Open Access Article
Open Access ArticleHighly efficient synthesis of chiral quaternary 3-aminooxindoles promoted by zinc(II) chloride via Et2Zn-catalysed addition of Grignard reagents to isaltin-derived N-tert-butanesulfinyl ketimines†
Shiwei Yangab,
Guangling Bian *a,
Zhongxiang Chena,
Xiaohan Xiaa,
Mi Zhoua,
Caiyan Cuia and
Ling Song*ab
*a,
Zhongxiang Chena,
Xiaohan Xiaa,
Mi Zhoua,
Caiyan Cuia and
Ling Song*ab
aThe Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: songling@fjirsm.ac.cn; glb@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, 100049, Beijing, P. R. China
First published on 3rd August 2017
Abstract
A highly efficient and practical approach to chiral quaternary 3-aminooxindoles was developed via Et2Zn catalyzed diastereoselective addition of Grignard reagents to isaltin-derived N-tert-butanesulfinyl ketimines giving good to excellent yields and diastereoselectivities with broad substrates and reagent scopes promoted by zinc(II) chloride.
Introduction
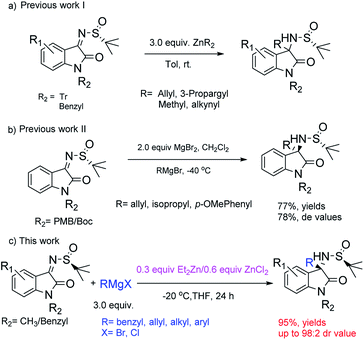
As a widely used chiral auxiliary due to its excellent stereoselectivity, facile preparation and low cost, the application of chiral tert-butanesulfinamide to build a chiral C–N center has been well documented.1 Although constructing chiral quaternary C–N centers is still a challenging task in asymmetric synthesis,2 using chiral tert-butanesulfinamide as a chiral auxiliary to construct tertiary chiral C–N centers via the addition of organometallic reagents to N-tert-butanesulfinyl aldimines has been reported.1a,3 The use of chiral tert-butanesulfinamide and organometallic reagents such as Grignard reagents provided a practical strategy to synthesize chiral quaternary carbon C–N bonds. For example, using isaltin-derived N-tert-butanesulfinyl ketimines to build quaternary C–N centers has been becoming a hot research topic in recent years.4 The core skeleton of chiral quaternary 3-aminooxindoles can be found in VIb receptor antagonist SSR-149415 (ref. 5) and the antimalarial drug candidate NITD609.6To synthesize chiral quaternary 3-aminooxindoles, several methodologies via metal-mediated diastereoselective addition of isaltin-derived N-tert-butanesulfinyl ketimines have been developed. Among them, methylation/terminal alkynylation and allylation/propargylation via alkyl zinc reagent were carried out by Wang4g and Xu4h (Scheme 1a); and MgBr2 mediated addition of the ketimines with Grignard reagents was first reported by Alessandra Silvani's group4i (Scheme 1b). Excessive ZnMe2, zinc powder and MgBr2 were needed for these methodologies accordingly. Herein, we report an effective Et2Zn catalyzed approach to chiral quaternary 3-aminooxindoles via diastereoselective addition of diverse Grignard reagents to a variety of N-tert-butanesulfinyl ketimines derived from isatin in mild conditions, giving satisfactory yields and diastereoisomeric ratios promoted by zinc(II) chloride (yields up to 95% and dr up to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (Scheme 1c).
2) (Scheme 1c).
Results and discussion
Ishihara7 showed that ZnCl2 could catalyze the addition of Grignard reagents to varied imines efficiently. And Yu8 also reported that stoichiometric amount of Et2Zn could promote the addition of RMgBr to N-tert-butanesulfinyl aldimines, but few to ketimines. Surprisingly, we found that ZnCl2 and Et2Zn could have synergistic effect in the synthesis of chiral quaternary 3-aminooxindoles via the addition of Grignard reagents to isaltin-derived N-tert-butanesulfinyl ketimines.Initially, we conducted the reaction in −78 °C without any additives. After the mixture was stirred for 3 days, good diastereoselectivity but low yield was obtained (Table 1, entry 1). When the temperature was increased to −55 °C, the product yield was enhanced a lot with decrease of the dr value. Surprisingly, when ZnCl2 was added the diastereoselectivity of the reaction was improved obviously (Table 1, entry 3). When catalytic amount Et2Zn was used respectively, the rate of reaction was greatly increased and 65% isolated yields after 48 hours (Table 1, entry 4). We then examined the efficiency of ZnCl2 and Et2Zn in the addition reaction between N-tert-butanesulfinyl ketimine 3a and benzylmagnesium bromide separately in −40 °C (Table 1, entries 5 and 6). In the presence of 0.3 equiv. of ZnCl2 only, although the reactivity was unsatisfied, the diastereoselectivity of the reaction was very high (>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr) at −40 °C for 24 h. Meanwhile, 0.3 equiv. of Et2Zn gave the desired product with 80% yields and 98
2 dr) at −40 °C for 24 h. Meanwhile, 0.3 equiv. of Et2Zn gave the desired product with 80% yields and 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr value under the same conditions. As we expected, when combining Et2Zn and ZnCl2, the product yield was increased to 86% without reduction in diastereoselectivity (Table 1, entry 7). Elevating the reaction temperature to −20 °C, the corresponding yield of the product was improved to 90%, but its dr value was decreased to 96
2 dr value under the same conditions. As we expected, when combining Et2Zn and ZnCl2, the product yield was increased to 86% without reduction in diastereoselectivity (Table 1, entry 7). Elevating the reaction temperature to −20 °C, the corresponding yield of the product was improved to 90%, but its dr value was decreased to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Table 1, entry 8). Further increasing the loading amount of ZnCl2 enhanced the de value of the product with lower yield (Table 1, entries 9 and 10). The loading amount of Et2Zn was critical and 0.3 equiv. of Et2Zn was shown to be the best (Table 1, entries 5, 11 and 12). Replacing ZnCl2 by ZnBr2, the product yields dropped a lot with slightly decreasing of the dr value (Table 1, entries 9 and 13). Using PhCH2MgCl instead of PhCH2MgBr in the presence of 0.3 equiv. of Et2Zn and 0.6 equiv. of ZnCl2 at −20 °C improved the yield to 92% and the dr value to 98.5
4 (Table 1, entry 8). Further increasing the loading amount of ZnCl2 enhanced the de value of the product with lower yield (Table 1, entries 9 and 10). The loading amount of Et2Zn was critical and 0.3 equiv. of Et2Zn was shown to be the best (Table 1, entries 5, 11 and 12). Replacing ZnCl2 by ZnBr2, the product yields dropped a lot with slightly decreasing of the dr value (Table 1, entries 9 and 13). Using PhCH2MgCl instead of PhCH2MgBr in the presence of 0.3 equiv. of Et2Zn and 0.6 equiv. of ZnCl2 at −20 °C improved the yield to 92% and the dr value to 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 (Table 1, entries 9 and 14). Further elevating the reaction temperature to 0 °C resulted in the decrease of the dr value to 94.5
1.5 (Table 1, entries 9 and 14). Further elevating the reaction temperature to 0 °C resulted in the decrease of the dr value to 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (Table 1, entry 15).
5.5 (Table 1, entry 15).
| Entry | T/°C | Additive/equiv. | Time/h | Yieldb % | Drc | |
|---|---|---|---|---|---|---|
| Et2Zn | ZnCl2 | |||||
| a Reaction conditions: 3a (0.2937 mmol), the Grigard reagents (0.8811 mmol), dried THF, N2.b Isolated yields.c Analyzed by NMR and HPLC.d SM did not react completely detected by TLC.e ZnBr2 was used instead of ZnCl2.f Benzylmagnesium chloride was used instead of benzylmagnesium bromide. | ||||||
| 1 | −78 | — | — | 72d | 19 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | −55 | — | — | 72d | 35 | 95.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5 |
| 3 | −55 | — | 0.3 | 72d | 42 | 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 4 | −55 | 0.3 | — | 48d | 65 | 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
| 5 | −40 | — | 0.3 | 24d | 39 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 6 | −40 | 0.3 | — | 24 | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7 | −40 | 0.3 | 0.3 | 24 | 86 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 8 | −20 | 0.3 | 0.3 | 24 | 90 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 9 | −20 | 0.3 | 0.6 | 24 | 88 | 97.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
| 10 | −20 | 0.3 | 1.0 | 24 | 70 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 11 | −20 | 0.4 | 0.6 | 24 | 61 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 12 | −20 | 0.2 | 0.6 | 24d | 63 | 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 13e | −20 | 0.3 | 0.6 | 24 | 79 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 14f | −20 | 0.3 | 0.6 | 18 | 92 | 98.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
| 15f | 0 | 0.3 | 0.6 | 24 | 93 | 94.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
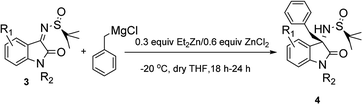
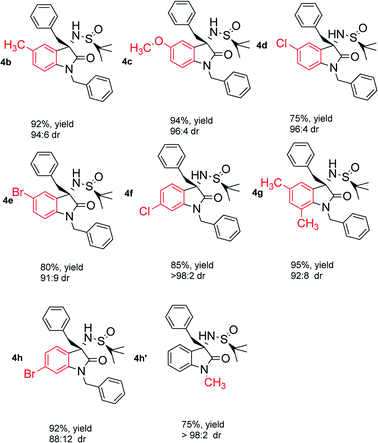
With the optimized conditions in hand, we then investigated the substrate scope of the reaction system. As shown in Table 2, this system worked very well for a variety of substrates with H-, CH3-, OCH3-, Cl-, Br-substitution on 3-, 4-, 5-position of the aromatic ring and Bn-, CH3-substitution on the nitrogen center of 3 giving 75–95% yields and up to 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 dr value of the desired products. Furthermore, our methodology was also applicable for diverse Grignard reagents, containing aryl, benzyl, alkyl and allyl Grignard reagents (Table 3).
2 dr value of the desired products. Furthermore, our methodology was also applicable for diverse Grignard reagents, containing aryl, benzyl, alkyl and allyl Grignard reagents (Table 3).
| a Reaction conditions: the corresponding substrates 3 (0.2937 mmol), benzylmagnesium chloride (0.8811 mmol), Et2Zn (0.08811 mmol), ZnCl2 (0.1762 mmol), dried THF, N2, −20 °C, 18–24 h. Yield is for isolated yields and dr is analyzed by NMR. |
|---|
 |
| Entry | No. | R- | Time/h | Yieldb/% | Drc |
|---|---|---|---|---|---|
| a Reaction conditions: 3a (0.2937 mmol), the Grignard reagents (0.8811 mmol), Et2Zn (0.08811 mmol), ZnCl2 (0.1762 mmol), dried THF, N2, −20 °C, 24 h.b Isolated yields.c Analyzed by NMR.d Only methyl addition product was isolated. The reason might be that Et2Zn was the catalytic amount and no or less ethyl addition product could be isolated. | |||||
| 1 | 4i | Isopropyl- | 24 | 84 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | 4j | Allyl- | 24 | 92 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 3 | 4k | Phenyl- | 24 | 85 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
| 4 | 4l | p-Methylphenyl- | 24 | 88 | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| 5 | 4m | Ethyl- | 24 | 95 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 6d | 4n | Methyl- | 24 | 94 | >98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
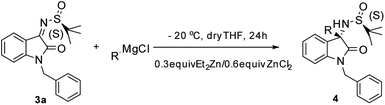
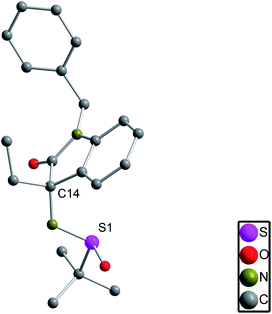
The S configuration of the new generated stereocenters of 4 was assigned on the basis of 4n and 4m. The relative configuration of the quaternary C–N center of 4n was confirmed by chemical transformation to a known compound 5 (ref. 4g) and the absolute configuration of 4m was determined by X-ray crystal structure (Fig. 1).11
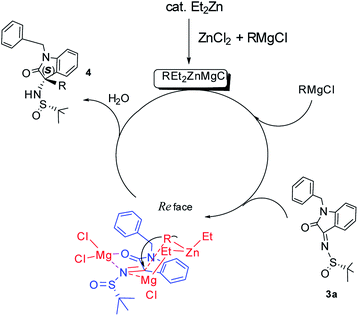
Based on our studies and previous published results by other groups,4i,7–9 we proposed the possible catalytic mechanism as follows (Fig. 2): catalytic Et2Zn reacted with ZnCl2 and RMgCl to generate active triorganozincate REt2ZnMgCl and MgCl2. The Lewis acid MgCl2 activated N-tert-butanesulfinyl ketimine 3a by coordinating with the oxygen of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the nitrogen of C
O and the nitrogen of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the ketimine. It facilitated the R-group transfer of REt2MgCl and favored the equilibrium of E configuration of the imine which preferred the Re attack of R-group of REt2MgCl to obtain the S configuration on the quaternary C–N center of the final product.
N in the ketimine. It facilitated the R-group transfer of REt2MgCl and favored the equilibrium of E configuration of the imine which preferred the Re attack of R-group of REt2MgCl to obtain the S configuration on the quaternary C–N center of the final product.
Conclusions
To summarize briefly, we developed a new and effective methodology for the diastereoselective addition of diverse Grignard reagents to a variety of N-tert-butanesulfinyl ketimines derived from isatin in good to excellent yields and diastereoselectivities. We provided a very practical synthetic approach to chiral quaternary 3-aminooxindoles. The rate of reaction and yield was greatly increased through the use of Et2Zn. The dr values of products were promoted obviously by zinc(II) chloride. The in situ generated active triorganozincate and rigid transition state are the key factors for the high reactivity and diastereoselectivity of the reaction.Acknowledgements
The authors gratefully acknowledge financial supports from the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20000000); The Key Laboratory of Coal to Ethylene Glycol and Its Related Technology, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences.Notes and references
- For reviews on the application of (R/S)-tert-butanesulfinamide, see: (a) M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600 CrossRef CAS PubMed; (b) F. Ferreira, C. Botuha, F. Chemla and A. Pérez-Luna, Chem. Soc. Rev., 2009, 38, 1162 RSC; (c) J. A. Ellman, Pure Appl. Chem., 2003, 75, 39 CrossRef CAS; (d) J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984 CrossRef CAS PubMed; for articles see: (e) C. Xie, Y. Dai, H. Mei, J. Han, V. A. Soloshonok and Y. Pan, Chem. Commun., 2015, 51, 9149 RSC; (f) L. Wu, C. Xie, H. Mei, V. A. Soloshonok, J. Han and Y. Pan, Org. Biomol. Chem., 2014, 12, 4620 RSC; (g) J. Cheng, L. Fu, C. Ling and Y. Yang, Heterocycles, 2010, 81, 2581 CrossRef CAS; (h) C. H. Ko, D. Y. Jung, M. K. Kim and Y. H. Kim, Synlett, 2005, 02, 304 Search PubMed; (i) D. R. Dragoli, M. T. Burdett and J. A. Ellman, J. Am. Chem. Soc., 2001, 123, 10127 CrossRef CAS PubMed; (j) H. B. Mei, C. Xie, J. L. Han and V. A. Soloshonok, Eur. J. Org. Chem., 2016, 2016, 5917 CrossRef CAS.
- (a) S.-G. Wang, X.-J. Liu, Q.-C. Zhao, C. Zheng, S.-B. Wang and S.-L. You, Angew. Chem., Int. Ed., 2015, 127, 15142 CrossRef; (b) Y. Liu and J. Zhou, Chem. Commun., 2013, 49, 4421 RSC; (c) L.-N. Jia, J. Huang, L. Peng, L.-L. Wang, J.-F. Bai, F. Tian and L.-X. Wang, Org. Biomol. Chem., 2012, 10, 236 RSC; (d) S. P. Marsden, E. L. Watson and S. A. Raw, Org. Lett., 2008, 10, 2905 CrossRef CAS PubMed; (e) Y. X. Jia, J. M. Hillgren, E. L. Watson, S. P. Marsden and E. P. Kündig, Chem. Commun., 2008, 34, 4040 RSC; (f) P. Fu, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2008, 130, 5530 CrossRef CAS PubMed; (g) T. Emura, T. Esaki, K. Tachibana and M. Shimizu, J. Org. Chem., 2006, 71, 8559 CrossRef CAS PubMed; (h) B. M. Trost and C. Jiang, J. Am. Chem. Soc., 2001, 123, 12907 CrossRef CAS PubMed; (i) T. Satoh, R. Matsue, T. Fujii and S. Morikawa, Tetrahedron Lett., 2000, 41, 6495 CrossRef CAS; (j) D. H. Hua, N. Lagneau, H. Wang and J. Chen, Tetrahedron: Asymmetry, 1995, 6, 349 CrossRef CAS; (k) D. H. Hua, S. W. Miao, J. S. Chen and S. Iguchi, J. Org. Chem., 1991, 56, 4 CrossRef CAS.
- (a) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS PubMed; (b) T. Vilaivan, W. Bhanthumnavin and Y. Sritana-Anant, Curr. Org. Chem., 2005, 9, 1315 CrossRef CAS.
- (a) H. H. Jung, A. W. Buesking and J. A. Ellman, Org. Lett., 2011, 13, 3912 CrossRef CAS PubMed; (b) J.-P. Chen, W.-W. Chen, Y. Li and M.-H. Xu, Org. Biomol. Chem., 2015, 13, 3363 CAS; (c) D. Chen and M.-H. Xu, J. Org. Chem., 2014, 79, 7746 CrossRef CAS PubMed; (d) V. B. Rao, A. P. Jadhav, D. Garad and R. P. Singh, Org. Lett., 2014, 16, 648 CrossRef CAS PubMed; (e) H. Takada, N. Kumagai and M. Shibasaki, Org. Lett., 2015, 17, 4762 CrossRef CAS PubMed; (f) S. Hajra, S. M. Aziz, B. Jana, P. Mahish and D. Das, Org. Lett., 2016, 18, 532 CrossRef CAS PubMed; (g) W. Yan, D. Wang, J. Feng, P. Li and R. Wang, J. Org. Chem., 2012, 77, 3311 CrossRef CAS PubMed; (h) D. Chen and M.-H. Xu, Chem. Commun., 2013, 49, 1327 RSC; (i) G. Lesma, N. Landoni, T. Pilati, A. Sacchetti and A. Silvani, J. Org. Chem., 2009, 74, 4537 CrossRef CAS PubMed.
- (a) H. Yin, T. Wang and N. Jiao, Org. Lett., 2014, 16, 2302 CrossRef CAS PubMed; (b) Y.-Q. Zhang, Y.-A. Yuan, G.-S. Liu and H. Xu, Org. Lett., 2013, 15, 3910 CrossRef CAS PubMed.
- (a) H. Zheng, X. Liu, C. Xu, Y. Xia, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 10958 CrossRef CAS PubMed; (b) T. N. Wells, Science, 2010, 329, 1153 CrossRef CAS PubMed.
- (a) M. Hatano, K. Yamashita and K. Ishihara, Org. Lett., 2015, 17, 2412 CrossRef CAS PubMed; (b) M. Hatano, R. Gouzu, T. Mizuno, H. Abe, T. Yamada and K. Ishihara, Catal. Sci. Technol., 2011, 1, 1149 RSC; (c) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, Chem. Commun., 2010, 46, 2674 RSC; (d) M. Hatano, O. Ito, S. Suzuki and K. Ishihara, J. Org. Chem., 2010, 75, 5008 CrossRef CAS PubMed; (e) M. Hatano, S. Suzuki and K. Ishihara, J. Am. Chem. Soc., 2006, 128, 9998 CrossRef CAS PubMed; (f) M. Hatano, M. Mizuno and K. Ishihara, Org. Lett., 2016, 18, 4462 CrossRef CAS PubMed.
- (a) R. Almansa, D. Guijarro and M. Yus, Tetrahedron Lett., 2009, 50, 3198 CrossRef CAS; (b) R. Almansa, D. Guijarro and M. Yus, Tetrahedron: Asymmetry, 2008, 19, 2484 CrossRef CAS; (c) R. Almansa, D. Guijarro and M. Yus, Tetrahedron: Asymmetry, 2008, 19, 603 CrossRef CAS.
- (a) M. Hatano, K. Yamashita and M. Mizuno, Angew. Chem., Int. Ed., 2015, 127, 2745 CrossRef; (b) F.-L. Li, H.-Y. Huang, H. Zong, G.-L. Bian and L. Song, Tetrahedron Lett., 2015, 56, 2071 CrossRef CAS; (c) H. Zong, H.-Y. Huang, J.-F. Liu, G.-L. Bian and L. Song, J. Org. Chem., 2012, 77, 4645 CrossRef CAS PubMed.
- The diastereoisomers 4a and 4a′ was separable, see: 1HNMR, 13CNMR and HRMS in ESI.† The dr value of 4a in optimization of experimental conditions was determined by NMR and HPLC.
- The X-ray crystal structure of 4m was confirmed by CCDC 1523303.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and spectral data for new compounds. CCDC 1523303. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra07692d |
| This journal is © The Royal Society of Chemistry 2017 |