic amines. The band observed at 660 cm−1 is due to the deformation of α-glucopyranose rings of carbohydrates.40,41 The FTIR spectrum confirms the presence of major plant compounds that are responsible for the antimicrobial, larvicidal and UV-protection properties.
O) stretching vibration in the amide linkage of the plant protein.38 The band at 1384 cm−1 is assigned to the primary amine (N–H) bending mode, which is the appropriate characteristic peak of flavanones and terpenoids present in A. indica plant leaves.39 The peaks at 1317 cm−1 and 1243 cm−1 signify the carboxylic acid (C–O) group40 and the obtained peak at 1060 cm−1 discloses the C–N stretching vibrations of aliphatic amines. The band observed at 660 cm−1 is due to the deformation of α-glucopyranose rings of carbohydrates.40,41 The FTIR spectrum confirms the presence of major plant compounds that are responsible for the antimicrobial, larvicidal and UV-protection properties.
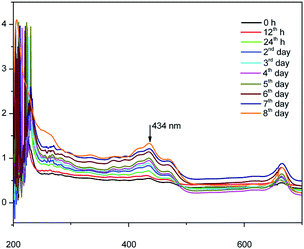
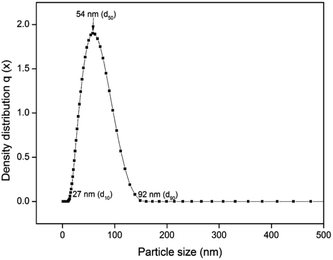
Fig. 4 shows the UV-vis spectrum of the synthesized herbal AINPs dispersed in water. In the present study, A. indica has an absorbance in the UV region at around 434 nm, which favours the anti-reflective and UV-adsorption properties for textile applications. Similar to silver nanoparticles, the plant based nanomaterials also show antimicrobial activity, but with lower toxicity. To determine the stability of the nanoparticles, we obtained the UV-vis spectra of the leaf nanoparticles at different time intervals for 8 days. It is interesting to see from Fig. 4 that there is no difference in absorption spectra, which confirms the higher stability of A. indica nanoparticles. The antireflective properties of the nanoparticles are favorable for developing UV-resistant biomaterials in an eco-friendly way. Fig. 5 shows the particle size distribution curve of the prepared AINPs. The average particle size of the prepared nanoparticles is around 54 ± 3 nm.
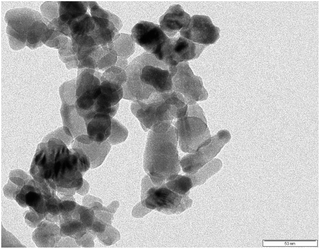
SEM and TEM images of the AINPs are shown respectively in Fig. 6 and 7. The topographical characterization of the nanoparticles observed in the SEM image shows that the herbal AINPs are uniform in structure. The SEM image shows the discrete distribution of herbal nanoparticles at higher magnification. The elemental composition of AINPs analyzed using EDX shows C and O peaks corresponding to elements such as Na, Mg, Si, Cl, K, and Ca metal ions, which confirm the inorganic compounds. A similar observation was reported in our previous study on obtaining the herbal nanoparticles from Tridax procumbens under different milling periods.26 The TEM (Fig. 7) image of the prepared AINPs confirms the spherical nature with high dispersion. The average particle size of the herbal AINPs obtained from the TEM image is about 50 nm. This is in close agreement with the measured particle size (54 nm) of the AINPs from the particle size distribution measurements.
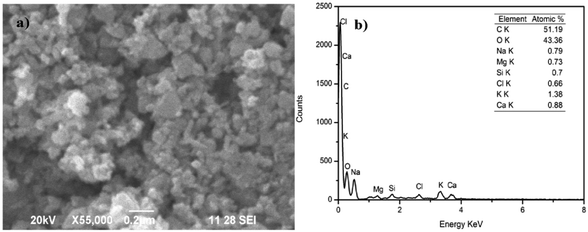 | ||
| Fig. 6 Particle size distribution of A. indica leaf nanoparticles (a) SEM image and (b) EDX analysis. | ||
Larvicidal activity
The larvicidal activities of the five different concentrations of AINPs to Aedes aegypti, Anopheles stephensi and Cx. quinquefasciatus are shown in Table 1. After 48 h of exposure, the AINPs were tested for their larvicidal activities according to the treatment concentrations. The larvicidal activities of the herbal nanoparticles were found to be high in terms of mortality rate at a higher concentration of AINPs against Aedes aegypti, Anopheles stephensi and Cx. Quinquefasciatus (LC50 = 500 mg L−1), as shown in Table 1. However, lower mortality was observed while exploiting both the Ist and IVth instars, due to the low amount of AINPs against these three mosquito vectors.| Mosquito species | Instar | A. indica nanoparticles at different concentrations (ppm) | |||||
|---|---|---|---|---|---|---|---|
| Prallithrin | |||||||
| (120 mg L−1) | 100 mg L−1 | 200 mg L−1 | 300 mg L−1 | 400 mg L−1 | 500 mg L−1 | ||
| Ae. aegypti | I | 100.0 ± 0.00 | 16.07 ± 2.71 | 33.12 ± 1.41 | 64.43 ± 3.17 | 97.15 ± 2.32 | 100.0 ± 0.00 |
| IV | 100.0 ± 0.00 | 8.11 ± 3.11 | 25.14 ± 2.04 | 47.03 ± 2.10 | 93.03 ± 1.15 | 100.0 ± 0.00 | |
| An. stephensi | I | 100.0 ± 0.00 | 19.07 ± 3.12 | 35.07 ± 2.51 | 63.14 ± 2.71 | 99.42 ± 2.11 | 100.0 ± 0.00 |
| IV | 100.0 ± 0.00 | 9.32 ± 2.05 | 27.16 ± 1.74 | 52.11 ± 1.76 | 94.01 ± 3.02 | 100.0 ± 0.00 | |
| Cx. Quinquefasciatus | I | 100.0 ± 0.00 | 19.07 ± 3.12 | 38.05 ± 2.72 | 69.32 ± 2.43 | 100.0 ± 0.11 | 100.0 ± 0.00 |
| IV | 100.0 ± 0.00 | 9.32 ± 2.05 | 26.42 ± 1.65 | 54.27 ± 1.54 | 96.01 ± 0.31 | 100.0 ± 0.00 | |
Several Euphorbiaceae plant extracts are known to exhibit larvicidal activity against these three mosquito vectors.42 Previously, extracts of A. indica using methanol, ethyl acetate, benzene and chloroform were studied for their larvicidal and ovicidal activities against Anopheles stephensi. In fact, crude nanoparticles of A. indica revealed comparatively higher larvicidal action than the extracted compounds of A. indica (by 15 percent), against the three mosquito vectors.43 The observed enhanced larvicidal activities are due to the exposure of the highly reactive surface area of the herbal AINPs. It is inferred from the present study that the obtained crude herbal nanoparticles with high surface areas act as an effective mosquito repellent and larvicide. The effectiveness of the prepared herbal A. indica nanoparticles was compared with a commercially used larvicide, prallethrin, with the least effective concentrations of A. indica nanopowder against mosquito larvae (120 mg L−1). Table 1 shows similar larvicidal properties for Prallethrin and prepared herbal nanoparticles. There are reports that show that Prallethrin is a poisonous material at lower concentration (25 mg mL−1).44 Our study shows that the use of herbal nanoparticles with good larvicidal action is amenable for mosquito control as an alternative larvicide to synthetic chemicals.
In vivo toxicity
The toxicity of AINPs was explored to determine the safe use for humans and other animals. Most herbs are non-toxic and good for health, due to the presence of numerous organic compounds. In order to explore the toxicity level of the nanoparticles, zebrafish embryos were treated with the AINPs and their developmental stages were monitored for 72 h as shown in Table 2. Among the five different concentrations of nanoparticles, particles of 200 mg L−1 concentration were highly effective with no mortality of the zebrafish embryos. In addition, a high concentration of nanoparticles does not affect the embryogenesis or hatching rate. Interaction of herbal particles associated with the biocompatibility of the plant compounds does not affect the fish developmental stages.| Nanoparticles | Concentrations (μg mL−1) | Mortality (%) | ||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | ||
| A. indica | Control | 0 | 0 | 0 |
| 25 | 0 | 0 | 3.3 | |
| 50 | 0 | 3.3 | 6.6 | |
| 75 | 0 | 6.6 | 13.3 | |
| 100 | 0 | 6.6 | 13.3 | |
| 200 | 0 | 6.6 | 6.6 | |
| Prallethrin | 25 | 100 | 0 | 0 |
A study investigating several metal oxide nanoparticles for their acute toxicity studies revealed significant toxicity at higher concentration when the embryos were tested for 72 h.45 Our observation reveals the non-toxic nature of the prepared AINPs against embryos, due to the samples being devoid of any processing chemicals like polar and non-polar solvents. On comparison of AINPs with other nano metal oxides, the herbal nanoparticles were found to be highly biocompatible with invertebrate animal model zebrafish by means a manifold reduction in mortality. Unfortunately, exposure concentrations of herbal nanoparticles are different from other metal oxide nanoparticles,41 since herbal nanoparticles are of biological origin and need to be tested at higher concentrations. Even at higher concentrations, the particles do not cause significant toxicity, which deserves wider use of the AINPs in the biomedical field for multidisciplinary applications as antimicrobial, mosquito repellent and biocompatible materials. On comparing the larvicidal activity of A. indica nanoparticles with the commercially used larvicide, prallethrin, it was observed that the synthetic oil prallethrin was highly toxic, since it showed 100% mortality at 24 h. From the aforementioned study, it can be said that A. indica nanoparticles can be used as a natural larvicide in place of prallethrin, due to its lower toxicity and better larvicidal properties.
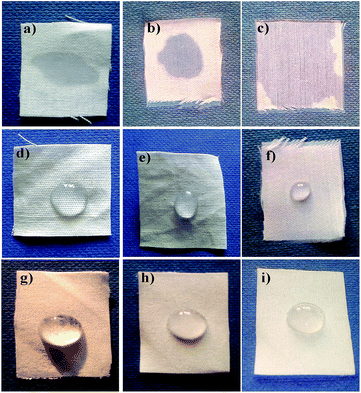
The surface characteristics of the AINPs like hydrophobicity and adherence properties were explored for biomedical applications, using cotton fabric. The ultraviolet protection factor (UPF) in the wavelength ranges from 280 to 400 nm was studied after coating the herbal particles onto the fabric, and the results are shown in Table 3. The percentage of transmittance for UV-A and UV-B is almost similar to that of the untreated fabrics.46 A significant reduction in transmittance was observed for herbal nanoparticle coated fabrics, which is ascribed to the blocking of UV-B and UV-A radiation due to the coating. In addition, the percentage blocking of UV-B radiation by the herbal nanocomposite is high (57%) as compared to that of chitosan-coating.47 Furthermore, the resistance rate of UV radiation for the herbal nanocomposite after the 5th and 10th washes is moderately reduced (4%) in herbal AINPs treated fabrics compared to that of the untreated cotton fabrics. On the basis of the ASTM D6603 standard data, the UPF value for the fabrics is more than 50%, demonstrating better protection of fabric from UV rays. The calculated UPF value for the AINPs coated fabrics was observed to be 57.7 ± 0.06, i.e., higher than the limitation (i.e., 50) ascribed to the higher resistance to UV irradiation. However, the values for un-coated and chitosan-coated fabrics exhibit lesser UV protection (<50) compared to those of the herbal nanocomposite coated fabrics. Thus, the herbal nanoparticles have an increment of nearly 50% in UV protection, compared to the un-coated fabric. This is attributed to the ability of herbal nanoparticles to provide protection from UV-rays, which would be an additional advantage for developing radiation protective clothing.48
| Sample names | UPF value | Contact angle (°) |
|---|---|---|
| Before wash | ||
| Un-coated fabrics | 13.9 ± 0.63 | 0 |
| Chitosan | 42.8 ± 0.46 | 119 ± 1 |
| Nanocomposite | 57.7 ± 0.06 | 151 ± 3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| After 5th wash | ||
| Un-coated fabrics | 11.2 ± 0.93 | 0 |
| Chitosan | 40.1 ± 0.77 | 101 ± 3 |
| Nanocomposite | 55.6 ± 0.70 | 135 ± 3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| After 10th wash | ||
| Un-coated fabrics | 10.8 ± 0.61 | 0 |
| Chitosan | 39.5 ± 0.55 | 93 ± 6 |
| Nanocomposite | 53.1 ± 0.47 | 124 ± 3 |
Hydrophobic activity
The water repellent property of the particles is one of the most interesting and desired properties of medical textiles, especially in pharmaceuticals and biomedicine.47,48 Researchers have reported that the hydrophobic or water repellent property is a function of the textile surface morphology and reduced surface energy.4,49–51 In our studies, we have investigated the water repellent efficiency of herbal nanoparticles, in terms of contact angle, by coating the herbal AINPs onto cotton fabrics. Generally, the fabrics with angle greater than 90° are considered to be hydrophobic, while those with angle greater than 150° are superhydrophobic. From Table 3, it is seen that herbal AINPs coated cotton fabrics show higher contact angle (151°) than the standard value of superhydrophobic materials. This in turn confirms the superhydrophobic behaviour of A. indica coated on the fabrics. The contact angle of un-coated, chitosan and nanocomposite coated cotton fabrics remains the same even after the 5th and 10th washes. This is due to the strong adherence of the particles and thereby the retention of the water repellent property even after 10 washes. It is interesting to note that the coating of herbal nanoparticles renders a superhydrophobic nature (151°) to the fabrics, thereby favoring self-cleaning applications.From the micrograph (Fig. 8), it is further evident that the chitosan and nanoparticle coated cotton fabrics have higher contact angles than the un-coated and the chitosan coated fabrics, owing to higher water repellent properties of the chitosan nanocomposite coated fabrics. In contrast, the liquid droplet on the un-coated fabric immediately seeps into the fabric, due to the very low contact angle and microspores in the materials, which make the materials more hydrophilic.47,49 This might also be attributed to the fact that the improved hydrophobicity of the fabrics is the result of the change in the surface morphology and surface energy, due the coating of nanomaterials,52 which is correlated with our observation for the superhydrophobic nature of the herbal nanoparticles.
Antimicrobial activity
The screening of the antibacterial activities of the prepared AINPs was carried out based on the zone of inhibition observed at different concentrations, namely 25, 50 and 100 mg mL−1 of AINPs (Fig. 9) and is summarized in Table 4. The agar well loaded with AINPs shows the maximum zone of inhibition against E. coli (23.5 mm) and S. aureus (22.7 mm) at a concentration of 100 mg mL−1. The magnitude of the inhibition zone is slightly higher in E. coli than S. aureus. In the case of treatment with the low concentration (25 mg mL−1) of AINPs, the inhibition zone was found to be 17.51 ± 0.23 and 16.72 ± 0.21 mm, respectively, against E. coli and S. aureus.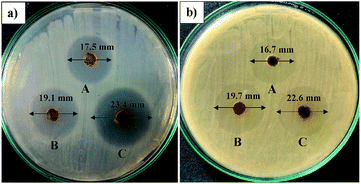 | ||
| Fig. 9 Antimicrobial activity of A. indica nanoparticles for different concentrations: (A) 25 mg mL−1, (B) 50 mg mL−1 and (C) 100 mg mL−1 against E. coli and S. aureus. | ||
| Herbal nanoparticles | Test organisms | Concentrations of AINPs, (zone of inhibition (mm)) | ||
|---|---|---|---|---|
| 25 mg mL−1 | 50 mg mL−1 | 100 mg mL−1 | ||
| A. indica | E. coli | 17.51 ± 0.23 | 19.14 ± 0.12 | 23.46 ± 0.01 |
| S. aureus | 16.72 ± 0.21 | 19.71 ± 0.12 | 22.68 ± 0.03 | |
A previous report on the aqueous extract of A. indica demonstrates the inhibition zone of 9 mm against E. coli and no inhibition zone against S. aureus.53 Similarly, acetone aqueous extract from AINPs shows the minimum antimicrobial properties against S. aureus (22 mm) and E. coli (15 mm).54 Quantitative evaluation of the antimicrobial activity of herbal nanoparticle coated fabrics for medical textile applications was also conducted, which is included with these results as a ESI file (S1).† The observed antimicrobial results of the tested herbal nanoparticles are comparatively higher than the organic extracts of the particles. This is due to the existence of intact reactive organic compounds such as acalyphine, triacetoneamine, cyanogenic glucosides, and alkaloids10 that are responsible for biochemical and cell wall reactions to inhibit bacterial growth. This is one of the superior properties of dry herbal nanoparticles possessing multifunctional characteristics like mosquito repellency, antimicrobial properties, and superhydrophobicity.
Conclusion
Herbal nanoparticles synthesized from A. indica leaves were screened and evaluated for their improved physico–mechanical and biological properties to explore their possible applications in biomedicine. The superhydrophobic nature and excellent UV-protection properties of the herbal particles are additionally proven to be favourable at higher concentrations (up to 200 μg mL−1) for medical textiles and self-cleaning applications. Larvicidal activity against major disease mosquito vectors is well demonstrated in the present investigation, which aids in the development of eco-friendly nanoparticles of biomedical importance. The prepared herbal nanoparticles exhibit exotic in vitro and in vivo biocompatibility, and antimicrobial activity that enhance the application of cost effective herbal nanoparticles as potent biomaterials to remediate life threatening problems in healthcare.Conflicts of interest
Authors have no conflicts to declare.Acknowledgements
The authors acknowledge the financial support provided by Board of Research and Nuclear Science (BRNS), Mumbai (Sanction no: 2013/34/30/BRNS/1127 dt.19.9.2013). One of the authors (Dr R. S.) is thankful to the University Grants Commission (UGC), New Delhi for the award of Post-Doctoral Fellowship for Women (F.15-1/2015-17/PDFWM-2015-17-TAM-36274 dt.12/10/2015).References
- S. Vijaya kumar, P. Mani, T. M. M. John Bastin and G. Ravikumar, Int. J. Med. Biosci., 2012, 1(3), 33–41 Search PubMed.
- S. Pawel, M. Magdalena and M. Elzbieta, Nano, 2011, 6, 509–539 CrossRef.
- A. Nasrollahi, K. Pourshamsian and P. Mansourkiaee, Int. J. Nano Dimens., 2011, 1, 233–239 CAS.
- N. R. Dhineshbabu, P. Manivasakan, A. Karthik and V. Rajendran, RSC Adv., 2014, 4, 32161–32173 RSC.
- S. C. Mccombie, Soc. Sci. Med., 1996, 43, 933–945 CrossRef CAS PubMed.
- Q. Daoming and N. K. Peter, Drugs R&D, 2003, 4, 1–18 Search PubMed.
- N. G. Das, I. Baruah, P. K. Talukdar and S. C. Das, J. Vector Borne Dis., 2003, 40, 49–53 CAS.
- K. Murugan, P. Murugan and A. Noortheen, Bioresour. Technol., 2007, 98, 198–201 CrossRef CAS PubMed.
- B. Gabriela, L. A. Ioana, B. Nicoleta, O. Cristina and M. Aurelia, Ind. Crops Prod., 2015, 67, 18–24 CrossRef.
- M. A. Rahman, C. B. Sitesh and R. Mohammed, Pak. J. Pharm. Sci., 2010, 23, 256–258 Search PubMed.
- H. Pao-Chuan, M. Jeng-Leun and H. Shu-Hui, Food Microbiol., 2001, 18, 35–43 CrossRef.
- A. Zahir Hussain and S. Kumaresan, Asian J. Plant Sci. Res., 2013, 3, 46–49 Search PubMed.
- M. Suresh, S. A. A. Mohammad, K. R. Pradipta, A. Panneerselvam and N. Thajuddin, Asian Pac. J. Trop. Biomed., 2016, 6(3), 185–191 CrossRef.
- S. Chandra Mohan, S. Dinakar, T. Anand, R. Elayaraja and B. S. Priya, Int. J. PharmTech Res., 2012, 4(3), 1050–1054 CAS.
- R. Borah, M. C. Kalita, A. Kar and A. K. Talukdar, Afr. J. Biotechnol., 2010, 9, 2527–2530 Search PubMed.
- E. J. Muturi, P. Burgress and R. J. Novak, Am. J. Trop. Med. Hyg., 2008, 78, 536–537 Search PubMed.
- R. Maheswaran, S. Sathish and S. Ignacimuthu, Int. J. Integr. Biol., 2008, 2, 214–217 Search PubMed.
- M. Pavunjarj, K. Baskar, V. Duraipandiyan, N. Abdullah Al-Dhabi, V. Rajendran and G. Benelli, J. Cluster Sci., 2017, 1–16 Search PubMed.
- R. Suriyaprabha, K. Gopalu, P. Muthusamy, Y. Rathinam, V. Rajendran and N. Kannan, RSC Adv., 2014, 4, 8461–8465 RSC.
- Y. M. Shivakar and V. L. Kumar, Pharm. Biol., 2003, 41, 263–265 CrossRef.
- M. Govindarajan, A. Jebanesan, D. Reetha, R. Anisath, T. Pushpanathan and K. Samidurai, Eur. Rev. Med. Pharmacol. Sci., 2008, 12, 299–302 CAS.
- P. Prasad and M. Estari, International Interdisciplinary Research Journal, 2014, 4, 175–182 Search PubMed.
- S. Sheila and W. Jakub, Antimicrob. Agents, 2012, 19, 387–406 Search PubMed.
- B. Venkatrajah, V. Vanitha Malathy, B. Elayarajah, S. Mohan, R. Rajendren and R. Rammohan, J. Med. Sci., 2012, 12, 148–160 CrossRef.
- R. Rajendran, R. Radhai, T. M. Kotresh and C. Emilia, Carbohydr. Polym., 2013, 91, 613–617 CrossRef CAS PubMed.
- N. R. Dhineshbabu, P. Manivasakan, R. Yuvakkumar, P. Prabu and V. Rajendran, J. Nanosci. Nanotechnol., 2013, 13, 4017–4024 CrossRef CAS PubMed.
- M. Vinoth, R. SuriyaPrabha, S. Arunmetha, A. Karthik, S. Karthik, P. Paramasivam, P. Prabu, P. Manivasakan, K. Saminathan and V. Rajendran, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2015, 46, 1445–1449 CrossRef.
- S. Karthik, R. Suriyaprabha, K. S. Balu, P. Manivasakan and V. Rajendran, IET Nanobiotechnol., 2016, 11, 12–17 CrossRef PubMed.
- S. Karthik, M. Vinoth, K. S. Balu, R. Suriyaprabha, P. Manivasakan, V. Rajendran and V. Suresh, J. Alloys Compd., 2017, 723, 698–707 CrossRef.
- K. Baskar, V. Sudha, G. Nattudurai, S. Ignacimuthu, V. Duraipandiyan, M. Jayakumar, N. Abdullah Al-Dhabi and G. Benelli, Physiol. Mol. Plant Pathol., 2017, 30, 1–5 Search PubMed.
- OECD, Guidelines for the Testing of Chemicals, No. 203: Fish Acute toxicity Test, 1992, Adopted 17/07/1992 Search PubMed.
- K. B. Dinesh and C. Desai, Indian J. Pharmacol., 2014, 46(3), 257–265 CrossRef PubMed.
- M. Prabhu, R. Suriyaprabha, V. Rajendran, P. Kulandaivelu and S. Valiyaveettil, RSC Adv., 2014, 4, 43630–43640 RSC.
- K. Kavitha, W. Chunyan, D. Navaneethan, V. Rajendran, S. Valiyaveettil and A. Vinoth, RSC Adv., 2014, 4, 43951–43961 RSC.
- B. Gupta, S. Saxena and A. Arora, J. Fibre Text. Res., 2011, 36, 272–280 CAS.
- S. S. Ugur, M. Sarıısik and A. H. Aktas, Nanotechnology, 2010, 21, 325603–325610 CrossRef PubMed.
- P. Prakash, P. Gnanaprakasam, R. Emmanuel, S. Arokiyaraj and M. Saravanan, Colloids Surf., B, 2013, 108, 255–259 CrossRef CAS PubMed.
- K. Rajathi and S. Sridhar, International Journal of Green Chemistry and Bioprocess, 2012, 2, 39–43 Search PubMed.
- B. Priya, S. Mantosh, M. Aniruddha and D. Papita, Bioresources and Bioprocessing, 2014, 1, 1–10 CrossRef.
- P. Logeswari, S. Silambarasan and J. Abraham, Sci. Iran., 2013, 20, 1049–1054 Search PubMed.
- P. Saravanan, G. Chandramohan, J. Mariajancyrani and P. Shanmugasundaram, Int. J. Environ. Sci., 2013, 2, 1–5 Search PubMed.
- A. A. Rahuman, G. Gopalakrishnan, P. Venkatesan and K. Geetha, Parasitol. Res., 2008, 102, 867–873 CrossRef PubMed.
- M. Govindarajan, A. Jebanesan, T. Pushpanathan and K. Samidurai, Parasitol. Res., 2008, 103, 691–695 CrossRef CAS PubMed.
- M. B. Emmanuel, S. Moorthy, G. Ganeshwala and G. Abraham, J. Med. Toxicol., 2010, 6, 7–30 Search PubMed.
- L. C. Wehmas, C. Anders, J. Chess, A. Punnose, C. B. Pereira, J. A. Greenwood and R. L. Tanguay, Toxicol. Rep., 2015, 2, 702–715 CrossRef CAS PubMed.
- S. Agnihotri, S. Mukherji and M. Suparna, RSC Adv., 2014, 4, 3974–3983 RSC.
- N. F. Attia, M. Moussa, A. M. F. Sheta, R. Taha and H. Gamal, Prog. Org. Coat., 2017, 106, 41–49 CrossRef CAS.
- N. F. Attia, M. Moussa, A. M. F. Sheta, R. Taha and H. Gamal, Prog. Org. Coat., 2017, 104, 72–80 CrossRef CAS.
- K. Autumn, Y. A. Liang, S. T. Hsieh, W. Zesch, W. P. Chan, T. W. Kenny, R. Fearing and R. J. Full, Nature, 2000, 405, 681–685 CrossRef CAS PubMed.
- S. Wang, C. Liu, G. Liu, M. Zhang, J. Li and C. Wang, Appl. Surf. Sci., 2011, 258, 806–810 CrossRef CAS.
- A. Nakajima, K. Hashimoto and T. Watanabe, Monatsh. Chem., 2001, 132, 31–41 CrossRef CAS.
- B. Liu, L. Wang, Y. Gao, T. Tian, J. Min, J. Yao, Z. Xiang, C. Huang and C. Hu, Text. Res. J., 2015, 85, 795–803 CrossRef CAS.
- S. S. Ugur, M. Sarıısik and A. H. Aktas, Nanotechnology, 2010, 21, 325603–325610 CrossRef PubMed.
- P. Mohanpuria, K. N. Rana and S. K. Yadav, J. Nanopart. Res., 2008, 10, 507–517 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra05697d |
| This journal is © The Royal Society of Chemistry 2017 |