Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
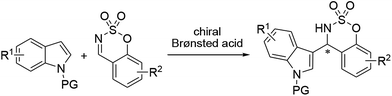
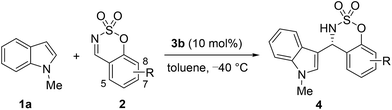
An asymmetric Brønsted acid-catalyzed Friedel–Crafts reaction of indoles with cyclic N-sulfimines†
Sang Gyu Lee and
Sung-Gon Kim *
*
Department of Chemistry, Kyonggi University, 154-42, Gwanggyosan-ro, Yeongtong-gu, Suwon 16227, Republic of Korea. E-mail: sgkim123@kyonggi.ac.kr
First published on 10th July 2017
Abstract
A highly enantioselective organocatalytic Friedel–Crafts reaction of indoles with cyclic N-sulfimines using a chiral phosphoric acid as an organocatalyst has been developed. This organocatalytic reaction provides for the first time 3-indolyl sulfamidate derivatives in good yields and with high enantioselectivities (up to 97% ee) with a broad range of functional groups and substitution patterns.
The indole skeleton is the most universal heterocycle structure in nature and is well established as a privileged scaffold; it is commonly encountered in many biologically active natural products and pharmaceutical compounds. Owing to the great structural diversity of biologically active indoles, indole units are of great importance in medicinal chemistry and are widely used in the pharmaceutical industry for the design of compounds with pharmacological properties.1 In particular, indoles bearing a nitrogen atom at the α-position are widely present as the structural core of many natural products and pharmaceuticals that exhibit a broad range of biological activities and have been the focus of extensive synthetic efforts for a long time.2 Because of their immense significance, numerous methods for the synthesis of chiral 3-indolyl methanamine structural scaffolds with careful stereochemical control have been developed. The asymmetric catalytic Friedel–Crafts reaction is one of the most powerful, straightforward and convenient methods for the preparation of these useful indole structures.3 To promote these transformations, significant progress has been made by employing both a chiral metal and an organocatalyst in the reaction of indoles with imines.4 However, to the best of our knowledge, an asymmetric Friedel–Crafts reaction with cyclic N-sulfimines that could enable the development of new strategies for the asymmetric synthesis of chiral indole derivatives has not yet been reported.5
Cyclic N-sulfimines have attracted much attention and have been proven to be powerful building blocks in the syntheses of functionalized benzosulfamidate heterocycles.6 These sulfamidate compounds exhibit important biological activities such as antibiotic, antiviral, anticancer, anticonvulsant, antiobesity, antiarthritis, and antiosteoporosis activities.7 Therefore, several reactions using cyclic N-sulfimines for the synthesis of sulfamidate derivatives have been reported, including allylation, annulation, cycloaddition, and the Mannich reaction.8 Herein, we report the first highly enantioselective Friedel–Crafts reaction of indoles with cyclic N-sulfimines catalyzed by a chiral Brønsted phosphoric acid (Scheme 1).9,10
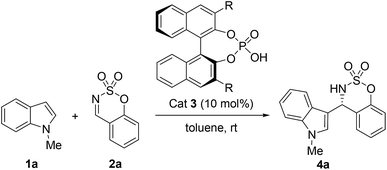
In our initial investigation, we began our studies on the Friedel–Crafts reaction between N-methylindole (1a) and benzoxathiazine 2,2-dioxide (2a) as the model substrates in the presence of a chiral BINOL-derived phosphoric acid catalyst 3 in toluene at room temperature (Table 1). The reaction proceeded smoothly to afford the desired product 4a in good yield (78%), but with 20% ee when phosphoric acid 3a (10 mol%) was used as the catalyst (Table 1, entry 1). Encouraged by this result, we investigated catalysts with various substitution patterns at the 3,3′-position of the binaphthyl scaffold (Table 1, entries 2–6), and chiral phosphoric acid 3b bearing two phenyl groups at the 3,3′-position of the binaphthyl scaffold proved to be the optimal catalyst, affording product 4a in 89% yield with 73% ee (Table 1, entry 2).
| Entry | 3, R | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a The reactions were carried out in toluene (0.2 M) with 1a (0.15 mmol) and 2a (0.1 mmol) in the presence of 10 mol% catalyst at room temperature.b Isolated yield after chromatographic purification.c Determined by chiral-phase HPLC analysis. | ||||
| 1 | 3a, H | 72 | 78 | 20 |
| 2 | 3b, Pheny1 | 18 | 89 | 73 |
| 3 | 3c, 4-Bipheny1 | 60 | 52 | 8 |
| 4 | 3d, 1-Naphthy1 | 48 | 43 | 7 |
| 5 | 3e, 2-Naphthy1 | 60 | 28 | 20 |
| 6 | 3f, 4-NO2-C6H4 | 48 | 36 | 3 |
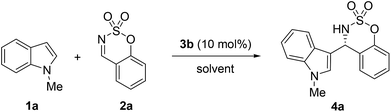
We further optimized the reaction conditions by using chiral phosphoric acid 3b as the catalyst. The results are summarized in Table 2. First, we screened a variety of solvents. Unfortunately, inferior results were generally observed (Table 2, entries 2–8). Then, the effect of the reaction temperature was investigated for this Friedel–Crafts reaction. The temperature was found to have a significant effect on the reaction. In general, lowering the temperature resulted in an increase of the enantioselectivity (73 to 91% ee, from room temperature to −40 °C, Table 2, entries 1, 9–11). Lower catalyst loadings were examined, and the use of 5 mol% of phosphoric acid 3b also led to the desired product in high yield and enantioselectivity, but a longer reaction time was required (Table 2, entry 12).
| Entry | Solvent | T (C) | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a The reactions were carried out in toluene (0.2 M) with 1a (0.15 mmol) and 2a (0.1 mmol) in the presence of 10 mol% catalyst at the indicated temperature.b Isolated yield after chromatographic purification.c Determined by chiral-phase HPLC analysis.d Reaction using 5 mol% catalyst 3b. | |||||
| 1 | Toluene | rt | 18 | 89 | 73 |
| 2 | CH2Cl | rt | 24 | 88 | 41 |
| 3 | ClCH2CH2Cl | rt | 24 | 73 | 57 |
| 4 | CHCl3 | rt | 24 | 95 | 55 |
| 5 | o-Xylene | rt | 18 | 87 | 71 |
| 6 | CH3CN | rt | 48 | 81 | 33 |
| 7 | THF | rt | 48 | 95 | 51 |
| 8 | MeOH | rt | 24 | 98 | 5 |
| 9 | Toluene | 0 | 24 | 93 | 78 |
| 10 | Toluene | −20 | 24 | 98 | 88 |
| 11 | Toluene | −40 | 24 | 93 | 91 |
| 12d | Toluene | −40 | 72 | 87 | 90 |
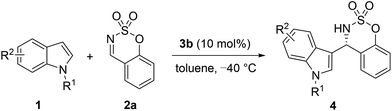
With the optimized reaction conditions in hand (1.5 equiv. of 1, 1 equiv. of 2, 10 mol% of catalyst 3b, in toluene at −40 °C), the substrate scope and generality of the reaction were investigated. First, we tested a variety of indole substrates 1 to examine the generality of the Friedel–Crafts reaction to yield 3-indolyl sulfamidate derivatives (Table 3).11 It appeared that N-protecting groups on the indole (such as Me, Bn, and allyl groups) were tolerated and the desired products were obtained in good yields (76–93%) with excellent enantioselectivities (90–92% ee, Table 3, entries 1–3). Both electron-donating and electron-withdrawing substituents on the indole ring were well tolerated, mostly leading to the desired products with good to excellent enantioselectivities. Electron-donating groups generally led to higher reaction activities and enantioselectivities than electron-withdrawing groups did (4d, 4e vs. 4f; 4g–4h vs. 4i–4l). Moreover, the position of the substituent on the indole ring was found to have a minimal impact on the reaction efficiency as well as the enantioselectivity, although the reaction with 6-chloroindole provided a moderate yield (Table 3, entries 13–15).
| Entry | R1 | R2 | Time (h) | Product | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
| a Unless otherwise noted, the reactions were carried out in toluene (0.2 M) with 1 (0.15 mmol) and 2a (0.1 mmol) in the presence of 10 mol% catalyst 3b at −40 °C.b Isolated yield after chromatographic purification.c Determined by chiral-phase HPLC analysis.d Reaction at 0 °C.e Reaction at RT. | ||||||
| 1 | Me | H | 24 | 4a | 93 | 91 |
| 2 | Bn | H | 48 | 4b | 76 | 90 |
| 3 | Allyl | H | 68 | 4c | 85 | 92 |
| 4 | Bn | 5-OMe | 72 | 4d | 95 | 94 |
| 5 | Bn | 5-OBn | 72 | 4e | 73 | 97 |
| 6d | Bn | 5-Br | 48 | 4f | 74 | 88 |
| 7 | Me | 5-OMe | 24 | 4g | 98 | 89 |
| 8 | Me | 5-OBn | 24 | 4h | 99 | 89 |
| 9d | Me | 5-Br | 48 | 4i | 87 | 68 |
| 10d | Me | 5-CN | 36 | 4j | 45 | 84 |
| 11d | Me | 5-CO2Me | 24 | 4k | 85 | 87 |
| 12e | Me | 5-NO2 | 168 | 4l | 88 | 78 |
| 13 | Me | 6-Cl | 72 | 4m | 48 | 78 |
| 14 | Me | 6-F | 72 | 4n | 88 | 85 |
| 15 | Me | 7-Me | 18 | 4o | 79 | 88 |
Encouraged by the excellent results obtained with various indoles, we then investigated the Friedel–Crafts reaction with respect to the cyclic N-sulfimines. As shown in Table 4, the 3-indolyl sulfamidate products were obtained in good yields with high enantioselectivities regardless of the electronic nature, bulkiness, and position of the substituent on the phenyl ring of the N-sulfimines. The cyclic N-sulfimines with electron-donating groups in the 6-position led to slightly higher enantioselectivities compare to those with electron-withdrawing groups (4p, 4q vs. 4r–4t). In addition, cyclic N-sulfimines bearing functional groups in the 8-position were also tolerated as substrates (Table 4, entries 8, 9 and 10).
| Entry | R | Time (h) | Product | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a The reactions were carried out in toluene (0.2 M) with 1a (0.15 mmol) and 2 (0.1 mmol) in the presence of 10 mol% catalyst 3b at −40 °C.b Isolated yield after chromatographic purification.c Determined by chiral-phase HPLC analysis. | |||||
| 1 | 6-Me | 72 | 4p | 95 | 91 |
| 2 | 6-OMe | 72 | 4q | 99 | 93 |
| 3 | 6-F | 72 | 4r | 96 | 84 |
| 4 | 6-Cl | 72 | 4s | 99 | 84 |
| 5 | 6-Br | 72 | 4t | 92 | 83 |
| 6 | 7-Me | 72 | 4u | 93 | 89 |
| 7 | 7-OMe | 72 | 4v | 45 | 80 |
| 8 | 6,8-Cl | 72 | 4w | 91 | 81 |
| 9 | 6,8-Br | 72 | 4x | 94 | 84 |
| 10 | 8-OMe | 72 | 4y | 96 | 85 |
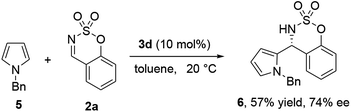
The absolute configuration was unambiguously determined by X-ray crystallographic analysis of 3-indolyl sulfamidate compound 4n and found to be S (Fig. 1a).12 The absolute configurations of the other products were assigned by analogy. On the basis of our experimental results and the transition state model of BINOL-phosphoric acids,13 we have proposed a simplistic plausible transition state to account for the observed stereoselectivity of the reaction (Fig. 1b). The cyclic N-sulfimine is activated by the phosphoric acid proton and then N-methylindole attacks the N-sulfimine from the Si-face preferentially, leading to an S-configuration adduct. Finally, an asymmetric catalytic Friedel–Crafts reaction of cyclic N-sulfimine with other nucleophiles have been developing. For example, the 2-pyrrolyl sulfamidate compound 6 was obtained in moderate yield and enantioselectivity (57% yield and 74% ee) when the catalytic Friedel–Crafts reaction between N-benzylpyrrole (5) and benzoxathiazine 2,2-dioxide (2a) in the presence of catalyst 3d in toluene at −20 °C (Scheme 2).
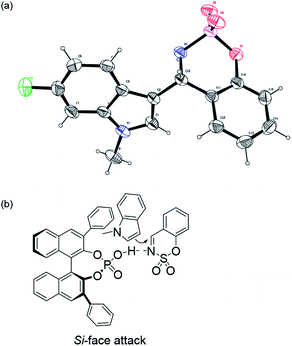 | ||
| Fig. 1 X-ray structure of 4n (a) with thermal ellipsoids at the 50% probability level and the proposed transition state model (b). | ||
Conclusions
In summary, we have developed a highly enantioselective Friedel–Crafts reaction of indoles with cyclic N-sulfimines catalyzed by a chiral phosphoric acid. This method represents the first aza-Friedel–Crafts reaction with cyclic N-sulfimines as electrophiles and provides the corresponding optically active 3-indolyl sulfamidate derivatives in good yields and with high enantioselectivities (up to 97% ee) with a broad range of functional groups and substitution patterns. Current work is focused on expanding the substrate scope of this asymmetric catalytic reaction. Studies on the biological activity of these 3-indolyl sulfamidate derivatives against diabetic peripheral neuropathy, in particular, are currently underway, and the results will be presented in due course.Acknowledgements
This research was supported by the Nanomaterial Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2012M3A7B4049645) and the Basic Science Research Program through NRF funded by the Ministry of Education (NRF-2013R1A1A2009850).Notes and references
- For recent reviews, see: (a) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742 RSC; (b) G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC; (c) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (d) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (e) S. Cacchi and G. C. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed.
- (a) P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley-VCH, Chichester, 2009 Search PubMed; (b) D. J. Faulkner, Nat. Prod. Rep., 2002, 19, 1–48 CAS; (c) D. H. R. Barton, K. Nakanishi, O. Meth-Cohn and J. W. Kelly, Comprehensive Natural Products Chemistry, Pergamon, Oxford, 1999 Search PubMed.
- For recent reviews for catalytic asymmetric Friedel–Crafts reaction, see: (a) I. P. Beletskaya and A. D. Averin, Curr. Organocatal., 2016, 3, 60 CrossRef CAS; (b) J. Vesely and R. Rios, Chem. Soc. Rev., 2014, 43, 611 RSC; (c) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626 CrossRef CAS PubMed; (d) M. Zeng and S.-L. You, Synlett, 2010, 1289 CAS; (e) V. Terrasson, R. M. de Figueiredo and J. M. Campagne, Eur. J. Org. Chem., 2010, 2635 CrossRef CAS.
- For the selected recent examples of asymmetric Friedel–Crafts reaction of indoles with imines, see: (a) T. Arai and J. Kakino, Angew. Chem., Int. Ed., 2016, 55, 15263 CrossRef CAS PubMed; (b) L. Osorio-Planes, C. Rodrŕguez-Escrich and M. A. Pericàs, Chem.–Eur. J., 2014, 20, 2367 CrossRef CAS PubMed; (c) T. Kano, R. Takechi, R. Kobayashi and K. Maruoka, Org. Biomol. Chem., 2014, 12, 724 RSC; (d) K.-F. Zhang, J. Nie, R. Guo, Y. Zheng and J.-A. Ma, Adv. Synth. Catal., 2013, 355, 3497 CrossRef CAS; (e) K. Wu, Y.-J. Jiang, Y.-S. Fan, D. Sha and S. Zhang, Chem.–Eur. J., 2013, 19, 474 CrossRef CAS PubMed; (f) J. Feng, W. Yan, D. Wang, P. Li, Q. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (g) Y. Qian, C. Jing, C. Zhai and W.-H. Hu, Adv. Synth. Catal., 2012, 354, 301 CrossRef CAS; (h) L.-Y. Chen, H. He, W.-H. Chan and A. W. M. Lee, J. Org. Chem., 2011, 76, 7141 CrossRef CAS PubMed; (i) C.-H. Xing, Y.-X. Liao, J. Ng and Q.-S. Hu, J. Org. Chem., 2011, 76, 4125 CrossRef CAS PubMed; (j) R. Husmann, E. Sugiono, S. Mersmann, G. Raabe, M. Rueping and C. Bolm, Org. Lett., 2011, 13, 1044 CrossRef CAS PubMed; (k) M. Rueping, S. Raja and A. Núñez, Adv. Synth. Catal., 2011, 353, 563 CrossRef CAS; (l) F. Xu, D. Huang, C. Han, W. Shen, X. Lin and Y. Wang, J. Org. Chem., 2010, 75, 8677 CrossRef CAS PubMed; (m) Y. Qian, G. Ma, A. Lv, H.-L. Zhu, J. Zhao and V. H. Rawal, Chem. Commun., 2010, 46, 3004 RSC; (n) G.-W. Zhang, L. Wang, J. Nie and J.-A. Ma, Adv. Synth. Catal., 2008, 350, 1457 CrossRef CAS.
- For the asymmetric Cu-catalyzed Friedel–Crafts reaction of indoles with cyclic N-sulfonyl ketimino esters, see: L. Wu, R.-R. Liu, G. Zhang, D.-J. Wang, H. Wu, J. Gao and Y.-X. Jia, Adv. Synth. Catal., 2015, 357, 709 CrossRef CAS.
- A. Kamal and P. B. Sattur, Synthesis, 1981, 272 CrossRef CAS.
- (a) S. J. Williams, Expert Opin. Ther. Pat., 2013, 23, 79 CrossRef CAS PubMed; (b) L. W. L. Woo, A. Purohit and B. V. L. Potter, Mol. Cell. Endocrinol., 2011, 340, 175 CrossRef CAS PubMed; (c) J.-Y. Winum, A. Scozzafava, J.-L. Montero and C. T. Supuran, Expert Opin. Ther. Pat., 2006, 16, 27 CrossRef CAS PubMed; (d) J.-Y. Winum, A. Scozzafava, J.-L. Montero and C. T. Supuran, Med. Res. Rev., 2005, 25, 186 CrossRef CAS PubMed.
- (a) L. De Munck, A. Monleón, C. Vila and J. R. Pedro, Adv. Synth. Catal., 2017, 359, 1582 CrossRef CAS; (b) M. Quan, L. Tang, J. Shen, G. Yang and W. Zhang, Chem. Commun., 2017, 53, 609 RSC; (c) H. B. Hepburn, G. Magagnano and P. Melchiorre, Synthesis, 2017, 49, 76 CAS; (d) L. De Munck, C. Vila, M. C. Muñoz and J. R. Pedro, Chem.–Eur. J., 2016, 22, 17590 CrossRef CAS PubMed; (e) J. Wang, F. Li, Q. Shen, W. Pei, W.-X. Zhao and L. Liu, Synthesis, 2016, 48, 441 CAS; (f) B.-N. Lai, J.-F. Qiu, H.-X. Zhang, J. Nie and J.-A. Ma, Org. Lett., 2016, 18, 520 CrossRef CAS PubMed; (g) M. Montesinos-Magraner, R. Cantón, C. Vila, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, RSC Adv., 2015, 5, 60101 RSC; (h) N. Gigant, E. Drège, P. Retailleau and D. Joseph, Chem.–Eur. J., 2015, 21, 15544 CrossRef CAS PubMed; (i) L. D. Munck, A. Monleón, C. Vila, M. C. Muñoz and J. R. Pedro, Org. Biomol. Chem., 2015, 5, 60101 Search PubMed; (j) T. Jiang, Z. Wang and M.-H. Xu, Org. Lett., 2015, 17, 528 CrossRef CAS PubMed; (k) C. Jiang, Y. Lu and T. Hayashi, Angew. Chem., Int. Ed., 2014, 53, 9936 CrossRef CAS PubMed; (l) H.-X. Zhang, J. Nie, H. Cai and J.-A. Ma, Org. Lett., 2014, 16, 2542 CrossRef CAS PubMed; (m) K. Parthasarathy, A. R. Azcargorta, Y. Cheng and C. Bolm, Org. Lett., 2014, 16, 2538 CrossRef CAS PubMed; (n) H. Yu, L. Zhang, Z. Yang, Z. Li, Y. Zhao, Y. Xiao and H. Guo, J. Org. Chem., 2013, 78, 8427 CrossRef CAS PubMed; (o) Y. Liu, T.-R. Kang, Q.-Z. Liu, L.-M. Chen, Y.-C. Wang, J. Liu, Y.-M. Xie, J.-L. Yang and L. He, Org. Lett., 2013, 15, 6090 CrossRef CAS PubMed; (p) Y.-Q. Wang, Y. Zhang, H. Dong, J. Zhang and J. Zhao, Eur. J. Org. Chem., 2013, 3764 CrossRef.
- For selected reviews on organocatalysis, see: (a) H. Pellissier, Recent Developments in Asymmetric Organocatalysis, RSC, Cambridge, 2010 Search PubMed; (b) S. Bertelsen and K. A. Jørgensen, Chem. Soc. Rev., 2009, 38, 2178 RSC; (c) A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638 CrossRef CAS PubMed; (d) S. Mukherjee, J. W. Yang, S. Hoffmann and B. List, Chem. Rev., 2007, 107, 5471 CrossRef CAS PubMed; (e) P. I. Dalko, Enantioselective Organocatalysis, Wiley-VCH, Weinheim, 2007 Search PubMed; (f) A. Erkkilä, I. Majander and P. M. Pihko, Chem. Rev., 2007, 107, 5416 CrossRef PubMed; (g) A. Berkessel and H. Gröger, Asymmetric Organocatalysis, Wiley-VCH, Weinheim, 2005 Search PubMed.
- For selected reviews on chiral BINOL-derived phosphoric acids, see: (a) H. Wu, Y.-P. He and F. Shi, Synthesis, 2015, 47, 1990 CrossRef CAS; (b) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS PubMed; (c) M. Rueping, A. Kuenkel and I. Atodiresei, Chem. Soc. Rev., 2011, 40, 4539 RSC; (d) A. Zamfir, S. Schenker, M. Freund and S. B. Tsogoeva, Org. Biomol. Chem., 2010, 8, 5262 CAS.
- The reaction of unprotected indole with benzoxathiazine 2,2-dioxide 2a under the optimized reaction conditions provided no desired 3-indolyl sulfamidate product, and no reaction was observed in the case of Boc- and Ts-protected indoles.
- CCDC 1540070 contains the supplementary crystallographic data for 4n.†.
- (a) M. Rueping, B. J. Nachtsheim, W. Ieawsuwan and I. Atodiresei, Angew. Chem., Int. Ed., 2011, 50, 6706 CrossRef CAS PubMed; (b) L. Simón and J. M. Goodman, J. Org. Chem., 2010, 75, 589 CrossRef PubMed; (c) I. D. Gridnev, M. Kouchi, K. Sorimachi and M. Terada, Tetrahedron Lett., 2007, 48, 497 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1540070. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra06244c |
| This journal is © The Royal Society of Chemistry 2017 |