Open Access Article
Open Access ArticleStudy of the estrogenic-like mechanism of glycosides of cistanche using metabolomics†
H. Songac,
W. L. Li*b,
B. M. Liud,
X. M. Suna,
J. X. Dinga,
N. Chena,
Y. B. Jiac and
Z. Xiang *a
*a
aInstitute of Materia Medica, Research Center of Life Sciences and Environmental Sciences, Harbin University of Commerce, Harbin, Heilongjiang 150076, China. E-mail: rainbowaftersnow@hotmail.com
bSchool of Pharmacy, Harbin University of Commerce, Harbin, Heilongjiang 150076, China. E-mail: lwldzd@163.com
cEngineering Research Center of Natural Anticancer Drugs of Ministry of Education, Harbin University of Commerce, Harbin, Heilongjiang 150076, China
dHeilongjiang Provincial Hospital, Harbin, Heilongjiang 150001, China
First published on 11th August 2017
Abstract
Cistanche deserticola, known as Rou Cong-Rong in China, has been used as a tonic for more than 1800 years, with previous studies demonstrating that glycosides of cistanche (GCs) are a main active component. In this study, a uterotrophic assay and histological analysis were utilized to confirm the estrogenic activity of GCs, and UPLC-MS/MS-based metabolomics was used to explore the estrogenic-like mechanism of GCs in serum and urine. Seven altered differential metabolites, including citric acid, taurine, proline, betaine, ornithine, pyroglutamic acid, and α-ketoglutaric acid, were of particular interest due to being present in both serum and urine. Moreover, the differential metabolites were categorized into several major pathways, including the citrate cycle (TCA cycle), glutathione metabolism, arginine and proline metabolism, D-glutamine and D-glutamate metabolism, phenylalanine, tyrosine, and tryptophan biosynthesis, and phenylalanine metabolism. The estrogenic-like mechanism of GCs could be concluded as closely related to the TCA cycle and glutathione metabolism due to these pathways being present in both serum and urine. Our results shed light on the estrogenic-like mechanism of GCs, which will be helpful for GC development and utilization.
Introduction
Estrogens play an important role in many developmental, physiological, and related processes, including uterus development.1–3 However, long-term use of estrogen can have many side effects, such as increasing the risk of breast cancer, endometrial cancer, and other gynecological tumors.4,5 Therefore, researchers have committed to seeking estrogen substitutes that could avoid these side-effects, known as selective estrogen receptor modulators, of which phytoestrogens comprise an important category.6 Phytoestrogen compounds naturally occur in plants with estrogenic potency and can bind to estrogen receptors to exert their activity. Therefore, phytoestrogens could stimulate uterus growth by combining with the abundant estrogen receptors in the uterus, meaning that the uterotrophic assay could be used for preliminary screening and evaluating phytoestrogens.7Cistanche deserticola (CD), known as Rou Cong-Rong in China, is allegedly effective for reproduction, development, and fertility functions, and has been used as a tonic for more than 1800 years.8,9 Recently, pharmacology studies have demonstrated that this tonic has broad medicinal functions, such as hormone regulation, aperient, immunomodulatory, anti-oxidative, anti-apoptotic, neuroprotective, anti-nociceptive, anti-inflammatory, anti-fatigue, and estrogenic activities.10 Glycosides of cistanche (GCs) extracted from CD are among the main active components and exhibit various biological activities.11 Although the active constituents of CD have been elucidated previously,12 the estrogenic-like mechanism of GCs has never been investigated.
In this continuing study, we aimed to confirm the possible use of GCs as phytoestrogens and carry out a metabolomics analysis to explore the estrogenic-like mechanism of GCs. First, a uterotrophic assay and histological analysis were utilized to confirm the estrogenic activity of GCs. Most importantly, we focused on the metabolic changes in rat serum and urine using UPLC-MS/MS-based metabolomics analysis. Due to the deficiencies of non-targeted metabolomics, such as repeatability and complicated matrix influence, an MRM mode-based pseudo method was used to specifically monitor the metabolites, and indexes (including energy metabolism, oxidative stress, lipid metabolism, and amino metabolism) related to estrogenic effects, growth, and development were selected as biomarkers for detection. Our results shed light on the estrogenic-like mechanism of GCs, which will aid the development and utilization of GCs.
Experimental
Reagents
L-Leucine, L-kynurenine, L-tryptophan, 5-HTP, cholic acid, N-phenylacetylglycine, 5-HT, glutathione (GSH), and 2,4-dinitrophenylhydrazine (≥99.0%, HPLC) were purchased from Dalian Melone Biology Technology Co. (Dalian, China). N-Ethylmaleimide (≥98%, HPLC), L-glutathione oxidized (GSSG, ≥98%, HPLC), and 1,1,3,3-tetraethoxypropane (TEP) were purchased from Sigma (Madrid, Spain). L-Phenylalanine (Ring-D5, 98%, DLM-1258-5) was purchased from Cambridge isotope laboratories (MA, USA). MS-grade methanol and acetonitrile were purchased from ACS (Houston, USA). Diethylstilbestrol (purity, ≥99.0%), formic acid, glacial acetic acid, and hematoxylin and eosin (H&E) were purchased from Sigma-Aldrich (Sigma Chemical Co., St. Louis, USA). Naringenin (purity, ≥98% (HPLC)) was purchased from Shanghai Jingchun Aladdin Reagent Co. (Shanghai, China). GCs were prepared in our laboratory, and their purity was determined to be 60% by ultraviolet spectrophotometry using acteoside as a marker for determination.Preparation of GCs
Briefly, cistanche powder (100 g) was soaked in 75% ethanol (1000 mL) for 1 h, then reflux extracted for 2.5 h three times. The supernatant was concentrated under vacuum to obtain cistanche extract (23.3 g). The extract was diluted with water to a concentration of 0.5 g mL−1 (solubility determined with crude drug). After adsorption with AB-8 macroporous resin for 8 h, the column was eluted sequentially with water and 85% ethanol. The 85% ethanol eluant was concentrated to obtain ethanol extract (8.4 g). Ethanol extract (0.02 g) was accurately weighed and dissolved in 50% methanol (10 mL). A 1 mL aliquot of the solution was diluted to 100 mL with methanol in a volumetric flask. Finally, the diluted solution was measured at 330 nm by ultraviolet spectrophotometry. The ultraviolet absorption was 0.341, the linear relationship for acteoside by UV was y = 24.905X + 0.0426 (R2 = 0.9982). When containing 5.04 g of GCs, the purification rate was 60% (acteoside was used as a marker for GC determination). Fingerprint evaluation of the GCs is shown in ESI Fig. S1.†Animal experiments and sample collection
Female SD rats of sexual immaturity (45–60 g) and sexual maturity (320–380 g) were provided by the Animal Center of Harbin Medical University, laboratory animal license, SCXK-(Army): 2013-001. The animals were housed under SPF laboratory conditions and provided with a standard laboratory diet and filtered tap water ad libitum. All animal procedures were approved by the Heilongjiang Provincial Animal Welfare and Care Guidelines, and performed according to the National Institute of Health guidelines regarding the principles of animal care (2004). All rats used in this experiment were acclimatized to the above environment for a week.The sexually immature SD rats were randomly divided into three groups of 10 rats: blank group, diethylstilbestrol group, and GC group. Meanwhile, 10 SD rats of sexual maturity were selected as the control group. The diethylstilbestrol group was i.g administered with diethylstilbestrol (0.35 mg kg−1, 1 mL/100 g), the GC group was i.g administered with the GC solution (30 g kg−1, 1 mL/100 g), and the blank group and control group received distilled water of the same volume twice a day (morning and evening) for 3 days. On the third day, the rats were housed in metabolic cages after the administration was finished and urine samples were continuously collected for 24 h. The rats were anesthetized using pentobarbital and blood samples were obtained and collected from abdominal aorta and centrifuged at 3000 × g (15 min, 4 °C) to obtain serum. All samples were stored at −20 °C. Furthermore, the uterus was separated, weighed, and fixed using 10% formalin.
Histological analysis
Serial 5 μm-thick tissues were cut from the fixed uterus. The tissue sections were then embedded in paraffin, stained with H&E using routine methods, and observed with an optical microscope (BZ-9000; Keyence, Osaka, Japan). The height of uterine epithelial cells was measured with a micrometer under the microscope.Metabolomics
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm for 20 min. The supernatant was filtered using Millipore express PES membranes (Merck Millipore, Ltd.) attached to a 20 mL syringe in the following sequence: 5 μm, 1.2 μm, and 0.45 μm. The “stripped” serum was confirmed to be free of biomarkers by LC-MS/MS.13,14
500 rpm for 20 min. The supernatant was filtered using Millipore express PES membranes (Merck Millipore, Ltd.) attached to a 20 mL syringe in the following sequence: 5 μm, 1.2 μm, and 0.45 μm. The “stripped” serum was confirmed to be free of biomarkers by LC-MS/MS.13,14During the analysis, a derivatization step was necessary to avoid GSH degradation, which improved the stability for detection and quantification of GSH. GSH was determined after reaction with NEM.15,16 According to the previous report, 50 mM NEM was selected for GSH.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, the supernatants (1000 μL) were transferred to 2 mL centritubes and evaporated to dryness. The dried residue was reconstituted in distilled water (100 μL) after centrifugation for 15 min at 4 °C and 13
000 rpm for 10 min, the supernatants (1000 μL) were transferred to 2 mL centritubes and evaporated to dryness. The dried residue was reconstituted in distilled water (100 μL) after centrifugation for 15 min at 4 °C and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm, and a 10 μL aliquot of the supernatant was injected for analysis.
500 rpm, and a 10 μL aliquot of the supernatant was injected for analysis.For urinary samples, 100 μL of sample was placed in a 2 mL tube, and 1 volume of PBS (m/v) containing 50 mM NEM was added to each urinary sample. Then, methanol (1000 μL containing IS at 10 ng mL−1) was added, and the sample was incubated at −20 °C for 20 min, and then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C. The supernatant (1000 μL) was evaporated to dryness under a gentle stream of nitrogen at room temperature, and then the residue was dissolved in 60 μL of mobile phase and vortexed for 1 min before centrifugation at 13
000 rpm for 10 min at 4 °C. The supernatant (1000 μL) was evaporated to dryness under a gentle stream of nitrogen at room temperature, and then the residue was dissolved in 60 μL of mobile phase and vortexed for 1 min before centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm and 4 °C for 15 min. A 10 μL aliquot supernatant was injected for analysis.
500 rpm and 4 °C for 15 min. A 10 μL aliquot supernatant was injected for analysis.
Results and discussion
Effect of GCs on the uterus
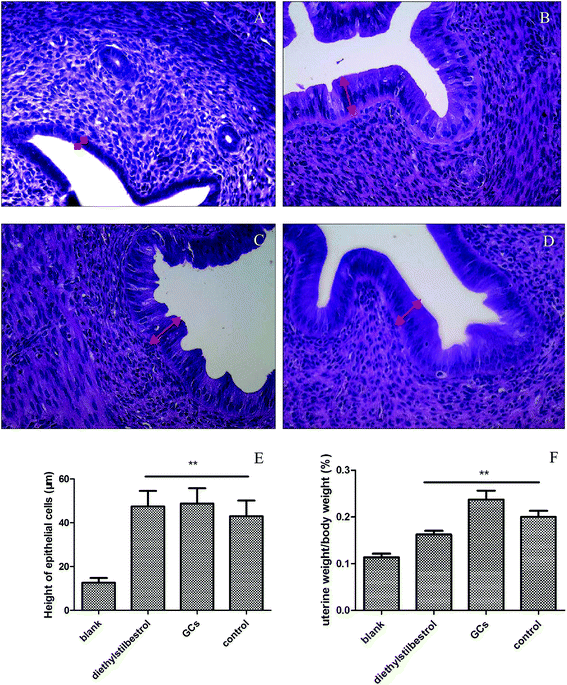
Uterine weight and histological analysis were used to evaluate the effect of GCs on the uterus. Uterine weight was significantly increased in the diethylstilbestrol, GC, and control groups relative to the blank group (P < 0.01). In the blank group, the endometrial epithelium was low columnar with small glands, a cubic-shaped glandular epithelium, and without interstitial inflammatory cells. In the diethylstilbestrol group, the glandular epithelium and endometrium were high columnar and showed hyperplasia, with a lot of interstitial inflammatory cells. In the GC group, the glandular epithelium and endometrium were high columnar serrated and showed hyperplasia with a lot of interstitial inflammatory cells and intimal thickening. In the control group, the endometrium was high columnar and the glandular epithelium was low columnar, with few interstitial inflammatory cells. Compared with the blank group, the height of the uterine epithelial cells was significantly increased in the other groups (P < 0.01) (Fig. 1).Method validation of detected biomarker by UPLC-MS/MS
| Sample | Compound | Standard curves | 1/X weight | R2 | Range |
|---|---|---|---|---|---|
| Serum | GSH | Y = 0.0002X − 0.0069 | Y = −0.0005X + 0.0001 | 0.9995 | 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
| GSSG | Y = 0.0027X + 0.3711 | Y = −0.0121X + 0.0023 | 0.9981 | 200–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| L-Leucine | Y = 0.0287X + 3.0001 | Y = −0.0287X + 0.0295 | 0.9983 | 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| L-Kynurenine | Y = 0.0412X + 0.0153 | Y = −0.0049X + 0.0344 | 0.9980 | 5–500 ng mL−1 | |
| L-Tryptophan | Y = 0.0021X + 0.9165 | Y = 1.6125X + 0.0048 | 0.9921 | 600–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| 5-HTP | Y = 0.0911X − 0.0099 | Y = 0.0005X + 0.0883 | 0.9919 | 0.2–20 ng mL−1 | |
| Cholic acid | Y = 0.00011 X − 0.0012 | Y = −0.3509X + 0.0153 | 0.9932 | 40–4000 ng mL−1 | |
| 5-HT | Y = 0.0495 X + 0.0001 | Y = −0.0131X + 0.0531 | 0.9986 | 0.8–80 ng mL−1 | |
| N-Phenylacetylglycine | Y = 0.0162X + 3.1554 | Y = −0.3321X + 0.0143 | 0.9932 | 250–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| Urine | GSH | Y = 0.0031X − 0.1633 | Y = −0.0249X + 0.0031 | 0.9970 | 100–1000 ng mL−1 |
| GSSG | Y = 0.00002X + 0.0012 | Y = −0.00004X + 0.00005 | 0.9977 | 200–2000 ng mL−1 | |
| L-Leucine | Y = 0.0289X − 2.0061 | Y = −0.0287X + 0.0295 | 0.9986 | 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| L-Kynurenine | Y = 0.0188X − 0.0315 | Y = 0.0013X + 0.0182 | 0.9983 | 5–500 ng mL−1 | |
| L-Tryptophan | Y = 0.0009X − 0.277 | Y = −0.0184X + 0.0009 | 0.9997 | 600–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
|
| 5-HTP | Y = 0.0061X + 0.0016 | Y = 0.00007X + 0.0065 | 0.9950 | 0.2–20 ng mL−1 | |
| Cholic acid | Y = 0.00118X − 0.0037 | Y = 0.0021X + 0.035 | 0.9928 | 40–4000 ng mL−1 | |
| 5-HT | Y = 0.0069X + 0.0312 | Y = 0.0044X + 0.0072 | 0.9995 | 0.8–80 ng mL−1 | |
| N-Phenylacetylglycine | Y = 0.0016X + 0.1058 | Y = −0.0009X + 0.0016 | 0.9949 | 250–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng mL−1 000 ng mL−1 |
Firstly, the PCA model was built to exhibit the metabolic distinction of the four groups. From multivariate analysis, there were obvious metabolic differences between the GC groups (including diethylstilbestrol and control groups) and the blank group. The QC samples clustered together tightly in the score plot of PCA, which indicated that the system stability was accommodative for this metabolomics study (Fig. 2).
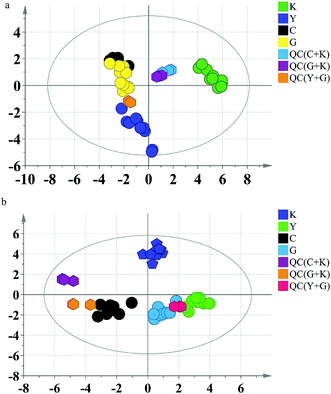 | ||
| Fig. 2 PCA score plots of rat data. (a) Serum; (b) urine. K: blank group; Y: diethylstilbestrol group; C: control group; G: GC group. | ||
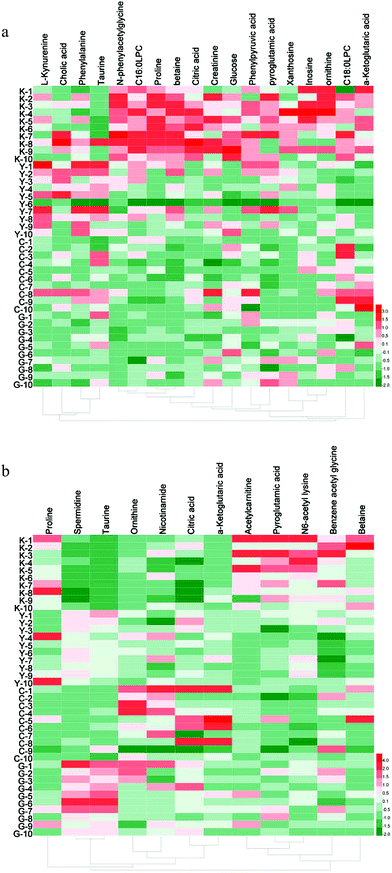
Then, the critical P-value was set to 0.05 for significantly differential metabolites in this research. Accordingly, as shown in Table 2, differential metabolites compared to the blank group were tentatively identified as follows: 17 in serum samples and 12 in urine samples for the GC group, 15 in serum samples and 9 in urine samples for the diethylstilbestrol group, 12 in serum samples and 11 in urine samples for the control group, and 11 in serum samples and 7 in urine samples that were simultaneously present in the GC, diethylstilbestrol, and control groups. To further understand the metabolic differences between different groups, a clustering heatmap was generated for all differential metabolites, demonstrating the relative increase (red) or decrease (green) (Fig. 3).
| Sample | Metabolites | Blank group | Diethylstilbestrol group | Control group | GCs group |
|---|---|---|---|---|---|
| a Compared to the blank group: *p < 0.05, **p < 0.01; compared to the diethylstilbestrol group: #p < 0.05, ##p < 0.01; compared to the control group: Δp < 0.05, ΔΔp < 0.01. | |||||
| Serum | Glucose | 50.54 ± 5.06##△△ | 27.91 ± 4.17** | 33.63 ± 3.08** | 33.41 ± 2.42** |
| Citric acid | 18.10 ± 2.49##△△ | 5.37 ± 0.70** | 7.16 ± 0.83** | 6.27 ± 0.84** | |
| Taurine | 15.66 ± 2.02## | 23.87 ± 2.40** | 20.59 ± 2.02 | 21.84 ± 1.37* | |
| Proline | 62.09 ± 3.70##△△ | 27.45 ± 3.26** | 24.40 ± 2.83** | 28.32 ± 2.61** | |
| Betaine | 8.30 ± 0.58##△△ | 5.53 ± 0.67**△△ | 2.89 ± 0.25**## | 3.56 ± 0.21**## | |
| Ornithine | 0.37 ± 0.071#△△ | 0.24 ± 0.048* | 0.12 ± 0.016** | 0.14 ± 0.021** | |
| Pyroglutamic acid | 0.61 ± 0.064 | 0.53 ± 0.062△△ | 0.31 ± 0.024**## | 0.44 ± 0.053* | |
| α-Ketoglutaric acid | 1.51 ± 0.16## | 0.66 ± 0.10**△△ | 1.38 ± 0.25## | 0.74 ± 0.12**△△ | |
| N-Phenylacetylglycine | 0.87 ± 0.086##△△ | 0.35 ± 0.061** | 0.23 ± 0.039** | 0.12 ± 0.019**## | |
| Phenylpyruvic acid | 1.34 ± 0.10#△ | 0.98 ± 0.12* | 1.05 ± 0.095* | 1.02 ± 0.061* | |
| Inosine | 0.96 ± 0.27##△△ | 0.11 ± 0.018** | 0.24 ± 0.10** | 0.25 ± 0.055** | |
| C18:0LPC | 1.31 ± 0.17## | 0.70 ± 0.094**△△ | 1.41 ± 0.14## | 0.82 ± 0.088*△△ | |
| C16:0LPC | 2.59 ± 0.25##△△ | 1.50 ± 0.23** | 1.00 ± 0.15** | 0.98 ± 0.13** | |
| Creatinine | 0.068 ± 0.011#△△ | 0.042 ± 0.0066* | 0.031 ± 0.008** | 0.034 ± 0.0061** | |
| Phenylalanine | 51.61 ± 4.14 | 62.66 ± 6.97△ | 46.27 ± 4.14# | 39.89 ± 1.96*## | |
| L-Kynurenine | 0.33 ± 0.036# | 0.53 ± 0.082*△△ | 0.26 ± 0.035## | 0.21 ± 0.021**## | |
| Xanthosine | 0.089 ± 0.023#△△ | 0.048 ± 0.0099*△ | 0.0070 ± 0.0027**# | 0.036 ± 0.0087* | |
| Urine | Benzene acetyl glycine | 8.17 ± 0.67##△ | 3.40 ± 0.62**△△ | 6.01 ± 0.72*## | 5.25 ± 0.71**# |
| Betaine | 11.33 ± 2.66# | 5.58 ± 0.84* | 6.63 ± 1.82 | 4.65 ± 0.77** | |
| Taurine | 7.91 ± 0.84##△△ | 30.48 ± 1.85**△△ | 17.01 ± 3.03**## | 44.29 ± 2.69**##△△ | |
| Citric acid | 81.70 ± 20.08△ | 116.34 ± 14.02 | 152.29 ± 35.27* | 144.44 ± 12.42* | |
| Ornithine | 0.12 ± 0.018#△△ | 0.19 ± 0.025*△△ | 0.37 ± 0.065**## | 0.26 ± 0.044* | |
| Pyroglutamic acid | 32.45 ± 3.99##△△ | 13.01 ± 1.74** | 12.15 ± 3.30** | 18.05 ± 2.26** | |
| Acetylcarnitine | 11.25 ± 1.74##△△ | 2.91 ± 0.26** | 4.27 ± 0.38** | 5.62 ± 1.06**# | |
| N6-Acetyl lysine | 4.02 ± 0.41##△△ | 2.66 ± 0.30**△△ | 1.19 ± 0.17**## | 2.14 ± 0.25**△ | |
| Nicotinamide | 3.08 ± 0.72△△ | 4.06 ± 0.79△ | 6.91 ± 1.01**# | 4.79 ± 0.93* | |
| α-Ketoglutaric acid | 27.88 ± 7.52#△△ | 50.44 ± 6.63* | 82.30 ± 17.99** | 52.21 ± 8.43* | |
| Spermidine | 1.29 ± 0.25##△ | 6.66 ± 0.31**△△ | 3.57 ± 0.98*## | 8.02 ± 0.63**△△ | |
| Proline | 65.14 ± 11.54△△ | 42.26 ± 10.24△ | 16.34 ± 5.23**# | 31.15 ± 5.88** | |
Eighteen differential metabolites simultaneously present in the GC, diethylstilbestrol, and control groups were described as follows: glucose, citric acid, proline, betaine, ornithine, N-phenylacetylglycine, phenylpyruvic acid, inosine, C16:0LPC, creatinine, and xanthosine in serum, and benzene acetyl glycine, pyroglutamic acid, acetylcarnitine, and N6-acetyl lysine in urine were observed to be significantly decreased (P < 0.05); while taurine, ornithine, α-ketoglutaric acid, and spermidine in urine were significantly increased (P < 0.05). Additionally, taurine (serum) was significantly increased, while α-ketoglutaric acid (serum), C18:0LPC (serum), and betaine (urine) were significantly decreased in the diethylstilbestrol and GC groups (P < 0.05); L-kynurenine (serum) was up-regulated in the diethylstilbestrol group, but down-regulated in the GC group (P < 0.05); pyroglutamic acid (serum) and proline (urine) were down-regulated, and citric acid (urine) and nicotinamide (urine) were markedly increased in control and GC group (P < 0.05); and phenylalanine was down-regulated in the GC group (P < 0.05).
In this study, the effects of GCs on the uterus of immature rats were investigated. The uterus is the most sensitive organ for assaying the ER-dependent effects of chemicals. Herba Cistanches have been reported to induce an increase in uterine weight by enhancing the lutropin-releasing function of the hypothalamic-pituitary-ovary,20 which was also observed for GCs in this study. This indicated that GCs have estrogenic-like activity. Recently, reports have mainly focused on the predominant mechanism by which estrogenic effects are expressed through binding to the ERs,21–23 but the metabolic mechanism has not been studied in depth. In this study, a pseudometabolomics method was used to explore the estrogenic-like mechanism of GCs.
Metabolic intermediates of a sequential series of reactions changed in a more pronounced fashion than enzymatic kinetics or individual fluxes.24 Seventeen metabolites in serum, including glucose, citric acid, taurine, proline, betaine, ornithine, pyroglutamic acid, α-ketoglutaric acid, N-phenylacetylglycine, phenylpyruvic acid, inosine, C18:0LPC, C16:0LPC, creatinine, phenylalanine, L-kynurenine, and xanthosine, and 12 metabolites in urine, including benzene acetyl glycine, betaine, taurine, citric acid, ornithine, pyroglutamic acid, acetylcarnitine, N6-acetyl lysine, nicotinamide, α-ketoglutaric acid, spermidine, and proline, were found to be involved in the estrogenic-like mechanism of GCs. Several altered metabolites were of special interest because they were present in both serum and urine. For instance, citric acid, formed by the condensation of acetyl coenzyme A and oxaloacetic acid and playing an important role in the citric acid cycle related to energy metabolism,25 was down-regulated in the serum of the GC group, but up-regulated in urine. Furthermore, α-ketoglutaric acid with a glutamine carbon frame, which can maintain total nitrogen balance and promote protein synthesis, and is the central material of the citric acid cycle, was down-regulated in the serum of the GC group, but up-regulated in urine.26 The citric acid cycle is not only the final metabolic pathway of three major nutrients (carbohydrates, lipids, and amino acids), but also the link among sugar, lipid, and amino acid metabolism, and the main way to obtain energy for the body.27,28 This showed that the estrogenic-like mechanism of GCs was related to energy metabolism, which is the same as diethylstilbestrol. Therefore, there was a clear link between the citric acid cycle and the increase in uterine weight in immature rats treated with GCs. As an important methyl donor, betaine plays an important role in fetus growth and development, indicating that betaine is involved in the increase in uterine weight.29 Interestingly, taurine was up-regulated in both serum and urine, which could promote lipid digestion and absorption, and cell uptake and utilization of glucose by promoting glucose metabolism. Taurine deficiency has also been reported to lead to weight loss in young animals, suggesting that taurine plays an important role in growth and development.30 Moreover, the regulation effect of betaine and taurine by GCs followed the same trend using diethylstilbestrol, but with an enhanced effect compared with diethylstilbestrol.
Furthermore, numerous amino acids were significantly altered. Our results suggested that GCs caused metabolic abnormalities in amino acids. Amino acid metabolism could be used for the synthesis of specific proteins, peptides, and other nitrogenous compounds, or decarboxylation by deamination, transamination, combined with ammonia decomposition, or for energy release through the citric acid cycle.31,32 Therefore, alterations of these compounds maybe involved in the important signaling events that trigger the increase in uterine weight.
Moreover, for a detailed pathway analysis, the differential metabolites were categorized into several major pathways including the citrate cycle (TCA cycle), glutathione metabolism, arginine and proline metabolism, D-glutamine and D-glutamate metabolism, biosynthesis of phenylalanine, tyrosine and tryptophan, phenylalanine metabolism, and other pathways using Pathway Analysis of MetaboAnalyst software (http://www.metaboanalyst.ca), as shown in Fig. 4. The energy metabolism-related pathway, including the TCA cycle and glutathione metabolism (both in serum and urine), was one of the main targets of estrogenic effects. The critical role of estrogenic chemicals in energy metabolism was verified by ERs regulating the genes required for mitochondrial function, TCA cycle, and more, according to previous studies.33,34 It could be concluded that the estrogenic-like mechanism of GCs was similar to that of diethylstilbestrol, with both related, to some extent, to the TCA cycle and glutathione metabolism, but with GCs performing better than diethylstilbestrol.
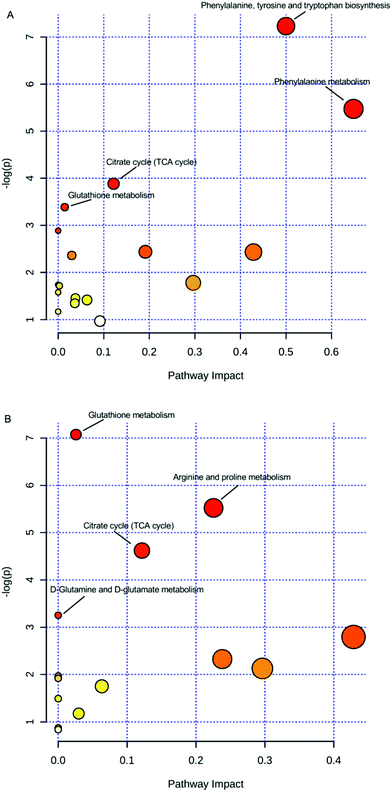 | ||
| Fig. 4 Pathways of differential metabolites. (A) Serum; (B) urine; small p values and large pathway impact factors indicate that the pathway is greatly influenced. | ||
Although the possible mechanisms could not be clarified in this study, some metabolites were selected that could be used to explore other estrogenic mechanisms of GCs in the uterus in the future. Therefore, to better explore the mechanism, other technology, such as proteomics, will be applied in future research.
Conclusions
To summarize, a serum and urine pseudotargeted metabolomics method based on UPLC-QTRAP MS was established for exploring the estrogenic-like mechanisms of GCs, which provided robust and reliable results. Using the established pseudotargeted approach, a holistic view of the changes in serum and urine metabolomics of the estrogenic-like mechanism (GCs) was revealed.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present study was supported by National Natural Science Foundation of China (81073015) and Harbin Applied Technology Research and Development Project (2015RQQXJ091). Young innovative talent training plan of College in Heilongjiang Province (UNPYSCT-2016182).References
- J. Cano-Nicolau, C. Vaillant, E. Pellegrini, T. D. Charlier, O. Kah and P. Coumailleau, Front. Neurosci., 2016, 10, 112 CrossRef PubMed.
- K. Dumasia, A. Kumar, S. Deshpande and N. H. Balasinor, Epigenetics, 2017, 31, 1–8 Search PubMed.
- G. Mirabolghasemi and Z. Kamyab, Int. J. Fertil. Steril., 2017, 11, 47–55 Search PubMed.
- L. J. Deleruyelle, Int. J. Pharm. Compd., 2016, 20, 447–454 Search PubMed.
- L. J. Deleruyelle, Int. J. Pharm. Compd., 2016, 20, 359–364 Search PubMed.
- T. Usui, Endocr. J., 2006, 53, 7–20 CrossRef CAS PubMed.
- M. D. Shelby, R. R. Newbold, D. B. Tully, K. Chae and V. L. Davis, Environ. Health Perspect., 1996, 104, 1296–1300 CrossRef CAS PubMed.
- L. Gu, W. T. Xiong, C. Wang, H. X. Sun, G. F. Li and X. Liu, Asian J. Androl., 2013, 15, 838–840 CrossRef CAS PubMed.
- F. Peng, R. Xu, X. Wang, C. Xu, T. Liu and J. Chen, Biol. Pharm. Bull., 2016, 39, 2066–2070 CAS.
- T. Wang, X. Zhang, W. Xie and Y. C. Ma, Am. J. Chin. Med., 2012, 40, 1123–1141 CrossRef CAS PubMed.
- Q. Chen, B. Yang, S. Gao and J. J. Zhang, Chin. Herb. Med., 2013, 5, 292–296 CrossRef.
- W. L. Li, X. M. Sun, H. Song, J. X. Ding, J. Bai and Q. Chen, J. Food Sci., 2015, 80, H2079–H2087 CrossRef PubMed.
- M. N. Samtani and W. J. Jusko, Biomed. Chromatogr., 2007, 21, 585–597 CrossRef CAS PubMed.
- Y. H. Kim, J. M. Kim, J. S. Lee, S. R. Gang, H. S. Lim, M. Kim and O. H. Lee, Food Chem., 2016, 190, 1086–1092 CrossRef CAS PubMed.
- R. Rossi, I. Dalle-Donne, A. Milzani and D. Giustarini, Clin. Chem., 2006, 52, 1406–1414 CAS.
- A. Carretero, Z. León, J. C. García-Cañaveras, A. Zaragoza, M. J. Gómez-Lechón, M. T. Donato and A. Lahoz, Anal. Bioanal. Chem., 2014, 406, 5465–5476 CrossRef CAS PubMed.
- M. Thompson, S. L. R. Ellison and R. Wood, Pure Appl. Chem., 2002, 74, 835–855 CrossRef CAS.
- Y. Huang, R. Shi, W. Gee and R. Bonderud, Bioanalysis, 2012, 4, 271–279 CrossRef CAS PubMed.
- G. Kaur, E. M. Leslie, H. Tillman, W. M. Lee, D. P. Swanlund and C. J. Karvellas, PLoS One, 2015, 10, e0139299 Search PubMed.
- P. W. Zhao, D. W. Wang, J. Z. Niu, J. F. Wang and L. Q. Wang, Zhongguo Zhongyao Zazhi, 2007, 32, 436–439 Search PubMed.
- S. J. Kim, S. W. Jin, G. H. Lee, Y. A. Kim and H. G. Jeong, Toxicol. Res., 2017, 33, 71–77 CrossRef PubMed.
- F. V. Le, S. Aït-Aïssa, M. Sonavane, J. M. Porcher, P. Balaguer, J. P. Cravedi, D. Zalko and F. Brion, Ecotoxicol. Environ. Saf., 2017, 142, 150–156 CrossRef PubMed.
- S. Zingue, C. B. Nde, T. Michel, D. T. Ndinteh, J. Tchatchou, M. Adamou, X. Fernandez, F. T. Fohouo, C. Clyne and D. Njamen, BMC Complementary Altern. Med., 2017, 17, 65 CrossRef PubMed.
- S. Fernández-Arroyo, A. Gómez-Martínez, L. Rocamora-Reverte, R. Quirantes-Piné, A. Segura-Carretero, A. Fernández-Gutiérrez and J. A. Ferragut, J. Pharm. Biomed. Anal., 2012, 63, 128–134 CrossRef PubMed.
- P. Lobit, M. Génard, B. H. Wu, P. Soing and R. Habib, J. Exp. Bot., 2003, 54, 2489–2501 CrossRef CAS PubMed.
- E. Riedel, M. Nündel and H. Hampl, Nephron, 1996, 74, 261–265 CrossRef CAS PubMed.
- O. E. Owen, S. C. Kalhan and R. W. Hanson, J. Biol. Chem., 2002, 277, 30409–30412 CrossRef CAS PubMed.
- R. S. Dalvi, T. Das, D. Debnath, S. Yengkokpam, K. Baruah, L. R. Tiwari and A. K. Pal, J. Therm. Biol., 2017, 65, 32–40 CrossRef CAS PubMed.
- M. K. Anas, M. A. Hammer, M. Lever, J. A. Stanton and J. M. Baltz, J. Cell. Physiol., 2007, 210, 266–277 CrossRef CAS PubMed.
- J. A. Sturman and J. M. Messing, J. Nutr., 1991, 121, 1195–1203 CAS.
- J. K. Cleal and R. M. Lewis, J. Neuroendocrinol., 2008, 20, 419–426 CrossRef CAS PubMed.
- M. A. Grillo, A. Lanza and S. Colombatto, Amino Acids, 2008, 34, 517–523 CrossRef CAS PubMed.
- C. R. Dufour, M. P. Levasseur, N. H. Pham, L. J. Eichner, B. J. Wilson, A. Charest-Marcotte, D. Duguay, J. F. Poirier-Héon, N. Cermakian and V. Giguère, PLoS Genet., 2011, 7, e1002143 CAS.
- C. Chaveroux, L. J. Eichner, C. R. Dufour, A. Shatnawi, A. Khoutorsky, G. Bourque, N. Sonenberg and V. Giguère, Cell Metab., 2013, 17, 586–598 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra06930h |
| This journal is © The Royal Society of Chemistry 2017 |