Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile shape-controlled synthesis of lanthanum oxide with different hierarchical micro/nanostructures for antibacterial activity based on phosphate removal
Jing Liu†
ab,
Ge Wang†ac,
Li Lua,
Yuming Guoab and
Lin Yang *ab
*ab
aCollaborative Innovation Center of Henan Province for Green Manufacturing of Fine Chemicals, Key Laboratory of Green Chemical Media and Reactions, Ministry of Education, School of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang, Henan 453007, P. R. China. E-mail: yanglin1819@163.com
bHenan Key Laboratory of Green Chemical Media and Reactions, Henan Key Laboratory of Boron Chemistry and Advanced Energy Materials, Henan Normal University, Xinxiang, Henan 453007, P. R. China
cSchool of Basic Medical Sciences, Xinxiang Medical University, Xinxiang, Henan 453003, P. R. China
First published on 22nd August 2017
Abstract
In this study, three La2O3 hierarchical micro/nanostructures, nanospindles, nanopolyhedra and nanospheres, were synthesized to remove phosphate from microbial growth media for bacterial inactivation as an antimicrobial strategy. The experiment results showed that the morphology of La2O3 hierarchical micro/nanostructures can be controlled by the concentration of the reactants, reaction temperature and cooling treatment. Meanwhile, the properties of the La2O3 were researched for phosphate removal and antibacterial activity. The results showed that the different morphologies of the La2O3 micro/nanostructures had different removal abilities for phosphate, and can differently inhibit growth of bacteria. Spherical La2O3 possesses the best removal ability and antibacterial activity, which indicates that the smaller the La2O3 hierarchical micro/nanoparticles are, the stronger the phosphate removal capacity. Compared with nanoparticles, the hierarchical micro/nanoparticles have a significant advantage: they not only have the properties of nanoparticles, but also are relatively stable, not easy to aggregate, and are easy to separate after the reaction. Therefore, the La2O3 hierarchical micro-nanomaterials may have very good application prospects for phosphate reduction in open water and to inhibit algae overgrowth.
1 Introduction
Recently, lanthanum compounds have attracted considerable attention in hydrogen storage, electrode and sorbent materials, gate insulators and superconductors due to their versatility and multifunctionality.1–4 Meanwhile, it is known that lanthanum compounds, such as lanthanum carbonate (La2CO3), lanthanum hydroxide (La(OH)3), lanthanum oxide carbonate (La2O2CO3),5–7 and mixtures of lanthanum compounds (La2O3, La2O2CO3 and La2(OH)3),8 can bind to phosphate so strongly that they can form LaPO4 and remove excess phosphate in bacterial or algal growth media,8 and lead to inhibit the growth of microorganisms and algae. As a typical representative, lanthanum carbonate has been developed as a medicine to reduce the excess phosphate in the human body.9 Based on the existing reports on bacterial and algal limitation utilizing lanthanum species,10–12 we here synthesize three La2O3 hierarchical micro/nanostructures and investigate their phosphate removal ability and antibacterial activity.It is widely accepted that phosphate is essential for growth of all organisms and serves as the main building block for nucleic acids, proteins, and energy carriers.13 Phosphate is the only form of phosphorus that can be directly assimilated by microorganisms. Firstly, the initial assimilation of inorganic phosphate for microorganisms proceeds via phosphorylation of ADP, which is affected by phosphate limitation in the environment.5 In addition, abundant phosphate is an essential condition to synthesize the cell wall of Gram-positive bacteria and the lipid composition of the membranes of Gram-negative bacteria. It could be affected strikingly within phosphate limitation environment.6 Consequently, the lack of phosphate often limits their growth, and efficient phosphate removal in water is expected to be a green strategy to prevent the growth of microorganisms and algae, which would avoid toxic substances released from antibacterial agents.14–16 On the other hand, reuse of secondary municipal effluent from wastewater treatment plants in water bodies could effectively alleviate freshwater resource shortage.9 During the wastewater treatment, excessive nutrients, e.g. phosphate, must be efficiently removed to prevent eutrophication.17
Considering the strong combination of lanthanum to phosphate and the valid antimicrobial strategy resulted from nutrient starvation of phosphate removal, it is meaningful to explore a facile, high quality and low cost method to synthesize La2O3 with micro/nanostructure. In the current study, shape-controlled La2O3 hierarchical micro/nanostructures were prepared through the regulation of the concentration of the reactants and the reaction temperature. Three morphologies of La2O3 micro/nanostructures were obtained, and were utilized to compare antimicrobial activity. The results indicated that lanthanum oxide can provide a broadly applicable antimicrobial strategy.
2 Experimental section
Material preparation
Series of morphologies micro-nanostructures of La2O3 were synthesized by a simple urea homogeneous precipitation process. All the chemicals, lanthanum nitrate hexahydrate La(NO3)3·6H2O, and urea were of analytical pure grade. The morphologies of La2O3 were controlled by the adjustment of the concentration of the reactants and the reaction temperature. The synthesis process consists of two steps: synthesis of La(OH)CO3 precursor and La2O3 product. Firstly, spindle La(OH)CO3 was synthesized at 90 °C with the concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 between La (NO3)3 and CO (NH2)2. While polyhedron La(OH)CO3 were prepared at 125 °C with the concentration ratio of 1
2 between La (NO3)3 and CO (NH2)2. While polyhedron La(OH)CO3 were prepared at 125 °C with the concentration ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 between La (NO3)3 and CO (NH2)2. In the same manner, when the concentration ratio between La (NO3)3 and CO (NH2)2 is 1
6 between La (NO3)3 and CO (NH2)2. In the same manner, when the concentration ratio between La (NO3)3 and CO (NH2)2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, spherical La(OH)CO3 was successfully synthesized at 85 °C. Subsequently, the La(OH)CO3 precursor was calcined from room temperature to 800 °C with a heating rate of 1 °C min−1 and maintained at this temperature for 4 h. Then spherical micro/nanostructures La2O3 were obtained. Other La2O3 (spindles, polyhedrons) samples were prepared by a similar procedure with different La(OH)CO3 precursors.
400, spherical La(OH)CO3 was successfully synthesized at 85 °C. Subsequently, the La(OH)CO3 precursor was calcined from room temperature to 800 °C with a heating rate of 1 °C min−1 and maintained at this temperature for 4 h. Then spherical micro/nanostructures La2O3 were obtained. Other La2O3 (spindles, polyhedrons) samples were prepared by a similar procedure with different La(OH)CO3 precursors.
Characterization
XRD measurements were determined using a D8 ADVANCE X-ray diffractometer at a scanning rate of 15° min−1 in the 2θ range from 10–80°, with graphite monochromatic Cu Ka radiation (λ = 0.15405 nm). Perking-Elmer 580B infrared spectrophotometer was used to measure Fourier transform infrared spectroscopy (FT-IR) spectra with the KBr pellet technique. Thermogravimetric and differential thermal analysis (TG-DTA) data were recorded with Thermal Analysis instrument (SDT 2960, TA Instruments, New Castle, DE) with the heating rate of 10 °C min−1 in an air flow of 100 mL min−1. SEM micrographs were obtained using a field emission scanning electron microscope (FE-SEM, S-4800, Hitachi). Microstructures and morphologies were investigated using scanning electronic microscopy (SEM, JEOL JSM-6390LV), transmission electron microscope (TEM, JEM-2100) and field emission scanning electron microscopy (FESEM, SUPRA40). All the measurements were performed at room temperature (RT). Brunauer–Emmett–Teller (BET) (Tristar Micromeritics, Norcross, GA, USA) method was used to determine the specific surface area at 77 K. The BET analysis was measured after out gassing for 90 minutes at 250 °C.Phosphate absorption capacity of lanthanum oxide nanoparticles and antibacterial activity
To evaluate the binding affinity of the nanoparticles to phosphate, PO43− was tested by colorimetry assay. Firstly, standard curve was drawn. Secondly, residual PO43− was determined. Specifically, 10% ascorbic acid was freshly prepared as follow: 10 g ascorbic acid was dissolved in 100 mL ddH2O. Then molybdate solution were prepared as follows: 13 g of (NH4)6Mo7O24·4H2O was dissolved in 100 mL of double distilled water (ddH2O). Meanwhile, 0.35 g K(SbO)C4H4O6·1/2H2O was dissolved in 100 mL of ddH2O. The prepared (NH4)6Mo7O24 and K(SbO)C4H4O6 solutions were added to 300 mL of dilute sulfuric acid successively. Whereafter the mixed solution was stirred and stored in 4 °C. Then La2O3 micro/nanoparticles with different morphologies were added to 15, 25, and 50 μg mL−1 phosphate solution, respectively. 1 mL of ascorbic acid (10%), 2 mL of molybdate solution and above La2O3 samples were mixed to determine residual PO43− according to absorbance at 710 nm by 752 Ultraviolet Spectrophotometer.18Escherichia coli (strain C43) and Staphylococcus aureus (strain TM300) were grown in Difco™ LB broth (Chemie Brunschwig) for 4 h at 37 °C and gently agitation to a concentration of about 108 CFU mL−1. This suspension was diluted to the required concentration with physiological saline (0.9 wt% NaCl in water) or where medium free (in particular phosphate free) was needed, repeatedly (5 times) centrifuged for 60 min at RCF = 1300 (Mistral 3000E, 2500 rpm). Subsequently, the supernatant was removed and the bacterial pellet was resuspended in minimal media. Minimal media was produced from 5 g glucose, 1 g NH4Cl, 5.4 g NaCl, 0.1 g MgSO4 and 0.02 g CaCl2 hexahydrate filled up to 1 liter with ddH2O and sterilized by autoclaving at 121 °C for 15 min. To quantify the CFU load in a sample, a dilution row (10−1–10−8) was plated in duplicate on dextrose agar plates (PDA, VWR BDH Prolabo). The plates were incubated at 37 °C for 24 h before readout.
3 Results and discussion
Phase structure, morphology and formation mechanism of La2O3
Polyhedral, spherical and spindles-like La2O3 micro/nanomaterials with hierarchical micro/nanostructures were synthesized by a simple precipitation method with urea and lanthanum nitrate. The synthesis process consists of two steps: synthesis of La(OH)CO3 and La2O3 (Scheme 1).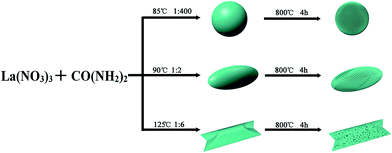 | ||
| Scheme 1 Schematic illustration for preparation of micro/nanostructures La2O3 with a simple precipitation method using urea and lanthanum nitrate. | ||
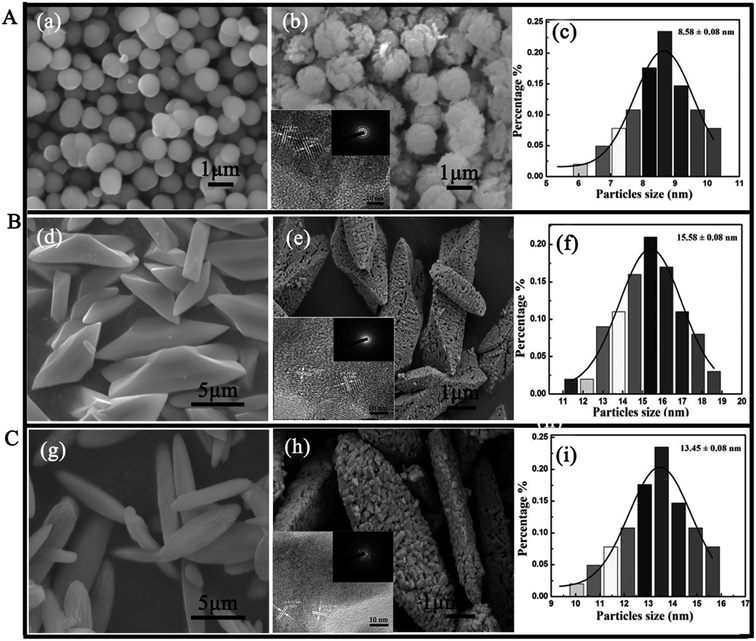
The morphology and microstructure of the products were characterized by SEM and TEM (shown in Fig. 1). The SEM images reveal that three different morphologies of La2O3 samples are successfully obtained, including polyhedral, spherical and spindles-like micro-nanostructures. The sizes of the samples are about 1 μm, 10 μm and 12 μm for spherical La2O3, polyhedral La2O3 and spindles La2O3 respectively. From Fig. 1 A(b), B(e) and C(h), the HRTEM images reveal that the surfaces of samples are very rough. It can be further observed that the samples consist of many even smaller nanoparticles with the size of 8–15 nm (inset of Fig. 1A(b), 2B(e) and 2C(h)), showing that the three morphologies of La2O3 possess hierarchical micro-nanostructures. The average diameters of the smaller nanoparticles are 8.58 ± 0.08 nm for spherical La2O3 (Fig. 1 A(c)), 15.58 ± 0.08 nm for polyhedral La2O3 (Fig. 1B(f)) and 13.45 ± 0.08 nm for spindles-like La2O3 (Fig. 1C(i)), respectively. In addition, the HRTEM shows that the values of inter planar lattice spacing are 0.29 nm, 0.31 nm and 0.34 nm, respectively, corresponding to the (101), (100) and (110) plane of La2O3. The results further show that the prepared La2O3 nanocrystals belong to cubic crystalline phase, which are in agreement with the XRD results (inset of Fig. 1A(b), 2B(e) and 2C(h)). In addition, the existence of detectable diffraction rings in the selected-area electron diffraction (SAED) pattern of three hierarchical nano-La2O3 (inset of Fig. 1A(b), 2B(e) and 2C(h)) further reveal the formation of polycrystalline products. These results further confirm the formation of the hierarchical nano-La2O3.
The growth mechanism of the nanostructures is investigated by FESEM and TEM in time-dependent experiments. The results reveal that three different morphologies of La2O3 samples, including polyhedral, spherical and spindles-like, are initially derived from spherical morphology, which show similar growth mechanisms to each other. We take spindles-like La2O3 for example to explain the growth mechanism in details (shown in Fig. 2). Initially, the La(OH)CO3 precursors of spherical morphology are formed within 0.5 hour reaction time (Fig. 2a), similarly to the formations of polyhedral and spherical La2O3 samples. Then, more La(OH)CO3 precursor products of spherical morphology assemble to form spindles-like morphology via oriented attachment within 1–3 hours (Fig. 2b–e). Meanwhile, non hollow structures are observed by characterization of TEM (inset of Fig. 2c). Then, we find spindles-like La(OH)CO3 precursors consist of small particles (inset of Fig. 2e). Finally, the La(OH)CO3 precursors are calcined from room temperature to 800 °C and maintained at this temperature for 4 h to form La2O3 samples with micro/nanostructures (Fig. 2f). The growth mechanisms of polyhedral and spherical products are also researched in time-dependent experiments. The similar growth mechanisms are observed.
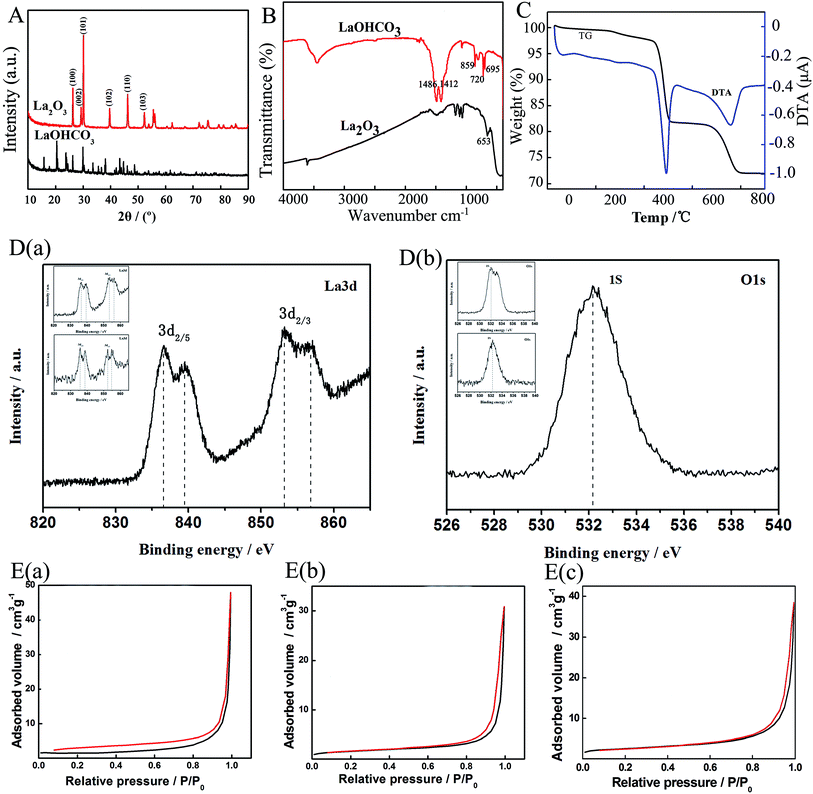
XRD patterns of three samples are very similar, one of which is depicted in Fig. 3A as a representation. From Fig. 3A, a strong intensity peak is detected at a diffraction angle of 30°, which is assigned to (101) plane of La2O3. The other five peaks are assigned to (100), (002), (102), (110) and (103) lattice planes belonging to cubic crystalline phase of La2O3 (JCPDS no. 26-0815). The results indicate that the pure La2O3 crystals are successfully prepared by the simple method. Representative FTIR spectra of spherical La2O3 and La(OH)CO3 precursor are shown in Fig. 3B. In the spectrum of La(OH)CO3, the bands at 1486 and 1412 cm−1 are attributed to the stretching vibration mode of the C–O bond, and the flexural vibration of CO32− appear at 859 cm−1, 720 cm−1, 695 cm−1. In the spectrum of La2O3, the La–O stretching vibration is at about 653 cm−1, showing La2O3 is prepared successfully. The thermogravimetric analysis (TGA) and differential temperature analysis (DTA) traces disclosed the formation process of La2O3 from pyrolysis of the La(OH)CO3 precursor. The TGA curves of the as-prepared La(OH)CO3 are shown in Fig. 3C. It can be noticed that there are three weight loss steps around 250 °C to 300 °C, 450 °C to 500 °C and 700 to 790 °C. For La(OH)CO3, the weight loss between 250 °C to 300 °C could be attributed to the removal of water, accompanying the formation of La2O(CO3)2 (see reaction (1)). Then La2O(CO3)2 decomposes to form La2O3 and release CO2, approximately to lose weight 28% (see reaction (2) and (3))
| 2LaOHCO3 → La2O(CO3)2 + 2H2O | (1) |
| La2O(CO3)2 → La2O2CO3 + CO2 | (2) |
| La2O2CO3 → La2O3 + CO2 | (3) |
Fig. 3D presents the X-ray photoelectron spectroscopies of La2O3. These spectra exhibit characteristic La 3d and O 1s peaks. As indicated in Fig. 3D(a), the La 3d spectrum shows two peaks at the binging energy 839.48 and 856.77 eV, corresponding to La 3d5/2 and 3d3/2, respectively. Meanwhile, the peaks located at a binding energy of around 528.9 and 531.65 eV are both corresponding to the La2O3 and H2O spectrum (Fig. 3D(b)). These results are in accordance with the previous reports on La2O3.19
Brunauer–Emmett–Teller (BET) gas adsorption measurements were used to characterize pore volume, pore diameter and specific surface area (see Fig. 3E). The N2 adsorption/desorption isotherms of the synthesized La2O3 exhibit typical type-IV hysteresis, indicating the presence of pores (Fig. 3E). The samples are mainly mesoporous (inset of Fig. 3E). The BET surface area is measured to be 18.76 m2 g−1 for spherical La2O3, 12.43 m2 g−1 for polyhedral La2O3 and 8.14 m2 g−1 for polyhedral La2O3, respectively.
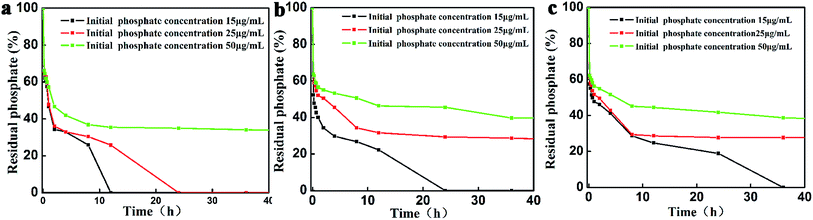
To determine absorption capacity of La2O3 micro-nanostructures for phosphate, ascorbic acid and molybdate solution were employed to measure phosphate group. The initial concentrations of phosphate were 15 μg mL−1, 25 μg mL−1 and 50 μg mL−1, respectively. As shown in Fig. 4, the residual phosphate reduce when La2O3 was added. It is worth highlighting that phosphate is absolutely removed by spherical La2O3 for only 12 h (Fig. 4a black wire) when initial phosphate concentration is 15 μg mL−1, while 24 h for spindles-like micro-nano La2O3 (Fig. 4b black wire), 35 h for polyhedral La2O3 (Fig. 4c black wire). Moreover, when initial phosphate concentration is 25 μg mL−1 (red wire), phosphate removal have been accomplished completely by spherical La2O3 after 24 h, but accomplished 68% by spindle-like La2O3 and 78% by polyhedral La2O3 in the meantime. Likewise, superior removal capacity of spherical La2O3 is observed, when initial phosphate concentration is 50 μg mL−1. The results show that spherical La2O3 has best phosphate removal capacity from water, which attributes to that the spherical La2O3 has the smallest particle sizes and the largest BET surface area.
To investigate the effect of phosphate addition and phosphate starvation on microorganism growth, the growth curve of Escherichia coli (a Gram-negative bacteria) and Staphylococcus aureus (a Gram-positive bacteria) were analyzed. Meanwhile, residual phosphate was calculated after microorganism grows for 8 h. As shown in Fig. 5a, the phosphate addition is beneficial to microorganisms growth (both Gram-positive and Gram-negative bacteria) (Fig. 5a and b). It is about 32 times and 26 times larger than the growth rate of E. coli in 2 h when adding phosphate (15 μg mL−1, red line and 50 μg mL−1, blue line), showing that phosphate addition to minimal medium could be beneficial to growth of Escherichia coli (Fig. 5a) and Staphylococcus aureus (Fig. 5b) when growth less than 5 h. However, the benefit of phosphate is suppressed following the addition of La2O3. The growth rates of Escherichia coli (Gram-negative bacteria) and Staphylococcus aureus (Gram-positive bacteria) were drawn in Fig. 5a–d. From Fig. 5a, it is only 5 and 2.5 times larger than the growth rate of E. coli in 2 h when adding La2O3 to medium in 4 h although the existence of phosphate (15 μg mL−1 and 50 μg mL−1). The results show that the growth rates of E. coli and S. aureus are obviously limited within 5 h when La2O3 is added, attributed to effective binding of La2O3 to phosphate, namely that phosphate starvation results in limited microbial growth. Given this, phosphate removal ability capacity of three morphology micro-nano La2O3 could be a key point to compare antimicrobial properties of La2O3. Due to superior removal capacity of spherical La2O3, spherical La2O3 exhibited better antibacterial property than other two morphologies La2O3. It means that phosphate absorption capacity of La2O3 is consistent with the antibacterial property of La2O3.
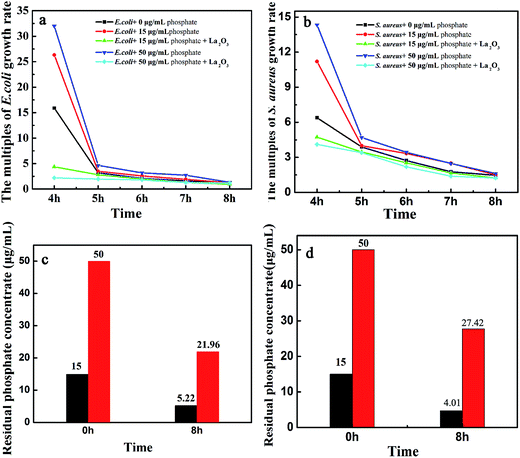 | ||
| Fig. 5 Antibacterial activity (Escherichia coli and Staphylococcus aureus) and phosphate adsorption of spherical La2O3 (a, c) Escherichia coli, (b, d) Staphylococcus aureus. | ||
4 Conclusion
We successfully obtained shape-controlled (spindle, polyhedron, and sphere) micrometer-scaled La2O3, hierarchically assembled by nanoparticles, through a facile, high quality and low prices method. Meanwhile, they have strong removal ability for phosphate and excellent antibacterial property, especially for sphere micro-nano structure La2O3. These hierarchical La2O3 micro-nanomaterials may have very good application prospects in water pollution of eutrophication, or reduce excess phosphate in human body in biomedical areas.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21571053), the Program for Innovative Research Team in Science and Technology in University of Henan Province (18IRTSTHN002), Key Scientific Research Project of Higher Education of Henan Province (18A150046), the 111 project (D17007).References
- S. I. Boldish and W. B. White, Vibrational spectra of crystals with the A-type rare earth oxide structure. La2O3 and Nd2O3, Spectrochim. Acta, Part A, 1979, 35, 1235 CrossRef.
- J. Gouteron, D. Michel, A. M. Lejus and J. Zarembowitch, Raman spectra of lanthanide sesquioxide single crystals: Correlation between A and B-type structures, J. Solid State Chem., 1981, 38, 288 CrossRef CAS.
- S. W. Kang and S. W. Rhee, Deposition of La2O3 Films by Direct Liquid Injection Metallorganic Chemical Vapor Deposition, J. Electrochem. Soc., 2002, 149, 345 CrossRef.
- A. M. De Asha, J. T. S. Critchley and R. M. Nix, Molecular adsorption characteristics of lanthanum oxide surfaces: the interaction of water with oxide overlayers grown on Cu (111), Surf. Sci., 1998, 405, 201 CrossRef CAS.
- W. Harder and L. Dijkhuizen, Physiological Responses to Nutrient Limitation, Rev. Microbiol., 1983, 37, 1 CrossRef CAS PubMed.
- T. F. Thingstad, U. L. Zweifel and R. F. Limnol, P limitation of heterotrophic bacteria and phytoplankton in the northwest Mediterranean T. Frede Thingstad, Limnol. Oceanogr., 1998, 43, 88 CrossRef CAS.
- C. V. Cardemil, D. R. Smulski, R. A. Larossa and A. C. Vollmer, Vollmer. Bioluminescent Escherichia coli Strainsfor the Quantitative Detection of Phosphate and Ammoniain Coastal and Suburban Watersheds, DNA Cell Biol., 2010, 29, 519 CrossRef CAS PubMed.
- L. C. Gerber, N. Moser, N. A. Luechinger, W. J. Stark and R. N. Grass, Phosphate starvation as an antimicrobial strategy: the controllable toxicity of lanthanum oxide nanoparticles, Chem. Commun., 2012, 48, 3869 RSC.
- M. Kasmi, M. Hamdi and I. Trabelsi, Eco-friendly process combining physical-chemical and biological technics for the fermented dairy products waste pretreatment and reuse, Water Sci. Technol., 2017, 75, 39 CrossRef PubMed.
- F. Haghseresht, S. Wang and D. D. Do, A novel lanthanum-modified bentonite, Phoslock, for phosphate removal from wastewaters, Appl. Clay Sci., 2009, 46, 369 CrossRef CAS.
- M. M. Gibbs, C. W. Hickey and D. Oezkundakci, Sustainability assessment and comparison of efficacy of four P-inactivation agents for managing internal phosphorus loads in lakes: sediment incubations, Hydrobiologia, 2011, 658, 253 CrossRef CAS.
- M. J. Barry and B. J. Meehan, The acute and chronic toxicity of lanthanum to Daphnia Carinata, Chemosphere, 2000, 41, 1669 CrossRef CAS PubMed.
- M. Lürling and F. V. Oosterhout, Controlling Eutrophication by Combined Bloom Precipitation and Sediment Phosphorus Inactivation, Water Res., 2013, 47, 6527 CrossRef PubMed.
- J. J. He, W. Wang, F. L. Sun, W. X. Shi, D. P. Qi, K. Wang, R. S. Shi, F. Y. Cui, C. Wang and X. D. Chen, Highly Efficient Phosphate Scavenger Based on Well-Dispersed La(OH)3 Nanorods in Polyacrylonitrile Nanofibers for Nutrient-Starvation Antibacteria, ACS Nano, 2015, 9, 9292 CrossRef CAS PubMed.
- J. Xie, Y. Lin, C. J. Li, D. Y. Wu and H. N. Kong, Removal and recovery of phosphate from water by activated aluminum oxide and lanthanum oxide, Powder Technol., 2015, 269, 351 CrossRef CAS.
- M. A. H. Johir, T. T. Nguyen, K. Mahatheva, M. Pradhan, H. H. Ngo, W. Guo and S. Vigneswaran, Removal of phosphorus by a high rate membrane adsorption hybrid system, Bioresour. Technol., 2016, 201, 365 CrossRef CAS PubMed.
- D. L. Correll, The Role of Phosphorus in The Eutrophication of Receiving Waters: A Review, J. Environ. Qual., 1998, 27, 261 CrossRef CAS.
- P. P. Veerle, J. B. Geert, R. B. An, E. D. B. Marc and C. D. Patrick, Lanthanum: A Safe Phosphate Binder, Semin. Dial., 2006, 19, 195 CrossRef PubMed.
- Y. Gao, Y. Zhang, Y. Zhou, C. Zhang, H. Zhang, S. Zhao, J. Fang, M. Huang and X. Sheng, Synthesis of ordered mesoporous La2O3-ZrO2 composites with encapsulated Pt NPs and the effect of La-dopping on catalytic activity, J. Colloid Interface Sci., 2017, 503, 178 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |