 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dehydration of glucose to 5-hydroxymethylfurfural and 5-ethoxymethylfurfural by combining Lewis and Brønsted acid
Haosheng Xinab,
Tingwei Zhang b,
Wenzhi Li
b,
Wenzhi Li *b,
Mingxue Sub,
Song Lic,
Qun Shao*a and
Longlong Mac
*b,
Mingxue Sub,
Song Lic,
Qun Shao*a and
Longlong Mac
aInstitute of Materials and Chemical Engineering, Anhui Jianzhu University, Hefei 230022, China
bLaboratory of Basic Research in Biomass Conversion and Utilization, Department of Thermal Science and Energy Engineering, University of Science and Technology of China, Hefei 230026, China. E-mail: liwenzhi@ustc.edu.cn
cCAS Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China
First published on 25th August 2017
Abstract
In this work, glucose was transformed into 5-hydroxymethylfurfural (HMF) and 5-ethoxymethylfurfural (EMF) in the presence of AlCl3·6H2O and a Brønsted solid acid catalyst (PTSA–POM). GVL (γ-valerolactone)–water and ethanol–water solvent systems were evaluated in the dehydration reaction of glucose into HMF and EMF, respectively. Water content and dosage of AlCl3·6H2O were examined in the conversion of glucose into HMF, and some valuable chlorides (FeCl3·6H2O, NiCl2·6H2O, CrCl3·6H2O etc.) were also used in contrast with AlCl3·6H2O. Some different organic solvents were added to the ethanol–water system to explore whether it would be beneficial to the generation of EMF. A high yield of HMF (60.7%) was obtained at 140 °C within 60 min in GVL–water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system, and total yield 42.1% of EMF and HMF (30.6% EMF, 11.5% HMF) was achieved at 150 °C after 30 min in an ethanol–water (9
1) solvent system, and total yield 42.1% of EMF and HMF (30.6% EMF, 11.5% HMF) was achieved at 150 °C after 30 min in an ethanol–water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent system.
1) solvent system.
Introduction
Urged by the rapid depletion of fossil fuels and increased environmental issues, great efforts have been devoted to the production of sustainable biofuels from biomass.1,2 In comparison with other sugars, glucose is the most abundant hexose contained in lignin biomass, considered as the most valuable bio-derived carbon resource, and it plays a vital role in the conversion of biomass to biofuels and chemicals.3–5 Thus, the high-efficiency transformation of glucose to platform chemicals has aroused much attention in recent years.6 Among those prospective chemicals, HMF and EMF are thought highly of as promising building blocks for the application of renewable resources.5-hydroxymethylfurfural (HMF), a valuable biomass-derived platform compound, referred as the bridge between renewable resources and chemistry chemicals.7–9 Glucose, a most widely distributed monosaccharide in nature, has been exploited in the production of HMF and furan compounds.10 There are two ways to convert glucose to HMF, one is direct dehydration of glucose to HMF; the other mainly comprises two steps, firstly, glucose is isomerized to fructose, after that, HMF can be obtained in the dehydration of fructose.11 Compared to the one-step method, the current two-step process is studied more extensively and Lewis acid plays a vital role for the isomerization of glucose into fructose in two-step process.12 Therefore, the combination of Lewis acid and Brønsted acid is extremely important in order to transform glucose efficiently.13 Nikolla et al. exploited the synthesis of HMF from glucose in the combination of HCl and Sn-beta catalyst in THF/H2O–NaCl system, HMF yield (56.9%) and glucose conversion (79%) was obtained after 70 min at 180 °C.14 In contrast to homogeneous catalyst (HCl, H2SO4, H3PO4), solid acid catalyst shows many advantages such as low corrosivity to the reactor, easy separation after reaction and more stable at high temperatures.15 Thus, the production of HMF over solid acid catalysts have been studied diffusely in recent years. Ohara et al. synthesized a catalyst with combination of Amberlyst-15 and hydrotalcite to catalyze glucose to HMF in DMF solvent system, HMF yield (41%) and glucose conversion (71%) was achieved at 373 K in 3 h.16 Thombal et al. prepared a solid acidic catalyst by mixing β-cyclodextrin and p-toluenesulfonic acid, with a reaction time of 5 h at 453 K, the HMF yield was 47% in DMSO.17 In addition, enormous attention was paid to ionic liquids (ILs) in recent period time. By combining and modifying the cations and anions properly, the properties of obtained ILs (polarity, hydrophobicity and dissolving capacity) could be fit better to adapt to the reaction.18–21 Detail works with careful design of IL has been done systemic which demonstrated that the transformation of glucose to HMF could be enhanced significantly.22,23 Zhang et al. prepared a heterogeneous catalyst (Cr-HAP) by combining hydroxyapatite and chromium chloride, and the catalyst was used in the dehydration of glucose in [BMIM]Cl, HMF yield of 40.2% with glucose conversion of 77.9% were obtained in 2.5 min with the aid of microwave irradiation.24 Chen et al. utilized Cr(CO)6 as catalyst for the transformation of glucose into HMF in [EMIM]Cl, after 6 h at 120 °C, affording the HMF yield of 50%.25 Although ILs has achieved great progress, its exorbitant price impose restrictions on the production of HMF and subsequent separation after reaction is also difficult. In this work, HMF is also a crucial intermediate product in the process of glucose conversion to EMF.
5-ethoxymethylfurfural (EMF), regarded as fuels or fuel additives, has high energy density (8.7 kW h L−1) close to diesel (9.7 kW h L−1) and regular gasoline (8.8 kW h L−1), far better than ethanol (6.1 kW h L−1).26 According to the previous literatures, EMF was mainly synthesized not directly from carbohydrates, the intermediate products such as HMF, 5-(chloromethyl)furfural (CMF) and 5-(bromomethyl)furfural BMF were firstly obtained, and then etherified into EMF.27–31 Liu et al. studied the EMF production from HMF, fructose and glucose, respectively.32 In accordance with expectation, the reaction efficient improved in the order: glucose < fructose < HMF. Compared with fructose and HMF, glucose has a good stability and pricing advantage in the formation of EMF. In the earlier time, Christopher M. Lew et al. used Sn-BEA and Amberlyst-131 as catalysts to catalyze glucose into EMF in a single reactor at 90 °C, 31% yield of EMF was achieved after 24 h.33 For the long time cost and low yield of EMF, people were inspired to hammer at ameliorating catalyst. In Yan's work, CrCl3 showed excellent catalytic efficiency in the conversion of carbohydrates.34 But as it known to us, Cr belongs to heavy metal and do harm to the environment with some toxicity. Recently, via the good deal of work of Yu Yang and Hu, Li, AlCl3·6H2O was also proved to be a preeminent catalyst in the production of EMF, high yield with expectation achieved.35,36
Both HMF and EMF are the vital platform chemicals in the conversion of carbohydrates, of which is worthy studied in depth, furthermore, EMF was obtained in the further etherification of HMF.14–21,24,25,27–36 And the study of direct conversion from glucose to EMF is particularly deserved. Thus, in this study, the dehydration of glucose to HMF was investigated by regulating reaction times, temperatures and the ratio of deionized water to organic solvent, some valuable chlorides (FeCl3·6H2O, SnCl4·5H2O, CrCl3·6H2O, etc.) were also been compared. The further etherification from HMF to EMF in the process of glucose conversion was also explored in ethanol/H2O. Apart from the influence of reaction times and temperatures, DMSO, dioxane, THF, MIBK, GVL were added extra to ethanol/H2O to explore the influence of added second organic solvent.
Previously, in the transformation of corn stalk into furfural, our group prepared a novel heterogeneous strong acid catalyst (PTSA–POM) by polymerizing p-toluenesulfonic acid (PTSA) and paraformaldehyde (POM) in the presence of H2SO4, and achieved significant results.37 Due to its easy preparation and excellent recyclability, the further application of PTSA–POM was explored in the dehydration of glucose in this paper. As for solvent, γ-valerolactone (GVL), a green solvent that can be obtained from biomass, was selected and proved to be a good solvent in the conversion of carbohydrates into furan compounds.38–40 Ethanol plays a decisive role in the etherification of HMF to EMF, not only acted as a green solvent, but also as a reactant.
Experimental
p-toluenesulfonic acid monohydrate (PTSA·H2O, 98.5%), paraformaldehyde (POM, AR), D-(+)-glucose (GC, 99.5%), HMF (99%), EMF (97%) were purchased from Aladdin Industrial, Inc. (Shanghai, China). GVL (95%) was obtained from LangFang Hawk Technology and Development Co, Ltd. AlCl3·6H2O(AR), CrCl3·6H2O(AR), CrCl2(AR), MgCl2·6H2O(AR), CaCl2(AR), NiCl2·6H2O(AR), NH4Cl(AR), FeCl3·6H2O(AR), CoCl2·6H2O(AR), MnCl2·4H2O(AR), SnCl4·5H2O(AR), SnCl2·2H2O(AR), H2SO4 (96–98%, AR), MIBK(AR), THF(AR), DMSO(AR) and dioxane(AR) were supplied by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All the chemicals were used without further purification.According to our reported literature,37 the details of synthesis of PTSA–POM as following: firstly, 0.2 ml sulphuric acid (98%) and 10.0 g PTSA–H2O were put to a three-necked round-bottomed flask with magnetic stirring and reflux condensation which was heated at 110 °C. Then 4 g POM added immediately after PTSA–H2O melted completely. The reaction temperature was kept at 110 °C for 8 h, then adjusted to 130 °C for 24 h to form a black solid. After that, the obtained solid was filtered and washed to pH = 7 with deionized water. Drying at 120 °C is necessary and then ground to powder. Finally, the resulting sample was calcined in muffle at 185 °C for 6 h.
For the production of HMF, reactions were carried out in a 48 ml pressure thick wall tube with oil-bath heating accompanied by 500 rpm magnetic stirring. As for EMF, because of the high temperature, a certain pressure will be produced in the reaction process by ethanol. Therefore, taking security into account, glucose, catalysts and solvents were loaded and sealed in a 25 ml autoclave. The autoclave was heated to desired temperature from room temperature in 30 min and also stirred magnetically at 500 rpm. After the reaction, both tube and autoclave were immersed in cold water immediately to terminate the reaction. Diluted and filtered liquid was analyzed using HPLC.
HPLC (waters 515 pump, equipped with an UV/Vis Detector (Waters 2489) using Waters Symmetry-C18 column(5 μm, 4.6 × 150 mm) and a Rrefractive Index Detector (Waters 2414) using Waters XBridge Amide column (5 μm, 4.6 × 150 mm)) was used to analyze diluted samples for HMF yield, EMF yield and glucose conversion.33–35 Authentic samples of HMF and EMF were used as standards, which calibration curves were applied to quantify.
HMF yield, EMF yield and glucose conversion were calculated as follows:
| HMF yield = (moles of HMF in products/moles of initial glucose) × 100%, |
| EMF yield = (moles of EMF in products/moles of initial glucose) × 100%, |
| HMF selectivity = (HMF yield/glucose conversion) × 100% |
| Glucose conversion = 1 − (moles of glucose in products/moles of initial glucose) × 100% |
Results and discussion
Except the dosage of AlCl3·6H2O, other parameters employed in the reaction are on the basis of previous literatures of our group.37,39 Initially, in order to make the dosage of AlCl3·6H2O appropriately, some probe trials at a mild temperature 140 °C with 20 min were explored in autoclave.The results are shown in Table 1, as it showing to us (entry 3), a maximum yield was obtained when 0.15 g AlCl3·6H2O was loaded. Herein, 0.15 g dosage of AlCl3·6H2O was adopted in the further reactions.
| Entry | Dosage | Yield/% |
|---|---|---|
| a Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 15 ml GVL and 1.5 ml DIW, 140 °C (30 min heating-up time), 20 min, 500 rpm. | ||
| 1 | 0.05 g | 50.3 |
| 2 | 0.10 g | 54.0 |
| 3 | 0.15 g | 57.8 |
| 4 | 0.20 g | 54.3 |
| 5 | 0.25 g | 51.7 |
| 6 | 0.30 g | 49.4 |
Production of HMF from the dehydration of glucose was carried out at 130 °C, 140 °C and 150 °C, with time from 30 min to 90 min (10 min interval), respectively, both HMF yield and glucose conversion are shown in Fig. 1. Obviously, according to the lines, it can be seen that temperature has profound impact on HMF yield. At 130 °C, HMF yield increased with the increase of reaction time, but relatively low HMF yield was obtained even in 90 min. By comparison, when the temperature are 140 °C and 150 °C, with reaction time goes by, HMF yield firstly increased, and decreased severally after reached the optimal values. Indicated that in higher temperatures, prolonged reaction time firstly promoted the transformation of glucose to HMF, after reached the optimal values, the effect of time on the reaction is not as obvious as at low temperature on HMF production. Throughout the whole reactions, highest yield 60.7% of HMF was achieved at 140 °C in a short time (60 min).
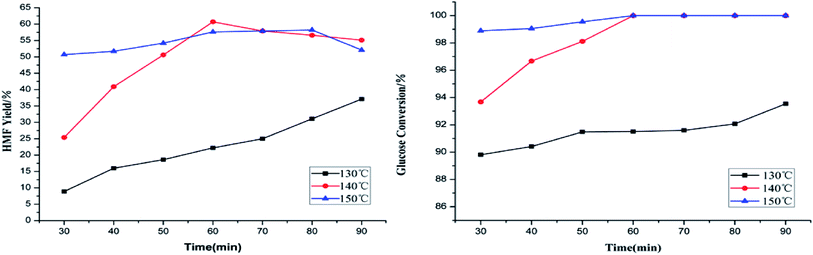 | ||
| Fig. 1 Effects of temperature and time on the conversion of glucose into HMF. Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 0.15 g AlCl3·6H2O, 15 ml GVL and 1.5 ml DIW, 500 rpm. | ||
Glucose is well converted in GVL/H2O solution and almost all the reactions conversion were over 90% even though the temperature was as low as 130 °C. As for higher temperature of 140 °C and 150 °C, complete conversion of glucose was achieved both in 60 min. It was clear that higher temperatures is more conducive to the conversion of glucose.
In this section, based on the optimal conditions found above, comparative experiment was also made on acid added. The results are shown in Table 2. From which we can learn that the yield of HMF was much promoted on the dehydration of glucose by combining PTSA–POM and AlCl3·6H2O, compared with only PTSA–POM or AlCl3·6H2O added. To our knowledge, compared with PTSA–POM, glucose is isomerized to fructose easily in the presence of AlCl3·6H2O, and some H+ will also be formed when AlCl3·6H2O dissolved in water, and the production of HMF from fructose is easy to carry out in GVL/H2O with H+. Then, with PTSA–POM added, the dehydration of fructose to HMF could be enhanced and the yield of HMF could be promoted. Therefore, the activity in the reaction may due to the conjunction of these two acids.
| Entry | Acid added | Yield/% |
|---|---|---|
| a Reaction conditions: 0.4 g glucose, 15 ml GVL and 1.5 ml DIW, 140 °C, 60 min, 500 rpm, PTSA–POM: 0.2 g, AlCl3·6H2O: 0.15 g. | ||
| 1 | PTSA–POM | 9.4 |
| 2 | AlCl3·6H2O | 50.8 |
| 3 | PTSA–POM/AlCl3·6H2O | 60.7 |
Apart from the effects of temperature and time, we judged that different water content could also has a significant influence on the dehydration of glucose to HMF. In view of this conjecture, great deal of efforts was made for the study of the effect of different water content. The results were summarized at Table 3, and results are highly consistent with our expectations, water added has a significant effect on HMF production. As for entry 1 and 2, we applied pure water and GVL as solvent under the optimum reaction condition, respectively. Apparently, very low yields of HMF were obtained both of them, only 1.2% in 16.5 ml water and 3.5% in pure GVL. Moreover, reaction with much water could pull down the conversion of glucose conspicuously, from 98.1% to 14.3%. From entry 3 to 11, different water content (0.5 ml, 1.5 ml, 3 ml) and fixed addition of 15 ml GVL solvent systems were evaluated at three time points (40 min, 60 min and 80 min). On the overall trend, the yield of HMF was increased with the increase of water content from 0.5 ml to 1.5 ml and decreased afterwards with the water content continue increase to 3 ml. Besides, higher water content in the reactions would do harm to the conversion, 100% conversion of glucose was achieved in lower water content of 0.5 ml and decreased with the increase of water content, which was in line with entry 1 and 2. Furthermore, the highest HMF yield was obtained under the optimized condition in 1.5 ml H2O/15 ml GVL system (entry 7). In fact, though glucose conversion decreased apparently with increase of water content from 1.5 ml to 3 ml, the selectivity was almost keep the same, which indicated that when the water content has arrivals a certain value there is no significant effect on selectivity of HMF production. And the comparison with previous catalytic system are shown in Table 4. From Table 4, we can also learn that low yields of HMF were obtained in pure water and GVL solvent (entry 1 and 2), which keeps consistent with the results we mentioned above.
| Entry | Solution | Time/min | HMF yield/% | Conversion/% | Selectivity/% |
|---|---|---|---|---|---|
| a Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 0.15 g AlCl3·6H2O, temperature: 140 °C, 500 rpm. | |||||
| 1 | 16.5 ml water | 60 | 1.2 | 14.3 | 8.4 |
| 2 | 16.5 ml GVL | 60 | 3.5 | 98.1 | 3.6 |
| 3 | 0.5 ml H2O + 15 ml GVL | 40 | 41.0 | 100 | 41 |
| 4 | 0.5 ml H2O + 15 ml GVL | 60 | 39.6 | 100 | 39.6 |
| 5 | 0.5 ml H2O + 15 ml GVL | 80 | 37.3 | 100 | 37.3 |
| 6 | 1.5 ml H2O + 15 ml GVL | 40 | 40.9 | 93.7 | 42.7 |
| 7 | 1.5 ml H2O + 15 ml GVL | 60 | 60.7 | 100 | 60.7 |
| 8 | 1.5 ml H2O + 15 ml GVL | 80 | 56.6 | 100 | 56.6 |
| 9 | 3 ml H2O + 15 ml GVL | 40 | 33.8 | 78.1 | 43.3 |
| 10 | 3 ml H2O + 15 ml GVL | 60 | 49.4 | 84.3 | 58.5 |
| 11 | 3 ml H2O + 15 ml GVL | 80 | 45.0 | 85.6 | 53 |
| Entry | Catalyst | Solvent | HMF yield/% | Glucose conversion/% | Ref. |
|---|---|---|---|---|---|
| a LCC = lignin-derived carbonaceous catalyst. | |||||
| 1 | TiO2 | Water | 18.6 | 63.8 | 43 |
| 2 | SO42−/ZrO2 | DMSO | 19.2 | 95.2 | 44 |
| 3 | [Sn,Al]-beta | H2O–DMSO | 37.3 | 100 | 45 |
| 4 | PTA–PCP(Cr)–SO3H(Cr3+) | H2O–THF–NaCl | 45.3 | 100 | 46 |
| 5 | Zr–P–Cr | [Bmim]Cl | 43.2 | 94.7 | 47 |
| 6 | LCCa | DMSO–[Bmim]Cl | 68 | 99 | 48 |
In addition to the effect of reaction temperature, time and water content, here some valuable chlorides are compared with AlCl3·6H2O and results were gathered in Table 5. As exhibited in Table 5, the HMF yield arranged from entry 1 to 11 in ascending order. Among those chlorides, FeCl3·6H2O performed the worst both in HMF yield and glucose conversion, lowest 4.5% HMF yield and 65.0% conversion were received. With regard to CoCl2·6H2O and MnCl2·4H2O, HMF yield were both below 10% while the conversion of glucose were over 90%. Entry 4, 5 and 8, 9, MgCl2·6H2O and NiCl2·6H2O, CaCl2 and NH4Cl shows alike catalytic effects with almost the same results in HMF yield and glucose conversion. Unfortunately, except CrCl2 and CrCl3·6H2O, low HMF yield were obtained in the presence of other chlorides in Table 5. HMF yield was obviously promoted to 46.1% in the presence of CrCl2, and higher glucose conversion 96.9% was obtained. Compared with CrCl2, higher HMF yield 60.4% was gained in the case of CrCl3·6H2O added, 91.8% of glucose conversion was observed in the meantime. Combined with previous reports illuminated that the hydrolyzed Cr(III) complex [Cr(H2O)5OH]2+ may provide a bifunctional site of Lewis acid–Brønsted base in glucose isomerization, and some water molecules in the first coordination sphere of Cr was displaced by glucose to promote the isomerization of glucose.41 Relate to AlCl3, the kinetics results in conjunction with reaction network expounded that the hydrolyzed Al(III) complex [Al(OH)2(aq)]+ is the most active Al species enable the isomerization of glucose.42
| Entry | Chloride | Dosage/g | HMF yield/% | Conversion |
|---|---|---|---|---|
| a Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 1.5 ml DIW, 15 ml GVL, temperature: 140 °C, time: 60 min, 500 rpm. | ||||
| 1 | FeCl3·6H2O | 0.15 | 4.5 | 65.0 |
| 2 | CoCl2·6H2O | 0.15 | 5.4 | 93.7 |
| 3 | MnCl2·4H2O | 0.15 | 7.0 | 92.9 |
| 4 | MgCl2·6H2O | 0.15 | 13.3 | 91.1 |
| 5 | NiCl2·6H2O | 0.15 | 13.8 | 90.1 |
| 6 | SnCl2·2H2O | 0.15 | 14.2 | 84.1 |
| 7 | SnCl4·5H2O | 0.15 | 16.3 | 79.1 |
| 8 | CaCl2 | 0.15 | 18.0 | 96.0 |
| 9 | NH4Cl | 0.15 | 18.8 | 92.8 |
| 10 | CrCl2 | 0.15 | 46.1 | 96.9 |
| 11 | CrCl3·6H2O | 0.15 | 60.4 | 91.8 |
Abovementioned, glucose was transformed into HMF in the first place and EMF was obtained with further etherification. In order to explore whether second organic solvent added will promote the production of EMF from glucose, in this section, DMSO, dioxane, THF, MIBK and GVL were added extra to ethanol/H2O system, respectively. As Table 6 demonstrated, highest HMF yield of 48% was obtained while lowest EMF yield of only 2.9% was achieved in entry 1 (DMSO added). Other organic solvents, including dioxane, THF, MIBK and GVL showed low selectivity towards HMF and EMF, and it is indicated that there is useless with organic solvent added in the production of EMF from glucose. Therefore, in the following study, ethanol/H2O solvent system without second organic solvent was employed in glucose-to-EMF.
| Entry | Organic solvent | HMF/% | EMF/% | Conversion/% |
|---|---|---|---|---|
| a Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 0.15 g AlCl3·6H2O, 1 ml DIW, 9 ml ethanol and all the additive amount of organic solvent is 9 ml, temperature: 150 °C, time: 30 min, 500 rpm. | ||||
| 1 | DMSO | 48 | 2.9 | 100 |
| 2 | Dioxane | 27.4 | 6.7 | 98.5 |
| 3 | THF | 28.6 | 6.8 | 96.8 |
| 4 | MIBK | 25.5 | 13.2 | 99.2 |
| 5 | GVL | 21.6 | 15.7 | 97.9 |
Table 7 exhibits the effects of temperature and time on EMF production from glucose by conducting the reactions in ethanol/H2O at 140 °C, 150 °C, and 160 °C. It can be observed that prolonged reaction time facilitated the production of EMF at 140 °C and 150 °C. In comparison to lower temperatures, the rate of EMF formation was rather quick when the temperature was raised to 160 °C, and EMF yield kept at a relatively stable value as time increased. Highest EMF yield 30.6% was obtained at optimal temperature and reaction time (150 °C, 30 min) with glucose conversion of 97.9%.
| Temperature/°C | Time/min | EMF yield/% | HMF yield/% | Glucose conversion/% |
|---|---|---|---|---|
| a Reaction conditions: 0.4 g glucose, 0.2 g PTSA–POM, 0.15 g AlCl3·6H2O, 1 ml DIW and 9 ml ethanol, 500 rpm. | ||||
| 140 | 10 | 11.8 | 19.9 | 61.7 |
| 140 | 15 | 15.6 | 19.2 | 65.9 |
| 140 | 20 | 18.5 | 17.9 | 71.9 |
| 140 | 25 | 19.9 | 17.8 | 78.6 |
| 140 | 30 | 22.5 | 15.1 | 86.0 |
| 150 | 10 | 21.0 | 19.5 | 93.5 |
| 150 | 15 | 25.8 | 14.6 | 94.9 |
| 150 | 20 | 26.4 | 14.2 | 96.3 |
| 150 | 25 | 27.9 | 11.3 | 97.8 |
| 150 | 30 | 30.6 | 11.5 | 97.8 |
| 160 | 10 | 26.7 | 15.6 | 95.6 |
| 160 | 15 | 28.8 | 11.7 | 98.2 |
| 160 | 20 | 28.4 | 9.8 | 98.4 |
| 160 | 25 | 28.5 | 8.8 | 99.2 |
| 160 | 30 | 27.9 | 7.7 | 99.0 |
Throughout the whole reaction progress, HMF was firstly produced and then etherified into EMF. As Table 7 showing, HMF yields gradually reduced with reaction time extended during all temperature. Both higher reaction temperature and longer reaction time facilitated the further conversion of HMF to EMF, but HMF yield of 11.5% was still retained under the optimum reaction conditions, which highest EMF yield was achieved. Consistent with previous reports35,36,49 that as an intermediate product, HMF could not etherified into EMF completely. EMF production process has similar glucose conversion trend with HMF production, the longer the reaction time and the higher the reaction temperature, the higher the conversion of glucose.
Conclusions
In conclusion, successfully furans' (HMF and EMF) preparation from glucose were carried out in different solvents in the presence of AlCl3·6H2O and a solid acid PTSA–POM, a series of influencing parameters were evaluated and significant results were acquired. The influence of water content has been studied and found that certain water content (1.5 ml) in reaction system is beneficial to HMF production. Some other available chlorides were also explored to compare with AlCl3·6H2O on the conversion of glucose into HMF, the efficiency of CrCl2 and CrCl3·6H2O are better than others. And we further discovered that extra organic solvent (DMSO, dioxane, THF, MIBK and GVL) added to ethanol/H2O system couldn't promote the production of EMF from glucose. Under the optimized conditions, 60.7% HMF yield with complete conversion of glucose was obtained in GVL/H2O, 30.6% EMF yield and 11.5% HMF yield were achieved directly from glucose in ethanol/H2O, respectively. Finally, due to the characteristics of cheap and nontoxic, described aluminum system shows a promising prospect for application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the State Key Program of National Natural Science Foundation of China (51536009), Science and Technological Fund of Anhui Province for Outstanding Youth (1508085J01), the National Key Technology R&D Program of China (No. 2015BAD15B06) and the international technology cooperation plan of Anhui (No. 1503062030).Notes and references
- A. Mukherjee, M.-J. Dumont and V. Raghavan, Biomass Bioenergy, 2015, 72, 143–183 CrossRef CAS.
- Y. S. Jang, B. Kim, J. H. Shin, Y. J. Choi, S. Choi and C. W. Song, et al., Biotechnol. Bioeng., 2012, 109, 2437–2459 CrossRef CAS PubMed.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044–4098 CrossRef CAS PubMed.
- M. Dashtban, A. Gilbert and P. Fatehi, RSC Adv., 2014, 4, 2037–2050 RSC.
- Y. Yang, W. T. Liu, N. N. Wang, H. J. Wang, Z. X. Song and W. Li, RSC Adv., 2015, 5, 27805–27813 RSC.
- J. Heltzel and C. R. F. Lund, Catal. Today, 2016, 269, 88–92 CrossRef CAS.
- F. W. Lichtenthaler and S. Peters, C. R. Chim., 2004, 7, 65 CrossRef CAS.
- W. Hou, Q. Wang and Z. Guo, et al., Catal. Sci. Technol., 2017, 7(4), 1006–1016 CAS.
- W. Zhang, J. Xie and W. Hou, et al., ACS Appl. Mater. Interfaces, 2016, 8(35), 23122–23132 CAS.
- B. Puértolas, Q. Imtiaz and C. R. Müller, et al., ChemCatChem, 2017, 9(9), 1579–1582 CrossRef.
- N. Shi, Q. Liu, L. Ma, T. Wang, Q. Zhang, Q. Zhang and Y. Liao, RSC Adv., 2014, 4, 4978–4984 RSC.
- P. Zhou and Z. Zhang, Catal. Sci. Technol., 2016, 6, 3694 CAS.
- T. F. Wang, M. W. Nolte and B. H. Shanks, Green Chem., 2014, 16, 548–572 RSC.
- E. Nikolla, Y. Román-Leshkov, M. Moliner and M. E. Davis, ACS Catal., 2011, 1, 408–410 CrossRef CAS.
- X. Tong, Y. Ma and Y. Li, Appl. Catal., A, 2010, 385, 1 CrossRef CAS.
- M. Ohara, A. Takagaki, S. Nishimura and K. Ebitani, Appl. Catal., A, 2010, 383, 149–155 CrossRef CAS.
- R. S. Thombal and V. H. Jadhav, Appl. Catal., A, 2015, 499, 213–216 CrossRef CAS.
- V. I. Pârvulescu and C. Hardacre, Chem. Rev., 2007, 107, 2615–2665 CrossRef PubMed.
- A. S. Amarasekara, Chem. Rev., 2016, 116, 6133–6183 CrossRef CAS PubMed.
- S. Zhang, J. Sun, X. Zhang, J. Xin, Q. Miao and J. Wang, Chem. Soc. Rev., 2014, 43, 7838–7869 RSC.
- C. Maton, N. De Vos and C. V. Stevens, Chem. Soc. Rev., 2013, 42, 5963–5977 RSC.
- S. Siankevich, Z. Fei and R. Scopelliti, et al., ChemSusChem, 2014, 7(6), 1647–1654 CrossRef CAS PubMed.
- S. Siankevich, Z. Fei and R. Scopelliti, et al., ChemSusChem, 2016, 9(16), 2089–2096 CrossRef CAS PubMed.
- Z. Zhang and Z. K. Zhao, Bioresour. Technol., 2011, 102, 3970–3972 CrossRef CAS PubMed.
- J. H. He, Y. T. Zhang and E. Y. X. Chen, ChemSusChem, 2013, 6, 61–64 CrossRef CAS PubMed.
- C. M. Lew, N. Rajabbeigi and M. Tsapatsis, Ind. Eng. Chem. Res., 2012, 51, 5364–5366 CrossRef CAS.
- M. Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47(41), 7924 CrossRef CAS PubMed.
- P. Lanzafame, D. M. Temi, S. Perathoner, G. Centi, A. Macario, A. Aloise and G. Giordano, Catal. Today, 2011, 175(1), 435 CrossRef CAS.
- P. H. Che, F. Lu, J. J. Zhang, Y. Z. Huang, X. Nie, J. Gao and J. Xu, Bioresour. Technol., 2012, 119, 433 CrossRef CAS PubMed.
- M. Mascal and E. B. Nikitin, ChemSusChem, 2009, 2(9), 859 CrossRef CAS PubMed.
- N. Kumari, J. K. Olesen, C. M. Pedersen and M. Bols, Eur. J. Org. Chem., 2011, 2011(7), 1266 CrossRef.
- B. Liu, et al., Fuel, 2013, 113, 625–631 CrossRef CAS.
- C. M. Lew, N. Rajabbeigi and M. Tsapatsis, Ind. Eng. Chem. Res., 2012, 51, 5364–5366 CrossRef CAS.
- Y. Yang, W. Liu, N. Wang and H. Wang, RSC Adv., 2015, 5, 27805 RSC.
- Y. Yang, C. Hu and M. M. Abu-Omar, Bioresour. Technol., 2012, 116, 190–194 CrossRef CAS PubMed.
- H. Li and K. Santosh Govind, Energy Convers. Manage., 2014, 88, 1245–1251 CrossRef CAS.
- Z. Xu, W. Li, Z. Du, H. Wu, H. Jameel, H.-m. Chang and L. Ma, Bioresour. Technol., 2015, 198, 764–771 CrossRef CAS PubMed.
- D. Martin Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584–595 RSC.
- T. Zhang, W. Li, Z. Xu, Q. Liu, Q. Ma, H. Jameel, H.-m. Chang and L. Ma, Bioresour. Technol., 2016, 209, 108–114 CrossRef CAS PubMed.
- W. Li, Z. Xu and T. Zhang, et al., BioResources, 2016, 11(3), 5839–5853 CAS.
- V. Choudhary, S. H. Mushrif and C. Ho, et al., J. Am. Chem. Soc., 2013, 135(10), 3997–4006 CrossRef CAS PubMed.
- J. Tang, L. Zhu and X. Fu, et al., ACS Catal., 2016, 7(1), 256–266 CrossRef.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Catal. Commun., 2008, 9, 2244–2249 CrossRef CAS.
- H. Pan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10, 1558–1563 CrossRef.
- L. Li, J. Ding, J.-G. Jiang, Z. Zhu and P. Wu, Chin. J. Catal., 2015, 36, 820–828 CrossRef CAS.
- D. Chen, F. Liang, D. Feng, M. Xian, H. Zhang, H. Liu and F. Du, Chem. Eng. J., 2016, 300, 177–184 CrossRef CAS.
- B. Liu, C. Ba, M. Jin and Z. Zhang, Ind. Crops Prod., 2015, 76, 781–786 CrossRef CAS.
- F. Guo, Z. Fang and T.-J. Zhou, Bioresour. Technol., 2012, 112, 313–318 CrossRef CAS PubMed.
- B. Liu and Z. Zhang, RSC Adv., 2013, 3, 12313–12319 RSC.
| This journal is © The Royal Society of Chemistry 2017 |
