Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New kinetic and mechanistic aspects of photosensitization of iodonium salts in photopolymerization of acrylates
Janina Kabatc *a,
Joanna Ortylb and
Katarzyna Kostrzewskaa
*a,
Joanna Ortylb and
Katarzyna Kostrzewskaa
aUTP, University of Science and Technology, Faculty of Chemical Technology and Engineering, Seminaryjna 3, 85-326 Bydgoszcz, Poland. E-mail: nina@utp.edu.pl; Fax: +48 52 374 9005; Tel: +48 52 374 91 12
bCracow University of Technology, Faculty of Chemical Engineering and Technology, Warszawska 24, 31-155 Cracow, Poland
First published on 29th August 2017
Abstract
1,3-Bis(p-bromophenylamino)squaraine which functions as an electron-transfer photosensitizer for a wide range of diphenyliodonium salts radical photoinitiators of triacrylate polymerization under UV-blue light irradiation is presented. The functionality of the photosensitizer is determined by the presence of a suitable co-initiator. For this purpose, several different diphenyliodonium salts were used as a source of active species for radical polymerization. The reactivity of the new iodonium salts was studied with ultraviolet-blue light initiated radical polymerization by photo-DSC using squaraine dye as a sensitizer. The iodonium salts functionated as radical initiators bearing different substitution patterns. Electron transfer from the excited state of the sensitizer to the iodonium salt results in initiating radicals. The ability to initiate free radical polymerization of TMPTA by iodonium salts studied is similar. Most of the iodonium salts exhibit no large differences regarding the reactivity in initiation of TMPTA polymerization. However, an introduction of a nitro group in the para position of the phenyl ring of the co-initiator results in a significant increase in the rate of polymerization and degree of monomer conversion. The dye-sensitized photoreaction mechanism of the SQ/I system was also confirmed.
1. Introduction
The most common photoinitiators acting in the visible light region for radical polymerization are two-component systems involving a dye and an electron donor (co-initiator) molecule. Active radicals are formed in a bimolecular electron transfer between the excited state of the dye and the co-initiator.Among the different co-initiators explored, a particular class corresponds to onium salts. The development of applications involving onium salts in the photoinitiation of polymerization has been a topic of interest since the late 60s.1,2 Studies have been reported on ammonium, phosphonium, sulfonium, iodonium, arsonium and pyridinium salts.1 Among them, diaryliodonium and triarylsulfonium are commercially important. These compounds are capable of undergoing photochemical decomposition, producing active species suitable to initiate polymerization. Most of them are characterized by high photolysis quantum yields and are efficient when the irradiation is carried out using light in the UV region from 230 nm to 300 nm.3 The most widely used onium salts are diaryliodonium salts with a chloride or perchlorate counterion. They have been employed extensively for cationic and vinyl polymerization.1,4–21 Extending their spectral response to visible wavelengths requires the presence of photosensitizers and/or free-radical photoinitiator.3 In last decade, polymethine dyes,4 cyanines with barbituryl group,22 erythrosine B,23 naphthalimide derivatives,24 coumarin chromophores,3 xanthenes,25 resazurin and resorufin,26 quinoxaline derivatives,19 isopropylthioxanthone27 as well as inorganic compounds, such as perovskites18 are used as photoinitiating systems containing diphenyliodonium salts. In generation, the dye sensitized photopolymerization is based on the formation of initiating species through either photoreduction or photooxidation reactions of dye with co-initiator.25,28
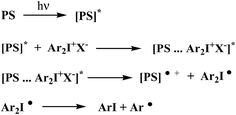
There are several mechanism by which the photosensitization of diphenyliodonium salts (onium salts) takes place.29,30 However, electron transfer photosensitization appears to be the most efficient and generally applicable process.31 Crivello et al. proposed the mechanism for an electron transfer photosensitization of diaryliodonium salts (Scheme 1).31
Electron transfer photosensitization involves first, absorption of light by the photosensitizer to give the corresponding excited species [PS]*. An excited state complex (exciplex) is often formed as an intermediate between the onium salt and the excited photosensitizer. Subsequently, the onium salt is reduced by a formal electron transfer between two reaction partners. The rapid decomposition of the resulting unstable diaryliodine radical prevents back electron transfer and renders the overall process irreversible.31 Generally, an electron transfer and/or energy transfer from the photoexcited sensitizer to the iodonium cation was suggested as the first reaction step in the initiation mechanism.
Squaraine dyes have unique photophysical and photochemical properties and have been found many specialized uses as a kind of important functional dyes for diversely technological applications in photodynamic therapy, xerography, optic recording, non-linear optic and organic solar cells, etc.32,33 In literature there are only few examples as photoinitiating systems for polymerization process combining squaraines.25,34
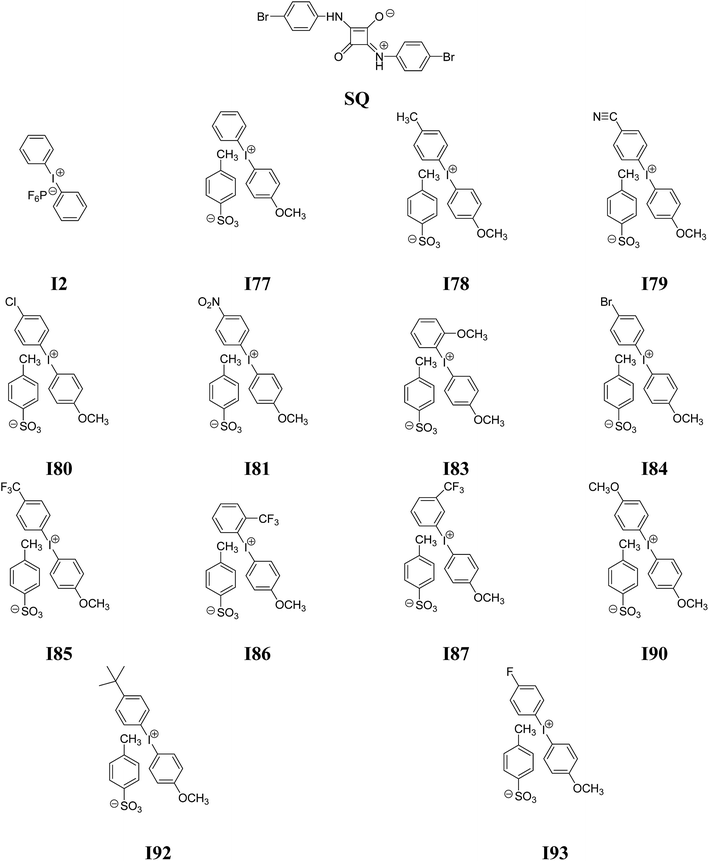
In the present paper, we will focus on the research carried out on the two-component photoinitiating systems for free radical polymerization containing nine diphenyliodonium salts differ type of a substituent in a para position of phenyl ring and squaraine dye. For this purpose, the possible role of squaraine dye as photoinitiators of the polymerization of TMPTA in the presence of onium salts was investigated and presented.
2. Experimental
2.1. Materials
2-Ethyl-2-(hydroxymethyl)-1,3-propanediol triacrylate (TMPTA) (monomer), 1-methyl-2-pyrrolidinone (MP), solvents (spectroscopic grade) and diphenyliodonium hexafluorophosphate (I2) were purchased from Aldrich (Poland) and used without further purification. The following diphenyliodonium salts: (4-methoxyphenyl)-phenyliodonium p-toluenesulfonate (I77), (4-methoxyphenyl)-(4-methylphenyl)iodonium p-toluenesulfonate (I78), (4-methoxyphenyl)-(4-cyanophenyl)iodonium p-toluenesulfonate (I79), (4-chlorophenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I80), (4-methoxyphenyl)-(4-nitrophenyl)iodonium p-toluenesulfonate (I81), (3-methoxyphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I83), (4-bromophenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I84), (4-trifluoromethylphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I85), (2-trifluoromethylphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I86), (3-trifluoromethylphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I87), bis(4-methoxyphenyl)iodonium p-toluenesulfonate (I90), (4-tert-butylphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I92), (4-fluorophenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I93) and 1,3-bis(p-bromophenylamino)squaraine (SQ) were synthesized in Cracow University Laboratory and University of Science and Technology Laboratory by methods described in literature.35 The structure of new co-initiators was confirmed using nuclear magnetic resonance spectroscopy. 1H NMR spectra were determined at room temperature in 5 mm o.d. tubes on a Bruker Avance III HD 400 MHz spectrometer. The 1H chemical shifts were referenced to the solvent peak DMSO-d6 (2.49 ppm) and presented below.2.2. Spectroscopic measurements
Absorption and emission spectra were recorded at room temperature using an Agilent Technology UV-Vis Cary 60 Spectrophotometer, a Hitachi F-7000 spectrofluorimeter and UV-VIS-NIR Fluorolog 3 Spectrofluorimeter (Horiba Jobin Yvon), respectively. The spectra were recorded in 1-methyl-2-pyrrolidinone (MP).The fluorescence quenching measurements were performed using a single-photon counting system UV-VIS-NIR Fluorolog 3 Spectrofluorimeter (Horiba Jobin Yvon). The apparatus uses a picosecond diode laser (370 nm) generating pulses of about 50 ps for the excitation. Short laser pulses in combination with a fast microchannel plate photodetector and ultrafast electronics make a successful analysis of fluorescence decay signals in the range of single picoseconds possible. The dye was studied at a concentration able to provide equivalent absorbance at 370 nm (0.2 in the 10 mm cell). The rate constant for fluorescence quenching was determined in 1-methyl-2-pyrrolidinone. The concentration of dye was 2 × 10−5 M and that of quenchers was in the range from 1 × 10−4 M to 5.0 × 10−3 M. The fluorescence quenching at 440 nm was measured in deaerated solution by bubbling with argon.
2.3. Cyclic voltammetry measurements
The electrochemical measurements were evaluated by Cyclic Voltammetry (CV) using ER466 Integrated Potentiostat System (eDAQ, Poland) in a three-electrode configuration. The electrolyte was 0.1 M tetrabutylammonium perchlorate in dry acetonitrile. Platinum 1 mm disk electrode was applied as working electrode, and platinum and Ag/AgCl were used as auxiliary and reference electrodes, respectively. All solutions were deoxygenated with N2 for at least 15 min prior to measurements. The computer-controlled potentiostat was equipped with EChem Software.2.4. Polymerization measurements
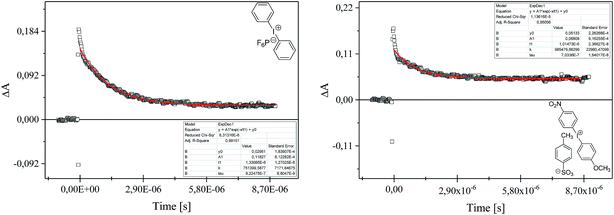
The kinetics of polymerization of 2-ethyl-2-(hydroxymethyl)-1,3-propanediol triacrylate photoinitiated by 1,3-bis(p-bromophenylamino)squaraine in a presence of diphenyliodonium salt was measured using Differential Scanning Calorimeter TA DSC Q2000 Instrument and TA Q PCA photo unit (Photo-DSC). The heat of polymerization reaction measured by means of a photo differential scanning calorimeter, is a good control of the reaction temperature. A high pressure mercury lamp as a UV-visible light source (300 nm < λ < 500 nm) was applied at a constant intensity of 30 mW cm−2 for several min under nitrogen flow of 50 mL min−1 at a prescribed temperature (isothermal mode). The weight of samples 30 ± 0.1 mg was placed into an open aluminum liquid DSC pan. The measurements were carried out under identical conditions. The sample was maintained at a prescribed temperature for 2 min before each measurement run began. Measurements were recorded at a sampling interval of 0.05 s per point. The polymerizing solution was composed of 1.8 mL of monomer, 0.2 mL of 1-methyl-2-pyrrolidinone and appropriate amount of photoinitiator (5 × 10−4 M, 1 × 10−3 M, 2 × 10−3 M, 5 × 10−3 M, 1 × 10−2 M and 2 × 10−2 M). 1-Methyl-2-pyrrolidinone was necessary due to poor solubility of sensitizer in the monomer.The reaction heat liberated in the polymerization is directly proportional to the number of acrylates reacted in the system. By integrating the area under the exothermic peak, the conversion of acrylate groups (C%) or the extent of reaction was determined according to eqn (1):
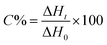 | (1) |
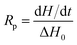 | (2) |
2.5. Nanosecond laser flash photolysis
Transient absorption spectra and decay kinetics were carried out using the nanosecond laser flash photolysis method. The nanosecond laser flash photolysis experiments were performed using a LKS.60 Laser Flash Photolysis apparatus (Applied Photophysics). Laser irradiation at 355 nm from the third harmonic of the Q-switched Nd:YAG laser from a Lambda Physik/model LPY 150 operating at 65 mJ per pulse (pulse width about 4–5 ns) was used for the excitation. Transient absorbances at preselected wavelengths were monitored by a detection system consisting of a monochromator, a photomultiplier tube (Hamamatsu R955) and a pulsed xenon lamp (150 W) as a monitoring source. The signal from the photomultiplier was processed by a Hewlett-Packard/Agilent an Agilent Infiniium 54810A digital storage oscilloscope and an Acorn compatible computer.3. Results and discussion
3.1. Initiators
2-Ethyl-2-(hydroxymethyl)-1,3-propanediol triacrylate as a trifunctional monomer was selected to study the reactivity of UV-blue photoinitiator system. A combination of a squaraine dye (sensitizer) and a diphenyliodonium salt (radical initiator) was selected as photoinitiator system. Several diphenyliodonium salts with distinct substitution pattern were investigated to check if there exists a relation between reactivity and structure. Chart 1 depicts the structure of photosensitizer and different structural variations of iodonium cations investigated.One of the preconditions of the polymerization process is an appropriate selection of a light source. The necessary is that the wavelength of the emitted light matches very well with the absorption range of sensitizer. Fig. 1 shows the absorption spectra of co-initiators and sensitizer studied.
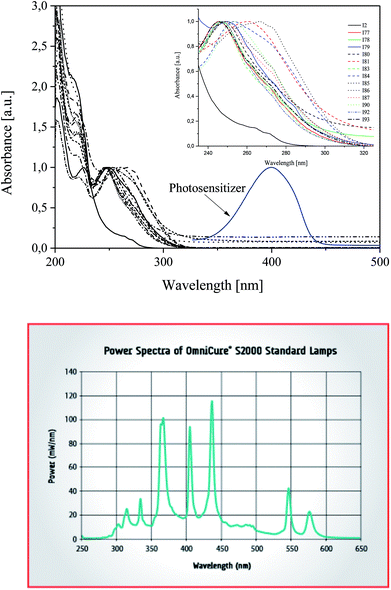 | ||
| Fig. 1 Top: Absorption spectra of co-initiators and photosensitizers in 1-methyl-2-pyrrolidinone solution recorded at room temperature. Bottom: Spectra of light source used. | ||
The UV-Vis absorption spectra of photosensitizer shows the main absorption band with maximum about 400 nm. As is seen, the emission spectra of high pressure mercury lamp used overlaps with the absorption region of squaraine. There is not any overlap between diphenyliodonium salts absorption and emission of a light source.
The irradiation of a dye with visible light leads to an excited singlet state formation. The dye in its excited state undergoes different deactivation processes. Generally, they are radiative or non-radiative processes. The fluorescence and phosphorescence belong to radiative but internal conversion and intersystem crossing are non-radiative. An excited singlet state may also be self-quenched or quenched by other molecule in bimolecular reaction. Basing on the Scheme 1, one can conclude that one of non-radiative processes, such as an electron transfer is necessary pathways to generate initiating radicals in the redox system comprising squaraine dye as electron donating molecule and the diphenyliodonium salt electron acceptor acting as radical initiator. The possibility of occurrence of electron transfer process is determine by the thermodynamic conditions of this process. In order to determine the value of free energy change for electron transfer process, the knowledge of the redox properties of all components of photoinitiating system is necessary.
Electrochemical properties of sensitizer and co-initiators were scrutinized by cyclic voltammetry in acetonitrile to evaluate thermodynamically about the possibility of electron transfer from the excited state sensitizer to the ground state diphenyliodonium salt. The reduction potentials of onium salts are listed in Table 1.
| Co-initiator | Substituent in para position | λab [nm] | Ered [eV] | ΔGel [kJ mol−1] | Rp [s−1] | Rp/Rpb [min] |
|---|---|---|---|---|---|---|
| a Substitution in other position than para.b The rate of polymerization observed for co-initiator I2. | ||||||
| I2 | H, H | 212 | −1.0 | −62.13 | 1.26 | 1.00 |
| I77 | H, CH3O | 246 | −0.206 | −138.10 | 1.38 | 1.10 |
| I78 | CH3, CH3O | 247 | −0.395 | −119.86 | 1.36 | 1.08 |
| I79 | CN, CH3O | 250 | −0.200 | −138.68 | 1.26 | 1.00 |
| I80 | Cl, CH3O | 248 | −0.292 | −129.80 | 1.39 | 1.11 |
| I81 | NO2, CH3O | 261 | −0.554 | −104.52 | 10.41 | 8.29 |
| I83 | o-CH3Oa, CH3O | 229 | −1.151 | −46.91 | 1.47 | 1.17 |
| I84 | Br, CH3O | 210 | −0.175 | −141.09 | 1.63 | 1.30 |
| I85 | CF3, CH3O | 230 | −0.106 | −147.75 | 1.43 | 1.14 |
| I86 | o-CF3a, CH3O | 230 | −0.260 | −132.89 | 1.72 | 1.37 |
| I87 | m-CF3a, CH3O | 230 | −0.254 | −133.47 | 1.38 | 1.10 |
| I90 | CH3O, CH3O | 225 | −0.342 | −124.98 | 1.26 | 1.00 |
| I92 | t-butyl, CH3O | 229 | −0.310 | −128.07 | 1.40 | 1.12 |
| I93 | F, CH3O | 229 | −0.302 | −128.84 | 1.48 | 1.18 |
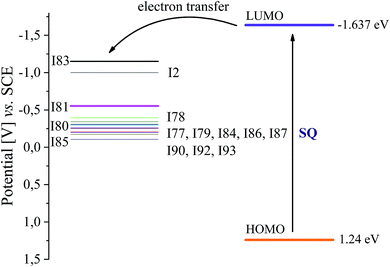
Iodonium salts under study possess reduction potentials (Ered) in the range from −1.151 eV to −0.106 eV, while sensitizer (SQ) exhibits an oxidation potential (Eox) of 1.24 eV. The singlet excited-state energy (E00) of squaraine dye determined from the crossover point between the normalized absorption and emission spectra was 277.63 kJ mol−1 (2.877 eV) for 1SQ*. Iodonium salts (I80, I84, I85, I93) possessing strong electron withdrawing substituents in para position of phenyl ring are more easily photosensitized by an electron transfer process than other salts studied because of lower reduction potentials.
As was mentioned above, in photoinitiator system, the sensitizer first absorbs the incident photon flux and is then excited from the ground state to the excited state, which transfers its electron to co-initiator. Fig. 2 shows a typical energy level diagram for photoinitiator system based on squaraine dye as sensitizer (SQ) and diphenyliodonium salt.
An energy gap more than 0.5 V between the LUMO level of the sensitizer and the HOMO level of iodonium salt is enough for effective electron transfer from excited state to ground state of co-initiator. Following data: Eox, Ered and E00 presented above, allow to calculate the value of free energy change for electron transfer (ΔGel) using eqn (3).
| ΔGel = F(Eox − Ered) − E00 | (3) |
3.2. Photoinitiating ability
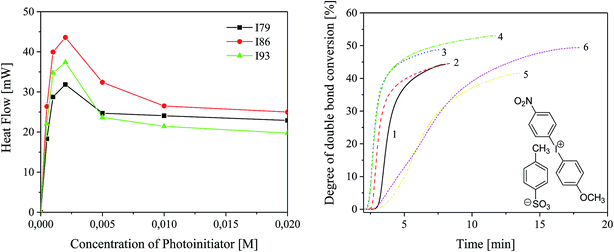
Photo-DSC was used to study the reactivity of UV-blue photoinitiator system comprising the sensitizer (SQ) and the radical initiator (I) in 2-ethyl-2-(hydroxymethyl)-1,3-propanediol triacrylate. The maximum of polymerization heat Rmaxp roughly describes the reactivity of a photoinitiating system because the system crosslinks during irradiation.5,36 It is a characteristic point where auto-acceleration and vitrification are equivalent.5 Thus, diffusion processes control the reactivity and reaction rate constants change with conversion.In order to choose the optimal concentration of photoinitiator under experimental conditions, first, the effect of the photoinitiator concentration on the rate of polymerization and degree of monomer conversion was studied. For this purpose, the polymerization was carried out using photoinitiator with different concentrations ranging from 5 × 10−4 M to 2 × 10−2 M. Fig. 3 shows the effect of concentration of photoinitiator on the kinetics of TMPTA polymerization and monomer conversion. Table 2 presents the changes in degree of monomer conversion with increasing concentration of photoinitiator.
| Co-initiator | Degree of monomer conversion [%] | |||||
|---|---|---|---|---|---|---|
| 5 × 10−4 M | 1 × 10−3 M | 2 × 10−3 M | 5 × 10−3 M | 1 × 10−2 M | 2 × 10−2 M | |
| I2 | 8.8 | 13.52 | 14.22 | 18.46 | 31.10 | 32.14 |
| I77 | 8.76 | 12.34 | 11.68 | 17.47 | 20.79 | 19.82 |
| I78 | 5.12 | 11.82 | 11.74 | 17.39 | 20.58 | 17.78 |
| I79 | 5.30 | 13.09 | 15.83 | 19.20 | 27.85 | 28.91 |
| I80 | 10.54 | 13.81 | 13.69 | 18.87 | 21.30 | 24.60 |
| I81 | 44.26 | 44.57 | 48.72 | 53.07 | 41.59 | 49.39 |
| I83 | 6.33 | 14.08 | 9.82 | 17.71 | 20.80 | 25.96 |
| I84 | 10.17 | 17.45 | 16.70 | 23.55 | 22.21 | 19.38 |
| I85 | 9.13 | 17.33 | 16.35 | 18.39 | 21.69 | 17.13 |
| I86 | 12.26 | 14.60 | 22.40 | 24.54 | 27.83 | 24.38 |
| I87 | 11.98 | 13.55 | 17.51 | 19.29 | 20.71 | 20.43 |
| I90 | 12.0 | 11.26 | 11.35 | 11.17 | 11.07 | 7.83 |
| I92 | 3.73 | 13.40 | 12.12 | 16.76 | 21.15 | 14.61 |
| I93 | 12.43 | 11.88 | 16.23 | 19.44 | 21.52 | 19.45 |
The concentration of all components of photoinitiator affects the rate of polymerization. As the concentration of photoinitiator increases from 5 × 10−4 M to 2 × 10−3 M, the maximum polymerization rate increases and reaches a maximum. Further increase in the concentration of photoinitiator causes a decrease in the rate of polymerization. This effect is attributed to the “inter filter effect” and becomes more significant for photoinitiators with high molar extinction coefficient (for squarine tested, ε is about 4 × 104 dm3 mol−1 cm−1). Forty-fold increase in the concentration of photoinitiator results in two- or four-fold increase in the rate of polymerization. The degree of TMPTA conversion observed under experimental conditions ranging from 3.73% to 53% for tert-butyl- and nitro-substituted diphenyliodonium salt with concentration 5 × 10−4 M and 5 × 10−3 M, respectively.
For the majority of the photoinitiators depending on their concentration, the degree of conversion of double bonds is from about 10% to about 30%. The presence of nitro group in para position of phenyl ring results in a rapid increase in the conversion of monomer to more than 44% and 53% for the following concentrations of photoinitiator: 5 × 10−4 M and 5 × 10−3 M.
Taking into account, the kinetic results it is seen that, the highest rate of polymerization was observed when the concentration of photoinitiator equals 2 × 10−3 M. Therefore, the reactivity of all photoinitiators have been studied for concentration of sensitizer and co-initiator equal 2 × 10−3 M.
It should be noted, that in absence of active species source (co-initiator), the photopolymerization of triacrylate (TMPTA) was carried out with 1,3-bis(p-bromophenylamino)squaraine (SQ) (2 × 10−3 M) at irradiation ranging from 300 nm to 500 nm and no monomer conversion was observed.
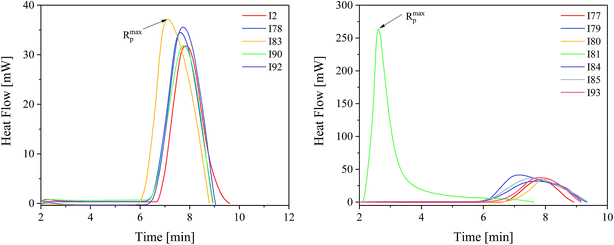
Fig. 4 depicts a characteristic profile obtained during exposure of polymerizing mixture composed of monomer and photoinitiator selected.
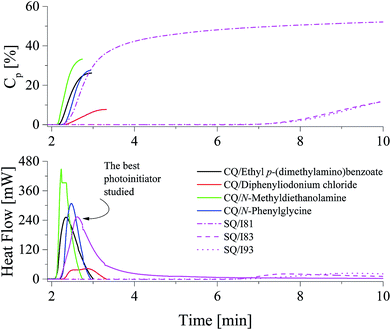
The kinetic results presented in Fig. 4 and Table 1 shown that the highest efficiency for the TMPTA polymerization was obtained for diphenyliodonium salt I81, whereas the lowest efficiency was obtained for I2, I79 and I90. It is obvious that there does not exist a clear relation between reactivity of photoinitiator and its structure. The rates of polymerization observed are in the range from 1.26 s−1 to 1.72 s−1 for almost all photoinitiators studied. With exception the photoinitiator composed of squaraine dye and diphenyliodonium salt possessing nitro group in para position of phenyl ring (I81). The maximum rate of polymerization achieved in this case is about 10.41 s−1 and is about 6 ÷ 8 times higher than that observed for other photoinitiators. These kinetic results also do not correlate with redox properties of components of photoinitiator. The majority of iodonium salts under study is reduced at the potential ranging from −0.395 eV to −0.106 eV. Thus, these salts can be readily reduced in comparison with (I81) (Ered = −0.554 eV) due to its lower reduction potential; therefore the photoredox systems that contain I77, I78, I79, I80, I84, I85, I86, I87, I90, I92 and I93 have higher values for ΔGel. However, kinetic studies show any relationship between the rate of polymerization and thermodynamic conditions of electron transfer process. Ability to initiate free radical polymerization of the TMPTA by iodonium salts studied is similar. In other words, most of the iodonium salts exhibit no large differences regarding the reactivity in TMPTA. Except to the onium salt comprising nitro group in para position of phenyl ring. Use of this type of photoinitiator results in about six-eight-fold increase in the rate of polymerization and high degree of TMPTA conversion of more than 53%. The highest reactivity of iodonium salt (I81) may be due to better solubility in the monomer used. It is well known, that in two-component photoinitiating systems, the rate of the photochemical reaction between photosensitizer and co-initiator depends not only on the free energy change but also on the environmental conditions.23 From the Strehmel's et al. studies it is known, that diphenyliodonium salts may exist as ion pairs, large associated ion pairs, incomplete dissociated in separated solvated ions or full dissociated in separated solvated ions.4 The nature of surrounding medium influences the dissociation process and determines the number of available separated solvated ions, that is also the solvated separated diphenyliodonium cation being available for the reaction with sensitizer in excited state. Thus, the high reactivity of a photoinitiator is a result of an effective electron transfer process due to the complete dissociation of iodonium salt in to well separated ions in monomer used.4 In order to really show the performance of the new photoinitiating systems under study the comparison with the reference initiating systems composed of commercially available camphorquinone (CQ) with different co-initiators was made. The photoinitiating ability of all photoinitiators is presented in Fig. 5.
The photoinitiating ability of systems composed of squaraine dye and (4-methoxyphenyl)-(4-nitrophenyl)iodonium p-toluenesulfonate (I81) is comparable with those observed for popular visible-light absorbing photoinitiator: camphorquinone/ethyl p-(dimethylamino)benzoate under experimental conditions. These redox pairs initiate radical polymerization of TMPTA with rate about 10.4 s−1. Camphorquinone in a presence of diphenyliodonium chloride is worst photoinitiator than the best photoinitiator proposed by us (SQ/I81). The efficiency of initiating the polymerization of acrylates by other new initiators studied is lower than that observed in the case of commercially available compounds. It should be also noted, that the degree of TMPTA conversion observed for commercial photoinitiators is relatively low and reaches value in the range from 12.4% to 33.41%. Polymerization initiates by the best photoinitiators under study leads to double bonds conversion above 50%.
3.3. Photochemical and photophysical characterization of photoinitiators
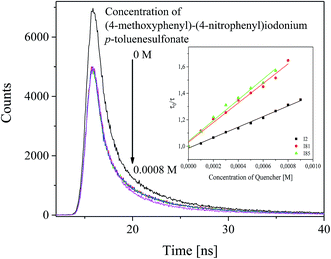
To elucidate the effect of iodonium salts on the polymerization rate, a detailed study of the photophysical and photochemical properties of these photoinitiators was carried out. As it was mentioned above and in our previous paper,37 the excited state of squaraine molecule may be quenched by different molecules in bimolecular reaction. For this purpose, selected iodonium salts: diphenyliodonium hexafluorophosphate (I2), (4-methoxyphenyl)-(4-nitrophenyl)iodonium p-toluenesulfonate (I81) and (4-trifluoromethylphenyl)-(4-methoxyphenyl)iodonium p-toluenesulfonate (I85) were used as a quencher. The fluorescence lifetimes (τ) of photosensitizer was measured in the absence and in the presence of iodonium salts.The influence of a co-initiator on fluorescence lifetime of photosensitizer and Stern–Volmer relationship are presented in Fig. 6.
For the sensitizer studied in a presence of quenchers (co-initiators) the shortening of average fluorescence lifetime was observed. This is interpreted as a result of interaction between dye and quencher molecule. The results obtained from fluorescence quenching experiments were analyzed with use the Stern–Volmer relationship eqn (4).
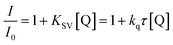 | (4) |
Form the data presented in Fig. 6, it is seen that the rate of dynamic quenching of the excited singlet state of sensitizer depends on the type of quencher used. The linear relationship between changes in the fluorescence lifetime and concentration of quencher is observed. The slopes of Stern–Volmer linear relationship (KSV) determined are as follows: 398.33, 738.10 and 781.97 for (I2) (I81), and (I85), respectively.
While, the rate constants of fluorescence quenching, kq were obtained to be 5.69 × 1010 M−1 s−1, 12.77 × 1010 M−1 s−1 and 13.53 × 1010 M−1 s−1 for (I2), (I81) and (I85), respectively, which are about two orders of magnitude higher than that of diffusion controlled bimolecular reaction constant (∼2 × 109 M−1 s−1). The values of the rate constants of fluorescence quenching calculated for p-substituted diphenyliodonium salts are about two-times higher than that calculated for unsubstituted diphenyliodonium salt, alkyltriphenylborate salt and N-alkoxypyridinium salt.37
These results confirmed that the co-initiators under study are very effective fluorescence quenchers for excited (SQ) dye and the fast quenching occurs predominantly through the intramolecular ion-pair pathway. The similar results were obtained by Y. He and et al.25 for other squaraine based photoinitiators composed of diphenyliodonium salts.
The influence of iodonium salt on the rate of fluorescence decay of sensitizer suggests that the primary photochemical process occurs between the excited dye and co-initiator in ground state. This phenomena may be a result of photoinduced electron transfer.38,39 During photoinduced electron-transfer process sensitizer plays a role of an electron donor, but iodonium salt acts as an electron acceptor.
As is mentioned above, an electron transfer process from excited sensitizer to iodonium cation was suggested as the first reaction step in the initiation mechanism. Another evidence supporting this reaction is the results obtained from the nanosecond laser flash photolysis experiment.
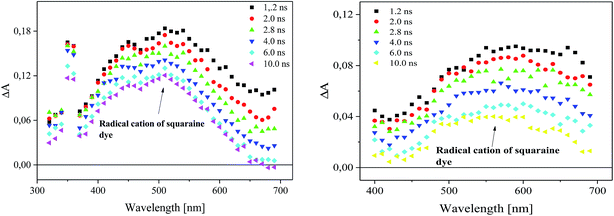
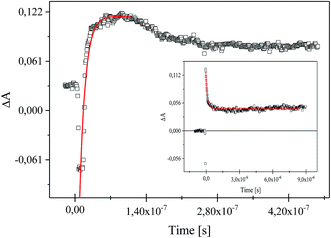
Following laser excitation of 1,3-bis(p-bromophenylamino)squaraine in presence of diphenyliodonium hexafluorophosphate (I2) and (4-methoxyphenyl)-(4-nitrophenyl)iodonium p-toluenesulfonate (I81) at 355 nm in acetonitrile, a transient absorption spectra show the appearance of two absorption bands at 380 nm and 510 nm, and 380 nm and about 550 nm, respectively (Fig. 7).
The irradiation of squaraine with visible light in acetonitrile with 5 ns laser pulse, leads to the excited singlet state formation observed at wavelength 380 nm. The rate constant of excited state formation is equal 2.48 × 108 s−1. The time of decay and the rate constant of the decay of SQ* are about 2.33 μs and 4.29 × 105 s−1, respectively. Next, the excited state of sensitizer (SQ*) is quenched by both iodonium salts, that results in decreasing of lifetime of SQ* with increase of concentration of iodonium salt. The new absorption bands observed about 510 nm and 550 nm assigned to the radical cation of squaraine are simultaneously formed as a result of electron transfer. At the same time the disappearance of the band corresponding to the absorption of the dye in the ground state is observed (Fig. 8).
The formation of radical cation of sensitizer as a result of an electron transfer process from sensitizer to iodonium salt (I81) and its disappearance are shown in Fig. 9.
The time of formation of new product and its disappearance is equal 6 ns and about 180 ns for both iodonium salts (I2) and (I81), respectively. It should be noted, that phenyl radical produced by fragmentation of diphenyliodonium salt are not readily detectable in the spectral region near UV and visible.26,40,41
The rate constant for the quenching of excited state of sensitizer, kq was determined by monitoring the excited state absorption decays of SQ* at 380 nm for various concentrations of iodonium salt using the Stern–Volmer equation. The established value of kq, is equal 0.163 × 106 mol−1 s−1 and 2.41 × 106 mol−1 s−1 for diphenyliodonium hexafluorophosphate (I2) and (4-methoxyphenyl)-(4-nitrophenyl)iodonium p-toluenesulfonate (I81), respectively. It is seen, that the value of rate constant for quenching of excited state of 1,3-bis(p-bromophenylamino)squaraine by iodonium salt possessing nitro-group in para position of phenyl ring is more than 10-times higher than others. This fact may be a reason such high reactivity of this co-initiator in initiation of free radical polymerization.
In summary, basing on the obtained results, one may conclude, that excited squaraine acts as an electron donor in the two-component photoinitiating system, composed of diphenyliodonium salt. It appears that singlet excited state of 1,3-bis(p-bromophenylamino)squaraine is oxidized by diphenyliodonium salts and following products are formed: sensitizer-based radical cation and diphenyliodonium radical. The co-initiator-based radical undergoes fast and irreversible C–I bond cleavage giving phenyl radical, active for initiation of radical polymerization and iodobenzene.
4. Conclusions
Electron-transfer photosensitization of onium salt in photoinitiated radical polymerization of acrylate monomer is a highly efficient process. The squaraine used as a photosensitizer is especially attractive since it is effective for the photosensitization of several structurally different iodonium salts photoinitiators. The polymerization rate enhancement effects in photosensitized polymerizations as compared to photopolymerizations conducted in the absence of such photosensitizer can be rationalized as due to the enhanced spectral absorption of these systems. Comparative studies of free radical photopolymerizations using different diphenyliodonium salts, showed that there are very little differences in the efficiencies of these photoinitiators. Except to onium salt comprising nitro group in para position of phenyl ring. Use of this type of photoinitiator results in about six-eight-fold increase in the rate of polymerization and a high degree of TMPTA conversion of more than 53%. In photoinitiating systems studied upon irradiation the photoinduced electron transfer reaction between excited SQ dye and diphenyliodonium salt occurs via intramolecular pathway, which have a strong driving force and high reaction rate as thermodynamic calculation and fluorescence quenching experiment shown. Moreover, such photoinitiating systems are interesting because they undergo bleaching at the excitation wavelength and open therefore the opportunity to cure coatings with a thickness up to several hundred micrometers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by The National Science Centre (NCN) (Cracow, Poland), Grant No. 2013/11/B/ST5/01281.References
- M. L. Gómez, C. M. Previtali and H. A. Montejano, Int. J. Photoenergy, 2012, 1–9 CrossRef.
- J. V. Crivello, UV Curing Science and Technology, Technology Marketing, Norwalk, Conn, USA, 1978, vol. 23 Search PubMed.
- H. Mokbel, J. Toufaily, T. Hamieh, F. Dumur, D. Campolo, D. Gigmes, J. P. Fouassier, J. Ortyl and J. Lalevée, J. Appl. Polym. Sci., 2015, 132 DOI:10.1002/app.42759.
- T. Brömme, D. Oprych, J. Horst, P. S. Pinto and B. Strehmel, RSC Adv., 2015, 5, 69915–69924 RSC.
- G. Manivannan, J. P. Fouassier and J. V. Crivello, J. Polym. Sci., Part A: Polym. Chem., 1992, 30, 1999–2001 CrossRef CAS.
- Y. Yağci and I. Reetz, Prog. Polym. Sci., 1998, 23, 1485–1538 CrossRef.
- Y. Yağci and Y. Hepuzer, Macromolecules, 1999, 32, 6367–6370 CrossRef.
- Y. Toba, J. Photopolym. Sci. Technol., 2003, 16, 115–118 CrossRef CAS.
- K. S. Padon and A. B. Scranton, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 2057–2066 CrossRef CAS.
- D. Kim and A. B. Scranton, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5863–5871 CrossRef CAS.
- E. W. Nelson, T. P. Carter and A. B. Scranton, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 247–256 CrossRef CAS.
- J. V. Crivello and M. Sangermano, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 343–356 CrossRef CAS.
- H. J. Timpe and A. G. Rajendran, Eur. Polym. J., 1991, 27, 77–83 CrossRef CAS.
- M. L. Gómez, H. A. Montejano and C. M. Previtali, J. Photochem. Photobiol., A, 2008, 197, 18–24 CrossRef.
- M. L. Gómez, C. M. Previtali, H. A. Montejano and S. G. Bertolotti, J. Photochem. Photobiol., A, 2007, 188, 83–89 CrossRef.
- J. P. Fouassier, X. Allonas and D. Burget, Prog. Org. Coat., 2003, 47, 16–36 CrossRef CAS.
- R. Podsiadły, A. Maruszewska, R. Michalski, A. Marcinek and J. Kolińska, Dyes Pigm., 2012, 95, 252–259 CrossRef.
- H. A. Mokbel, F. Dumur, B. Raveau, F. Morlet-Savary, C. Simonnet-Jégat, D. Gigmes, J. Toufaily, T. Hamieh, J. P. Fouassier and J. Lalevée, Tetrahedron, 2016, 72, 7686–7690 CrossRef CAS.
- U. Bulut, G. E. Gunbas and L. Toppare, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 209–213 CrossRef CAS.
- C. Selvaraju, A. Sivakumar and P. Ramamurthy, J. Photochem. Photobiol., A, 2001, 138, 213–226 CrossRef CAS.
- S. P. Pappas, B. C. Pappas and L. R. Gatechair, J. Polym. Sci., Part A: Polym. Chem., 1984, 22, 69–76 CrossRef CAS.
- T. Brömme, Ch. Schmitz, N. Moszner, P. Burtscher, N. Moszner, P. Burtscher, N. Strehmel and B. Strehmel, ChemistrySelect, 2016, 3, 524–532 CrossRef.
- X. Nan, Y. Huang, Q. Fan and J. Shao, Prog. Org. Coat., 2015, 81, 11–18 CrossRef CAS.
- N. Zivic, J. Zhang, D. Bardelang, F. Dumur, P. Xiao, T. Jet, D.-L. Versace, C. Dietlin, F. Morlet-Savary, B. Graff, J. P. Fouassier, D. Gigmes and J. Lalevée, Polym. Chem., 2016, 7, 418–429 RSC.
- Y. He, W. Zhou, F. Wu, M. Li and E. Wang, J. Photochem. Photobiol., A, 2004, 162, 463–471 CrossRef CAS.
- M. L. Gómez, C. M. Previtali, H. A. Montejano and S. G. Bertolotti, J. Photochem. Photobiol., A, 2007, 188, 83–89 CrossRef.
- J. Christmann, S. Shi, A. Ibrahim, Ch. Ley, C. Croutxé-Barghorn, M. Bessieres and X. Allonas, J. Phys. Chem. B, 2017, 121, 1972–1981 CrossRef CAS PubMed.
- T. Tanabe, A. Torres-Filho and D. C. Neckers, J. Polym. Sci., Part A: Polym. Chem., 1995, 36, 1691–1703 CrossRef.
- J. W. Knapczyk, J. J. Lubinkowski and W. E. Mc Even, Tetrahedron Lett., 1972, 35, 3739–3742 CrossRef.
- A. Kunze, U. Müller, K. Tittes, J. P. Fouassier and F. Morlet-Savary, J. Photochem. Photobiol., A, 1997, 110, 213–226 CrossRef.
- J. Hua and J. V. Crivello, Macromolecules, 2001, 34, 2488–2494 CrossRef.
- K. Y. Law, J. S. Facci, F. C. Bailey and J. F. Yanus, J. Imaging Sci., 1990, 34, 31–38 CAS.
- A. B. Karl, Synth. Met., 2001, 124, 385–391 CrossRef.
- P. F. Santos, L. V. Reis, I. Duarte, J. P. Serrano, P. Almeida, A. S. Oliveira and L. F. V. Ferreira, Helv. Chim. Acta, 2005, 88, 1135–1143 CrossRef CAS.
- S.-Y. Park, K. Jun and S.-W. Oh, Bull. Korean Chem. Soc., 2005, 26, 428–432 CrossRef CAS.
- J. Liu, G. D. Howard, S. H. Lewis, M. D. Barros and J. W. Stransbury, Eur. Polym. J., 2012, 48, 1819–1828 CrossRef CAS PubMed.
- J. Kabatc, K. Kostrzewska, K. Jurek, R. Dobosz and Ł. Orzeł, Dyes Pigm., 2016, 127, 179–186 CrossRef CAS.
- Y. Yağci, S. Jockusch and N. J. Turro, Macromolecules, 2010, 43, 6245–6260 CrossRef.
- S. Deniizligil, Y. Yağci and C. MacArdle, Polymer, 1995, 36, 3093–3098 CrossRef.
- D. Weldon, S. Holland and J. C. Scaiano, J. Org. Chem., 1996, 61, 8544–8546 CrossRef CAS.
- J. G. Radziszewski, Chem. Phys. Lett., 1999, 301, 565–570 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |