Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of wheat peptide supplementation on anti-fatigue and immunoregulation during incremental swimming exercise in rats
Zhiqiang Zhengab,
Xiaoxue Yangc,
Jin Liub,
Ping Qianb,
Limin Haob,
Zhenyu Wangc and
Shuntang Guo *a
*a
aCollege of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China. E-mail: guoshuntang@sina.com; Tel: +86-10-66727124
bThe Quartermaster Equipment Institute of Logistic Support Department, CMC, Beijing 100010, China
cSchool of Chemistry and Chemical Engineering, Harbin Institute of Technology, Harbin 150001, China
First published on 7th September 2017
Abstract
This study elucidated the effects of wheat peptide administration on anti-fatigue and immunoregulation functions in rats. Wheat peptides were separated and the fraction with the highest radical scavenging activity in vitro was subjected to mass spectrometry to identify the peptide sequences. Sixty rats were randomized into 5 groups: no exercise control group (C), no exercise with low dose [20 mg kg−1 d−1] group (M), exercise control group (E), exercise with low dose group (Z), and exercise with high dose [100 mg kg−1 d−1] group (D). After training for 4 weeks with incremental swimming exercise, bodyweight and exhaustive time were tested and serum, small intestine, skeletal muscle and brain tissues of the rats were collected. A total of four peptide sequences from the highest active fraction were identified. The exhaustive time of group D was significantly longer than groups E and Z. The malondialdehyde content of group M was significantly lower than group C (p < 0.01), but secretory immunoglobulin A and 5-hydroxytryptamine (5-HT) were higher (both p < 0.01). Compared to group E, the activities of superoxide dismutase in skeletal muscle and acetylcholinesterase were significantly higher in groups Z and D (p < 0.01 or 0.05), but caspase-3 was lower (p < 0.01). Glutathione peroxidase and 5-HT in group D were both significantly higher than in group E (p < 0.01 or 0.05), but interleukin-6 and interleukin-8 were lower (P < 0.05). Supplementation of wheat peptide could effectively improve the ability of one-time exhaustive exercise of rats, remove free radicals from skeletal muscle in time, and alleviate the intestinal and blood inflammatory responses.
1. Introduction
Wheat protein is a byproduct of wheat starch processing, and is mainly composed of gliadin and glutenin.1 Wheat protein contains abundant hydrophobic amino acids and uncharged amino acids, and leads to a large area of intramolecular hydrophobicity and poor solubility in water,2 which consequently hinders the utilization of wheat protein. Enzymatic modification increases the solubility of wheat protein, which in turn improves the processing performance and functional properties of wheat protein. Enzymatic hydrolysis of wheat produces a large amount of peptide material with different molecular weights. In recent years, plant-derived peptides, including soy peptides, corn peptides, etc., have been widely used as raw food materials in the food industry. But wheat peptide has not been widely used as a new source of raw food material. The development of deep processing of wheat protein showed good application prospects for wheat peptide in food industry.Current research is focusing on the functional properties of wheat peptides. Wheat peptides have a variety of biological functions, such as antioxidant activities. Žilić et al.3 have reported that the free radical scavenging potential of wheat peptide was stronger than that of legume protein hydrolysate. Similarly, few other studies4,5 have also indicated that wheat peptides have stronger antioxidant activity. On the other hand, a large number of studies6–9 indicated that wheat peptides can significantly inhibit ACE activity and have anti-hypotensive effect. As early as 1979, Ziadrou et al.10 have obtained opioid-like active peptides from wheat protein hydrolysate. Fukudome et al.11 have also shown that high concentrations of wheat encephalin could be obtained in the wheat protein hydrolysates. In addition, few other studies have shown the tumor suppressor activity of wheat protein. Calzuola et al.12 isolated acidic peptides from the chromatin of wheat germ by using infrared spectroscopy and mass spectrometer, and demonstrated its role in controlling cell proliferation. Jeong et al.13 also reported that the tumor suppressor peptide extracted from the wheat protein inhibited the acetylation of nucleoprotein.
Wheat peptide has a variety of functional properties, but few studies have demonstrated its anti-fatigue and regulation of immune functions. In the studies that reported anti-fatigue function of plant-derived peptides, soybean and corn peptides tend to have a certain anti-fatigue effects.14,15 Yimit et al.,16 demonstrated that soybean peptide regulates immune function well. Wheat peptides, soybean peptides, as well as corn peptides were obtained in plant protein hydrolysates, and therefore, wheat peptide might be associated with the anti-fatigue and immune regulation functions. High intensity exercise causes a series of physiological and biochemical changes in muscle, intestine, blood, and nervous system, leading to the occurrence of fatigue and decreased immunity. These mainly reflect in the declination of glycogen amount, increase oxidative stress, descent intestinal mucosal permeability, muscle damage, inflammatory responses, etc.17 Animal testing is a good way to validate both anti-fatigue and immune regulation functions of wheat peptide, and is the essential part before human clinical trials. Therefore, animal experiments were conducted in this study to elucidate the effects of wheat peptide on the growth of rats, as well as the related indexes of serum, small intestine, skeletal muscle, and brain tissue in rats. Exhaustive time that reflects the direct impact of rat exercise capacity was tested, and the mechanism of anti-fatigue and immune regulation of wheat peptide was also discussed. Hence, the aim of this study was to provide a theoretical basis for the functional diversity of wheat peptides, and further develop a kind of functional food ingredient with anti-fatigue and immunoregulation functions for processing military food. This can alleviate the fatigue and disturbances of immune regulation in a large number of physical training of soldiers.
2. Materials and methods
2.1 Materials
Alkaline protease (2.2 × 105 U mL−1) and flavor protease (3.1 × 104 U mL−1) were provided by Novozymes Biotechnology Co., Ltd.
2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55
55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1. Detection wavelength: 220 nm. Flow rate: 0.5 mL min−1. Column temperature: 30 °C. Injection volume: 10 μL. The standard calibration curve of relative molecular mass was prepared with bacitracin (Mw 1500 Da), cytochrome C (Mw 12
0.1. Detection wavelength: 220 nm. Flow rate: 0.5 mL min−1. Column temperature: 30 °C. Injection volume: 10 μL. The standard calibration curve of relative molecular mass was prepared with bacitracin (Mw 1500 Da), cytochrome C (Mw 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da), aprotinin (Mw 6500 Da), glycine-glycine-tyrosine-arginine (Mw 451 Da) and glycine-glycine-glycine (Mw 189 Da). The regression equation between the logarithm of relative molecular mass and retention time is y = −0.2095x + 6.6081 (R2 = 0.9975). The relative molecular mass and distribution range of the sample were obtained by substituting the chromatographic data of the sample into the calibration curve equation.
500 Da), aprotinin (Mw 6500 Da), glycine-glycine-tyrosine-arginine (Mw 451 Da) and glycine-glycine-glycine (Mw 189 Da). The regression equation between the logarithm of relative molecular mass and retention time is y = −0.2095x + 6.6081 (R2 = 0.9975). The relative molecular mass and distribution range of the sample were obtained by substituting the chromatographic data of the sample into the calibration curve equation.| Weeks | Monday (min) | Tuesday (min) | Wednesday (min) | Thursday (min) | Friday (min) | Saturday (min) |
|---|---|---|---|---|---|---|
| a Note: the number after each “*” was the ratio of weight-bearing to weight of each rat (%). | ||||||
| 1 | 20 | 30 | 40 | 50 | 60 | 60 |
| 2 | 70 | 75 | 80 | 85 | 90 | 90 |
| 3 | 95 | 100 | 105 | 110 | 115 | 120 |
| 4 | 120*1 | 120*1 | 120*1 | 120*2 | 120*2 | 120*2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 to 16
00 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 every day from Monday to Saturday, and stopped on Sunday. The mass concentration of wheat peptide solution was 25 mg mL−1. The modest gavage dose of 20 mg kg−1 body weight per day was given to the rats of M and Z groups, and the gavage dose of 100 mg kg−1 body weight per day was given to the rats of group D.
00 every day from Monday to Saturday, and stopped on Sunday. The mass concentration of wheat peptide solution was 25 mg mL−1. The modest gavage dose of 20 mg kg−1 body weight per day was given to the rats of M and Z groups, and the gavage dose of 100 mg kg−1 body weight per day was given to the rats of group D.2.3 Statistical analysis
Data were processed using SPSS 13.0 statistical software. Results were expressed as mean ± standard deviation (means ± SD). Using single factor analysis of variance, P < 0.05 showed significant difference, P < 0.01 indicated that the difference was very significant.3. Results and discussion
3.1 Characterization and identification of wheat peptide
As shown in Table 2, wheat protein was digested by alkaline protease and flavor protease to prepare wheat peptide samples, and components with molecular weight less than 1000 Da accounted for 82.15%. Molecular weight less than 180 Da is usually considered as a free amino acid component. Therefore, the ratio of wheat peptide with molecular weight less than 1000 Da was 71.94% and the major molecular weight was located at 180–500 Da (54.66%) in this experiment. These results indicated that wheat peptide was mainly composed of small peptides. It was reported that small active peptides were more easily absorbed and demonstrated higher biological activities than longer peptides in vivo as they were less susceptible to undergo gastrointestinal hydrolysis.20| Molecular weight range/Da | Content/% |
|---|---|
| a Data are shown as mean ± SD. | |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.42 ± 0.24 |
5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.65 ± 0.36 |
| 3000–5000 | 2.19 ± 0.61 |
| 2000–3000 | 3.05 ± 0.12 |
| 1000–2000 | 9.54 ± 0.44 |
| 500–1000 | 17.28 ± 0.83 |
| 180–500 | 54.66 ± 1.89 |
| <180 | 10.21 ± 0.58 |
The amino acid composition of wheat peptide sample was analyzed and the results were shown in Table 3. Results demonstrated that the most abundant amino acids were glutamic acid and glutamine (37.7 g/100 g in total amino acids). It was reported that glutamine is beneficial for improving the body's immune function,21 so wheat peptide may also have its role in immunoregulation. Saito et al. have shown that His, Pro and Tyr could contribute to the higher radical scavenging ability.22 In this study, the total content of His, Pro and Tyr was 10.5 g/100 g in total amino acids, which may provide the wheat peptide with strong antioxidant capacity. These results suggest that the biological activities of wheat peptide seemed to be due to these specific amino acids.
| Amino acid | Total amino acid | Free amino acid | Bound amino acid |
|---|---|---|---|
| a Aspartic acid + asparagine.b Glutamic acid + glutamine.c Not determined. | |||
| Aspa | 2.3 | 0.1 | 2.2 |
| Glub | 37.7 | 0.4 | 37.3 |
| Ser | 3.7 | 0.2 | 3.5 |
| His | 1.6 | 0.2 | 1.4 |
| Gly | 3.0 | 0.2 | 2.8 |
| Thr | 1.7 | 0.3 | 1.4 |
| Arg | 2.7 | 0.8 | 1.9 |
| Ala | 1.9 | 0.5 | 1.4 |
| Tyr | 2.6 | 0.5 | 2.1 |
| Cys | 0.7 | n.dc | 0.7 |
| Val | 3.1 | 0.3 | 2.8 |
| Met | 1.5 | 0.4 | 1.1 |
| Phe | 4.6 | 0.6 | 4.0 |
| Ile | 2.7 | 0.2 | 2.5 |
| Leu | 5.0 | 1.2 | 3.8 |
| Lys | 1.1 | 0.4 | 0.7 |
| Pro | 6.3 | 0.1 | 6.2 |
| Trp | 0.6 | n.dc | 0.6 |
| Total | 82.8 | 6.4 | 76.4 |
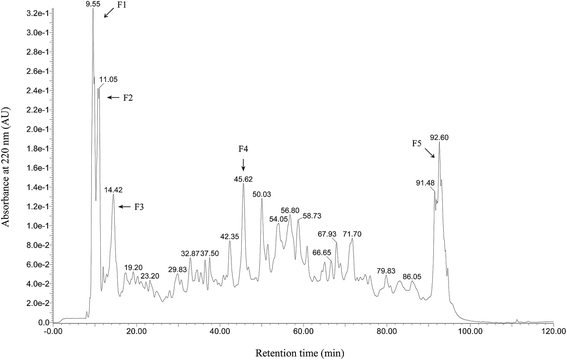
Wheat peptide with molecular weight less than 1000 Da was separated by RP-HPLC, and the chromatogram was shown in Fig. 1. Five fractions were selected and designated as F1–F5. Research suggested that fatigue was positively correlated with free radical increase.23 Thus, DPPH radical scavenging activities of these five fractions were measured in vitro at a concentration of 3.0 mg mL−1. As shown in Fig. 2, fraction F4 (86.61 ± 3.22%) showed the highest DPPH radical scavenging activity. Therefore, fraction F4 was selected for further identification of amino acid sequences.
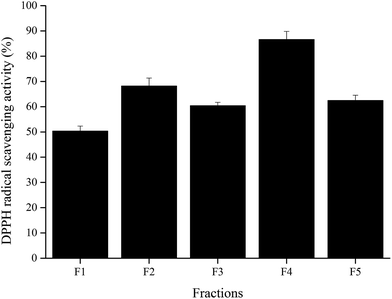 | ||
| Fig. 2 DPPH radical scavenging activities of the collected fractions from RP-HPLC system at a concentration of 3 mg mL−1. The data are shown as mean ± SD. | ||
MALDI-TOF-MS for peptide identification was performed using the highest active fraction F4. A total of four peptide sequences were identified (Table 4). The molecular masses of all these identified peptides are less than 500 Da. All of them were peptides with 2–4 amino acid residues. The biological activities of these peptides depend on their amino acid composition and structure. Suetsuna et al.24 demonstrated that hydrophobic amino acid residues, such as Ala or Leu, were helpful for radical scavenging. In addition, the active peptides usually have specific amino acid sequences. One study demonstrated strong antioxidant activity of the peptides containing Leu residues at the N-terminus and Arg residues at the C-terminus (Leu-Asp-Arg).25 The study of Saito et al. also proved higher antioxidant property of Leu-Trp-Arg.22 In this study, Leu-Ala-Arg was identified from fraction F4, and meant that this was associated with higher radical scavenging activity, which may be due to a tripeptide with specific amino acid sequence.
3.2 Effect of wheat peptides on exercise ability and fatigue resistance
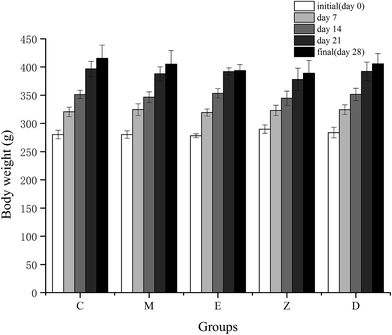
Long-term high-intensity training may lead to endocrinal imbalance, movement system strain, energy metabolism disorder, and further may affect the ability to exercise and recovery ability after fatigue. It has been reported that excessive training would increase blood viscosity,26 and inhibit brain absorption of complex ammonia, leading to emotional depression.27The indicators for determining the declination of body function includes TTE and COR. TTE is the main male androgen and it increases the protein synthesis metabolism and improves athletic ability. COR is mainly generated in stress conditions, which is related to the body's catabolism, leading to the declination of exercise capacity. Under normal circumstances, synthesis and decomposition of blood TTE and COR maintains balance. However, long-term high-intensity training would lead to hypothalamic dysfunction, and gradual decline of TTE in the blood. The reason could be due to the decrease in the cleavage enzyme activity of cholesterol side chain in the testicular interstitial cells.28 At the same time, the concentration of COR was gradually increased, and the protein catabolism was increased, resulting in the decrease ratio of blood TTE to COR.29,30 In this study, the model of increasing load intensity swimming exercise in rats was used. Results showed the weight gain of the rats in the exercise group was slightly slower than that of the control group (Fig. 3), proving that the long-term and high-intensity exercise training hindered the normal growth of the body. However, the level of TTE and COR in this study showed no significant changes between each group (Table 6), which may be due to the loss of endocrine system dysfunction with training. Another reason for this may be that the serum was taken 24 h after the rat's exhaustive test, and the fatigue of the exercise stress in the rats was fully restored, resulting in a stable level of TTE and COR. Rahimi et al.31 have shown that during high-intensity endurance training, TTE/COR ratio of long-term resting was higher than that of short-term resting, which was helpful in the stable maintenance of TTE and COR levels. In addition, supplementation of wheat peptides in this study showed no significant effect on TTE and COR levels. Although TTE and COR are associated with the synthesis and decomposition of proteins, additional protein supplementation may not have any effect on both the substances.
| C | M | E | Z | D | |
|---|---|---|---|---|---|
| a Data are shown as mean ± SD. *p < 0.05, **p < 0.01 vs. C; #p < 0.05, ##p < 0.01 vs. M; &p < 0.05, &&p < 0.01 vs. E; @p < 0.05, @@p < 0.01 vs. Z. C, no exercise control group; M, no exercise with low dose group; E, exercise control group; Z, exercise with low dose group; D, exercise with high dose group. | |||||
| SOD (U mg−1) | 5.31 ± 0.45 | 5.96 ± 2.08 | 5.46 ± 2.57 | 5.59 ± 1.33 | 6.43 ± 1.34 |
| MDA (nmol mg−1) | 1.2 ± 0.31 | 0.63 ± 0.19** | 0.91 ± 0.31 | 1.12 ± 0.47## | 1.07 ± 0.37## |
| IL-6 (pg mg−1) | 11.05 ± 11.23 | 23.57 ± 15 | 34.26 ± 11.03** | 29.08 ± 8.26** | 11.04 ± 4.44&&@@ |
| IL-8 (pg mg−1) | 6.1 ± 1.24 | 11.44 ± 3.72** | 12.59 ± 3.5** | 7.24 ± 1.43#&& | 7.18 ± 2.09#&& |
| Caspase-3 (ng mg−1) | 0.09 ± 0.05 | 0.16 ± 0.06 | 0.19 ± 0.07* | 0.08 ± 0.03##&& | 0.05 ± 0.03##&& |
| sIgA (μg mg−1) | 0.23 ± 0.04 | 0.3 ± 0.06** | 0.25 ± 0.07 | 0.22 ± 0.04## | 0.25 ± 0.05 |
| C | M | E | Z | D | |
|---|---|---|---|---|---|
| a Data are shown as mean ± SD. #p < 0.05 vs. M; &p < 0.05 vs. E. C, no exercise control group; M, no exercise with low dose group; E, exercise control group; Z, exercise with low dose group; D, exercise with high dose group. | |||||
| CK (U L−1) | 794.75 ± 273.71 | 644.44 ± 136.95 | 598.38 ± 263.13 | 718.88 ± 259.59 | 741.79 ± 175.28 |
| TTE (ng mL−1) | 0.51 ± 0.11 | 0.67 ± 0.29 | 0.55 ± 0.05 | 0.55 ± 0.03 | 0.6 ± 0.13 |
| COR (ng mL−1) | 296.65 ± 25.37 | 290.11 ± 24.34 | 299.37 ± 8.29 | 298.84 ± 17.16 | 285.06 ± 17.47 |
| ET (EU mL−1) | 0.71 ± 0.17 | 0.84 ± 0.06 | 0.78 ± 0.23 | 0.72 ± 0.14 | 0.64 ± 0.15# |
| IL-6 (pg mL−1) | 192.19 ± 29.15 | 198.23 ± 22.31 | 209.23 ± 17.23 | 197.53 ± 18.11 | 191.84 ± 21.65& |
| IL-8 (pg mL−1) | 44.59 ± 6.79 | 46.15 ± 7.74 | 50.82 ± 5.63 | 43.6 ± 10.89 | 42.08 ± 5.83& |
In Table 6, there was no significant difference in CK between each group. CK is a non-functional enzyme present in human serum that does not catalyze in the blood and reflects only the destruction of organ cells or cell permeability. CK is mainly present in skeletal muscle, myocardium and brain tissues. Changes in CK levels are often used as an important indicator for the assessment of skeletal muscle load, injury, adaptability and recovery.32,33 Results of this study indicated that the high-intensity training did not cause skeletal muscle injury, and that supplemented with wheat peptides had no significant effect on skeletal muscle injury. According to the previous studies, supplementation of protein or protein hydrolysates showed no significant preventive effect on skeletal muscle injury and recovery during exercise,34,35 and these results were consistent with our study findings.
Glycogen is mainly stored in the skeletal muscle and liver, and serves as a form of energy storage. Long-term exercise can promote the body's capacity of uptake and utilization of sugar, and increase muscle glycogen and liver glycogen storage.36 In Table 7, muscle glycogen of group M was significantly lower than that in group E (p < 0.05), group Z (p < 0.05) and group D (p < 0.01). Results of our study showed that the muscle glycogen of exercise group was significantly higher than the quiet group, indicating that exercise can improve muscle glycogen storage. In the exercise groups, there was no significant effect with wheat peptide supplementation on muscle glycogen storage, indicating that supplementation of wheat peptide did not have a significant effect on muscle glycogen storage during exercise. Glycogen is mainly composed of excess glucose, and wheat peptide is the hydrolysate derived during the wheat protein hydrolysis. The addition of protein may not have a significant effect on the synthesis of glycogen, but the excess protein and carbohydrate could be helpful in the maintenance of glycogen storage. Morifuji et al.37 reported that the addition of glucose and whey protein hydrolysate significantly reduced the consumption of muscle glycogen compared with glucose supplementation alone during exercise. Ivy et al.38 also showed that the addition of carbohydrate and protein was more helpful in improving the ability of high-intensity exercise than carbohydrate supplementation alone. Supplementation of carbohydrates alone can maintain the level of muscle glycogen during exercise,39 and supplementation of carbohydrates with additional protein may show better results. Bringing up the results of this study, the additional protein or protein hydrolysate alone has no significant influence on glycogen storage, but it may play a complementary role in promoting supplementation effects of carbohydrates. The reason may be the protein or protein hydrolysate is more capable of activating the key protein kinase in the skeletal muscle, which determines the absorption of glucose and the synthesis of glycogen during exercise.37
| C | M | E | Z | D | |
|---|---|---|---|---|---|
| a Data are shown as mean ± SD. *p < 0.05, **p < 0.01 vs. C; #p < 0.05, ##p < 0.01 vs. M; &p < 0.05, &&p < 0.01 vs. E. C, no exercise control group; M, no exercise with low dose group; E, exercise control group; Z, exercise with low dose group; D, exercise with high dose group. | |||||
| SOD (U mg−1) | 3.52 ± 0.99 | 3.49 ± 0.66 | 3.32 ± 0.84 | 4.2 ± 0.62& | 4.62 ± 0.83**##&& |
| MDA (nmol mg−1) | 0.82 ± 0.09 | 0.7 ± 0.12* | 0.89 ± 0.21## | 0.71 ± 0.11& | 0.78 ± 0.18 |
| GSH-Px (U mg−1) | 106.56 ± 27.07 | 78.28 ± 7.17 | 70.94 ± 13.34* | 93.4 ± 25.76 | 102.6 ± 18.69#&& |
| MG (mg g−1) | 3.1 ± 0.88 | 2.49 ± 0.47 | 3.32 ± 0.67# | 3.2 ± 0.92# | 3.38 ± 0.77## |
| IL-6 (pg mg−1) | 6.61 ± 2.45 | 8.37 ± 0.85 | 7.93 ± 2.67 | 8.11 ± 2.76 | 9.37 ± 4.38 |
In the long-term study of fatigue and injury mechanism, the relationship between generation/removal of free radical and fatigue-injury of body were paid more attention. Many studies23,40–44 have shown that exercise fatigue was associated with increased free radicals in the body. In the process of movement, generation and removal of free radicals play a decisive role in the levels of free radicals in vivo, thus affecting the occurrence and elimination of exercise-induced fatigue, and the extent of oxidative stress injury.23,41
Because of the differences in the blood distribution of respective tissues during exercise, as well as different sensitivities to exercise stimulation, there were differences in free radical metabolism and oxidative damage of different tissues on the same exercise. Skeletal muscle involves strong metabolic organization in the body, which is the driving force of movement, and is the organ of maximal consumption of energy and oxygen. So, skeletal muscle represents to be the most severe lipid peroxidative tissue that is affected with acute strenuous exercise, and is the most vulnerable tissue to free radical damage. SOD is an important antioxidant enzyme in the body, which can remove oxygen free radicals. MDA is the main product of lipid peroxidation, reflecting the lipid peroxidation level of body tissues and cells. GSH-Px is an important peroxide decomposition enzyme in the body, which can protect the body from peroxide damage. In Table 7, the SOD activity of skeletal muscle in group D was significantly higher than that in groups C, M and E (p < 0.01), and group Z was significantly higher than that in group E (p < 0.05). The content of MDA in group M was significantly lower than groups C (p < 0.05) and E (p < 0.01), and group Z was significantly lower than that in group E (p < 0.05). GSH-Px activity in group E was significantly lower than that in group C (p < 0.05), and group D was significantly higher than that in groups M (p < 0.05) and E (p < 0.01). The activities of SOD and GSH-Px in skeletal muscle of exercise group rats fed with wheat peptide were significantly higher than the exercise control groups. MDA content was significantly lower than that of exercise control group, which had an important effect on scavenging free radicals in skeletal muscle tissue. Rats not fed with wheat peptide demonstrated significantly lower activity of GSH-Px in the exercise group and the content of MDA was significantly higher than those in the quiet group. This indicated that the antioxidant enzyme system of skeletal muscle tissue was affected by exercise stress, while its function was significantly improved with wheat peptide supplementation. These results suggest that long-term exercise training would increase the level of oxidative stress in skeletal muscle and increase oxygen free radicals, and was an important factor in the occurrence of exercise-induced fatigue. The supplement of wheat peptide significantly improved the function of antioxidant enzyme system of the body, and thus the oxygen free radicals were eliminated in time.
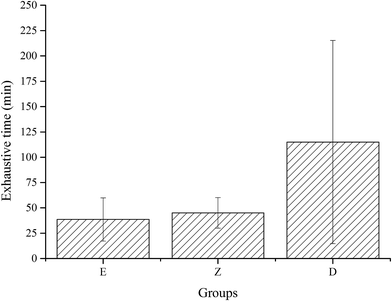
Exhaustive experiments are the most direct way to test the exercise capacity of animals. In this study, exhaustive experiment was conducted in E, Z, and D groups, with 12 rats in each group. Results showed that the exhaustive time of group D with high dose wheat peptide supplementation was longer than group E (p = 0.028) and group Z (p = 0.055), but no significant differences between groups Z and E were observed (Fig. 4). Although there were large individual differences, the effects of high-dose wheat peptide supplementation on prolonging the exhaustive time and improving the exercise ability were obvious. The positive effects may be due to the improvement of the situation of oxidative stress in the skeletal muscle system. Yu et al.14 found that soybean peptide had a strong free radical scavenging ability. Rats fed with 400 mg kg−1 body weight of soybean peptide daily for 20 days demonstrated an increase of 70% in the swimming exhaustive time of rats than the control group. In combination with the results of exhaustive experiments in this study, it can be concluded that the enhancement of exercise capacity in wheat peptide nutrition group was related to the enhancement of antioxidant capacity of skeletal muscle, suggesting that anti-fatigue may be related to anti-oxidation.
Long-term exercise stress can lead to the dysfunction of central nervous system. 5-HT as a neurotransmitter is mainly involved in the neurological regulation of mood, energy, appetite, sleep, body temperature and so on. Studies found that lower levels of 5-HT were more prone to anxiety, depression, suicide, impulse, alcoholism, assault and violence.45,46 Acetylcholine is a common neurotransmitter in the central nervous system and the peripheral nervous system. It participates in nerve conduction in the autonomic nervous system and the kinetic nervous system. Acetylcholine is released when the nerve endings are excited by stimulation. AchE is a key enzyme in the conduction of biological nerve impulse, and mainly exists in the synaptic space of cholinergic nerve endings. AchE mainly hydrolyzes acetylcholine, terminate excitatory function of neurotransmitters on the synaptic membrane, and ensure the normal transmission of nerve signals in organisms.47 In Table 8, the AchE of group E was significantly lower than that of groups Z (p < 0.01) and D (p < 0.05), and group C was significantly lower than that in group Z (p < 0.05). 5-HT of group M was significantly higher than that of groups C, E (p < 0.05) and Z (p < 0.01), while group D was significantly higher than that of groups C, E (p < 0.05) and Z (p < 0.01). In this study, there were no significant changes in AchE and 5-HT in the exercise control group compared with the quiet control group. But the levels of the two substances in the exercise group with wheat peptide supplementation were significantly higher than the non-supplemented group. It is possibly due to the long-term intake of glutamine-rich wheat peptide, which may improve the serotonergic system and cholinergic system of central nervous system, and also make emotions stable, ease the movement stress, and ensure the normal functioning of the central nervous system. This study demonstrated that supplementation of wheat peptide may modulate neurotransmitters in the central nervous system. According to the previous reports,16 soybean peptides regulated neurotransmitters, and also enhancing the brain function.
| C | M | E | Z | D | |
|---|---|---|---|---|---|
| a Data are shown as mean ± SD. *p < 0.05, **p < 0.01 vs. C; #p < 0.05, ##p < 0.01 vs. M; &p < 0.05, &&p < 0.01 vs. E; @p < 0.05, @@p < 0.01 vs. Z. C, no exercise control group; M, no exercise with low dose group; E, exercise control group; Z, exercise with low dose group; D, exercise with high dose group. | |||||
| AchE (μg mg−1) | 23.49 ± 5.74 | 25.76 ± 6.61 | 21.2 ± 6.58 | 29.25 ± 3.4*&& | 27.74 ± 5.93& |
| 5-HT (pg mg−1) | 13.18 ± 3.65 | 18.82 ± 5.12* | 14.12 ± 5.19# | 10.3 ± 2.3## | 19.14 ± 5.74*&@@ |
3.3 Effect of wheat peptides on intestinal immune function
Long-term exercise stress can cause declination in body function, affecting the immune function, and is considered as an important law of motor immunology. Especially, intestinal immune function has an important impact on the body's immune system. Intestine is the largest organ of bacteria and endotoxin repository. Under normal circumstances, the bacteria and endotoxins were harmless to the body, which is entirely dependent on the body's intestinal mucosal barrier function. However, under stress conditions, the intestinal immune system is affected, which decrease the epithelial cell integrity, and increase the intestinal permeability. This in turn lead to intestinal bacteria and endotoxin breakthrough of the intestinal mucosal barrier into the mesenteric lymph node or portal vein system, and get into the organs away from the intestine, resulting in the translocation of intestinal bacteria, and increase the blood endotoxins, and further arising the blood immune response.48,49 As shown in Table 6, the endotoxin levels of exercise control group showed no significant difference compared with the quiet control group from the serum test, indicating no obvious occurrence of intestinal bacterial translocation during exercise. There was no significant difference between the nutrition group and control group of endotoxin level, which indicated that edible wheat peptide had no significant effect on the inhibition of endotoxin level and intestinal bacterial translocation. Endotoxin is a component of the Gram-negative bacterial cell wall, and its main chemical ingredient is lipopolysaccharide, which is toxic to the host. Studies have shown that antibacterial agents do not necessarily be effective against endotoxins.50 As a result, wheat peptide might not necessarily have significant anti-endotoxin effects in the blood.Caspase-3 is the most important terminal cleavage enzyme in the process of apoptosis and plays an irreplaceable role in apoptosis.51 In Table 5, the activity of caspase-3 was significantly higher in group E than that in group C (p < 0.05), which indicated that exercise stress could accelerate the process of intestinal epithelial cell apoptosis, and was consistent with the results of Hoffman-Goetz et al. study.52 The activity of caspase-3 in the exercise group with supplementation of wheat peptide was significantly lower than that in the control group, which indicated that wheat peptide supplementation had an important effect on the apoptosis of intestinal epithelial cells. The mechanism of wheat peptide inhibition on caspase-3 activity may be that the wheat peptide is a pseudo-substrate of caspase-3, and after cleavage by caspase-3, wheat peptide still binds to active protease and hinders the active site of caspase-3. Intestinal epithelial cells acts as an important barrier in the intestine. This study showed that exercise may have adverse effects on the intestinal barrier, while wheat peptide can protect the intestinal barrier during exercise. Ostaszewska et al.53 showed that compared with the wheat protein supplementation alone, the mixture of wheat protein and lysine-glycine (Lys-Gly) dipeptide could play a significant inhibitory effects on the apoptosis of intestinal caspase-3 positive cells, suggesting that supplementation of small peptides may be beneficial for slowing the apoptosis of intestinal epithelial cells.
IgA is classified as serotype IgA and secretory IgA (sIgA). Serotype IgA does not show significant immune function in the serum, while sIgA is the main antibody that is produced against the local mucosal anti-infection immunity.54 High intensity exercise showed adverse effects on the intestinal immune system, leading to changes in the ultrastructure of intestinal mucosa, decreased IgA levels, decreased intestinal mucosal immunity and digestive system function, and diminished the capacity of resistance to pathogen.55 In addition, after high-intensity exercise, skeletal muscle protein catabolism is increased, and several amino acids were released, where glutamine accounted for the largest proportion. Under stress conditions, the demand for glutamine increases in the intestinal tissue, resulting in a sharp declination in the blood glutamine concentration. Glutamine deficiency also resulted in a significant decrease in the sIgA content in the intestine, which was associated with a decrease in the number of plasma cells secreting sIgA in the intestinal mucosa. Therefore, glutamine supplementation can significantly improve the body's immune function after high-intensity exercise.21 Glutamine can improve the body's immune function and protect the intestinal mucosal barrier. The most abundant amino acids in wheat peptide are glutamine and glutamic acid, which constitutes to 37.7 g/100 g in this study. Therefore, supplementation of wheat peptide may play an important role in improving the immune function of the body. Although sIgA in this study was not significantly associated with exercise, wheat peptide can significantly enhance the level of intestinal sIgA during the absence of exercise (Table 5). Zhou et al.56 showed that alanyl-glutamine dipeptide significantly increased sIgA activity in calf intestine and reduced the incidence of diarrhea. Fan et al.57 also found that supplementation of glutamine increased the number of intestinal lamina IgA plasma cells, and improved the immune function of the intestinal mucosa.
Interleukin is produced by activated monocytes – macrophages and lymphocytes, which is a cytokine that regulates immunity and eliminates inflammation. The main functions of interleukin-6 (IL-6) are: mediating T and B cell activation, proliferation and differentiation, induction of acute phase response during protein synthesis, promoting bone marrow hematopoietic function. Interleukin-8 (IL-8) is a strong chemotactic factor of specific and non-specific immune cells, as well as induces chemotaxis and activation of neutrophils.58 Table 5 showed that IL-6 in group E and group Z was significantly higher than that in groups C and D (p < 0.01). The levels of IL-8 in groups M and E were significantly higher than those in group C (p < 0.01), and groups Z and D were significantly lower than those in groups M and E (p < 0.05 or 0.01). These results indicated that the inflammatory response of intestinal tract was more obvious after the exercise intervention, and the inflammatory reaction was obviously eliminated after supplementing wheat peptide. In addition, IL-6 and IL-8 levels of group D were significantly lower than those in group E (P < 0.05, Table 6). Serum test results showed that exercise did not cause significant increase in the inflammatory response in blood, but the addition of wheat peptide significantly reduced the levels of blood inflammatory factors. Teixeira-Lemos et al.59 reported that long-term high-intensity exercise may lead to systemic inflammatory response, and glutamine supplementation can reduce the inflammatory response, which is consistent with the results of our study. In addition, the activity of SOD showed no significant differences in each group. The content of MDA in group M was significantly lower than that in groups C, Z and D (p < 0.01, Table 5). The present study showed that the levels of SOD and MDA in the intestinal tissue without wheat peptide supplementation were not significantly changed after exercise, which means that the level of oxidative stress on the intestinal tissue was not affected by training. After wheat peptide supplementation, the level of MDA was reduced in the absence of exercise intervention, but the antioxidant effect was lost in the training groups. The above results indicated that wheat peptide could play a significant role in slowing the inflammatory response caused by long-term exercise, which may have no association with oxidative stress in the intestine.
4. Conclusion
In summary, from the results of exercise capacity and anti-fatigue ability analysis showed that long-term supplementation of wheat peptide could effectively improve the exercise capacity of one-time exhaustive exercise, remove free radicals in skeletal muscle in time, maintain long-term emotional stability, but could not improve the ability of glycogen storage in skeletal muscle or prevent skeletal muscle injury. The intestinal immune function analysis showed that long-term supplementation of wheat peptide inhibited apoptosis of intestinal epithelial cells, improved the intestinal sIgA level at rest, and alleviated the inflammatory response in the intestine and in the blood effectively. However, wheat peptide supplementation could not reflect the ability to improve the antioxidant capacity of the intestinal tract at the state of motion or prevent the displacement of bacteria.Conflicts of interest
All the authors declare that they have no conflict of interest.Abbreviations
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| GSH-Px | Glutathione peroxidase |
| TTE | Testosterone |
| COR | Cortisol |
| CK | Creatine kinase |
| ET | Endotoxin |
| MG | Muscle glycogen |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| Caspase-3 | Cysteinyl aspartate specific proteinase-3 |
| sIgA | Secretory immunoglobulin A |
| 5-HT | 5-Hydroxytryptamine |
| AchE | Acetylcholinesterase |
Acknowledgements
This work was supported by a key project of the Chinese Army (No. AX110C002). We would like to thank participants of this study without whom this research wouldn't have been accomplished.References
- H. Wieser, Food Microbiol., 2007, 24, 115–119 CrossRef CAS PubMed.
- M. C. Gianibelli, O. R. Larroque, F. MacRitchie and C. W. Wrigley, Cereal Chem., 2001, 78, 635–646 CrossRef CAS.
- S. Žilić, G. Akıllıoğlu, A. Serpen, M. Barać and V. Gökmen, Food Res. Int., 2012, 49, 1–6 CrossRef.
- K. X. Zhu, H. M. Zhou and H. F. Qian, Process Biochem., 2006, 41, 1296–1302 CrossRef CAS.
- J.-s. Wang, M.-m. Zhao, Q.-z. Zhao and Y.-m. Jiang, Food Chem., 2007, 101, 1658–1663 CrossRef CAS.
- J. Jia, H. Ma, W. Zhao, Z. Wang, W. Tian, L. Luo and R. He, Food Chem., 2010, 119, 336–342 CrossRef CAS.
- B. G. Thewissen, A. Pauly, I. Celus, K. Brijs and J. A. Delcour, Food Chem., 2011, 127, 1653–1658 CrossRef CAS.
- A. Pihlanto-Leppala, P. Koskinen, K. Piilola, T. Tupasela and H. Korhonen, J. Dairy Res., 2000, 67, 53–64 CrossRef CAS PubMed.
- H. Motoi and T. Kodama, Nahrung, 2003, 47, 354–358 CrossRef CAS PubMed.
- C. Ziadrou, R. A. Streaty and W. Klee, J. Biol. Chem., 1979, 254, 2446–2449 Search PubMed.
- S. Fukudome, Y. Jinsmaa, T. Matsukawa, R. Sasaki and M. Yoshikawa, FEBS Lett., 1997, 412, 475–479 CrossRef CAS PubMed.
- I. Calzuola, F. Giavarini, P. Sassi, L. De Angelis, G. L. Gianfranceschi and V. Marsili, Peptides, 2005, 26, 2074–2085 CrossRef CAS PubMed.
- H. J. Jeong, J. B. Jeong, D. S. Kim, J. H. Park, J. B. Lee, D.-H. Kweon, G. Y. Chung, E. W. Seo and B. O. de Lumen, Cancer Lett., 2007, 255, 42–48 CrossRef CAS PubMed.
- B. Yu, Z.-X. Lu, X.-M. Bie, F.-X. Lu and X.-Q. Huang, Eur. Food Res. Technol., 2008, 226, 415–421 CrossRef CAS.
- W. Yuanxiu, S. Xiaoyan, Z. Guixiang, S. Naxin and Z. Mingyang, International Conference on Biomedical Engineering and Biotechnology, 2012 Search PubMed.
- D. Yimit, P. Hoxur, N. Amat, K. Uchikawa and N. Yamaguchi, Nutrition, 2012, 28, 154–159 CrossRef CAS PubMed.
- N. Mach and D. Fuster-Botella, J. Sport Health Sci., 2017, 6, 179–197 CrossRef.
- R. Z. Gu, W. Y. Liu, F. Lin, Z. T. Jin, L. Chen, W. X. Yi, J. Lu and M. Y. Cai, Food Res. Int., 2012, 49, 326–333 CrossRef CAS.
- X. Li, J. Lin, Y. Gao, W. Han and D. Chen, Chem. Cent. J., 2012, 6, 140 CAS.
- R.-Z. Gu, C.-Y. Li, W.-Y. Liu, W.-X. Yi and M.-Y. Cai, Food Res. Int., 2011, 44, 1536–1540 CrossRef CAS.
- L. M. Castell, Nutrition, 2002, 18, 371–375 CrossRef CAS PubMed.
- K. Saito, D. Jin, T. Ogawa, K. Muramoto, E. Hatakeyama, A. Tadashi Yasuhara and K. Nokihara, J. Agric. Food Chem., 2003, 51, 3668 CrossRef CAS PubMed.
- M. B. Reid, Free Radicals Biol. Med., 2008, 44, 169–179 CrossRef CAS PubMed.
- K. Suetsuna and J. R. Chen, Food Sci. Technol. Res., 2002, 8, 227–230 CrossRef CAS.
- G. Hang, Y. Kouzuma and M. Yonekura, Food Chem., 2009, 113, 238–245 CrossRef.
- E. Varlet-Marie, F. Maso, G. Lac and J. F. Brun, Clin. Hemorheol. Microcirc., 2004, 30, 211–218 Search PubMed.
- A. L. T. Uusitalo, M. Valkonen-Korhonen, P. Helenius, E. Vanninen, K. A. Bergstrom and J. T. Kuikka, Int. J. Sports Med., 2004, 25, 150–153 CrossRef CAS PubMed.
- E. P. Murono, R. C. Derk and Y. Akgul, Reprod. Toxicol., 2006, 21, 148–153 CrossRef CAS PubMed.
- A. Urhausen, T. Kullmer and W. Kindermann, Eur. J. Appl. Physiol. Occup. Physiol., 1987, 56, 528–533 CrossRef CAS PubMed.
- A. Urhausen, H. H. Gabriel and W. Kindermann, Med. Sci. Sports Exercise, 1998, 30, 407–414 CAS.
- R. Rahimi, H. Rohani and M. Ebrahimi, Apunts Medicina De Lesport, 2011, 46, 145–149 CrossRef.
- L.-L. Huang, C.-T. Huang, M.-L. Chen and I. F. Mao, Chin. J. Physiol., 2010, 53, 254–261 CrossRef PubMed.
- P. Brancaccio, N. Maffulli and F. M. Limongelli, Br. Med. Bull., 2007, 81–82, 209–230 CrossRef PubMed.
- J. Lee,
 ,
,  and
and  , Korean Journal of Sport Science, 2010, 21, 1298–1314 Search PubMed.
, Korean Journal of Sport Science, 2010, 21, 1298–1314 Search PubMed. - B. Knechtle, P. Knechtle, C. Mrazek, O. Senn, T. Rosemann, R. Imoberdorf and P. Ballmer, J. Int. Soc. Sports Nutr., 2011, 8, 6 Search PubMed.
- K. Sakamoto, D. E. W. Arnolds, I. Ekberg, A. Thorell and L. J. Goodyear, Biochem. Biophys. Res. Commun., 2004, 319, 419–425 Search PubMed.
- M. Morifuji, A. Kanda, J. Koga, K. Kawanaka and M. Higuchi, Nutrition, 2011, 27, 833–837 Search PubMed.
- J. L. Ivy, P. T. Res, R. C. Sprague and M. O. Widzer, Int. J. Sport Nutr. Exercise Metab., 2003, 13, 382–395 Search PubMed.
- G. McConell, R. J. Snow, J. Proietto and M. Hargreaves, J. Appl. Physiol., 1999, 87, 1083–1086 Search PubMed.
- Y. Lecarpentier, J. Appl. Physiol., 2007, 103, 1917–1918 Search PubMed.
- S. K. Powers and M. J. Jackson, Physiol. Rev., 2008, 88, 1243–1276 Search PubMed.
- R. A. Nazeer, N. S. S. Kumar and R. J. Ganesh, Peptides, 2012, 35, 261–268 Search PubMed.
- J.-F. Ding, Y.-Y. Li, J.-J. Xu, X.-R. Su, X. Gao and F.-P. Yue, Food Hydrocolloids, 2011, 25, 1350–1353 Search PubMed.
- J. J. Wang, M. J. Shieh, S. L. Kuo, C. L. Lee and T. M. Pan, Appl. Microbiol. Biotechnol., 2006, 70, 247–253 Search PubMed.
- L. A. W. Jans, W. J. Riedel, C. R. Markus and A. Blokland, Mol. Psychiatry, 2007, 12, 522–543 Search PubMed.
- G. P. A. Placidi, M. A. Oquendo, K. M. Malone, Y. Y. Huang, S. P. Ellis and J. J. Mann, Biol. Psychiatry, 2001, 50, 783–791 Search PubMed.
- K. A. Modesto and C. B. R. Martinez, Chemosphere, 2010, 78, 294–299 Search PubMed.
- G. A. P. Nieuwenhuijzen, E. A. Deitch and R. J. A. Goris, Eur. J. Surg., 1996, 162, 259–273 Search PubMed.
- B. J. Rowlands, C. V. Soong and K. R. Gardiner, Br. Med. Bull., 1999, 55, 196–211 Search PubMed.
- K. Brandenburg, L. Heinbockel, W. Correa and K. Lohner, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 971–979 Search PubMed.
- G. Nunez, M. A. Benedict, Y. M. Hu and N. Inohara, Oncogene, 1998, 17, 3237–3245 Search PubMed.
- L. Hoffman-Goetz and P. A. Spagnuolo, J. Neuroimmunol., 2007, 187, 94–101 Search PubMed.
- T. Ostaszewska, K. Dabrowski, M. Kamaszewski, P. Grochowski, T. Verri, M. Rzepkowska and J. Wolnicki, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2010, 157, 158–169 Search PubMed.
- B. Moldt, K. Saye-Francisco, N. Schultz, D. R. Burton and A. J. Hessen, Methods, 2014, 65, 127–132 Search PubMed.
- M. Godinez-Victoria, M. Elisa Drago-Serrano, H. Reyna-Garfias, M. Viloria, E. Lara-Padilla, A. A. Resendiz-Albor, L. E. Sanchez-Torres, T. R. Cruz-Hernandez and R. Campos-Rodriguez, Brain, Behav., Immun., 2012, 26, 1300–1309 Search PubMed.
- Y. Zhou, P. Zhang, G. Deng, X. Liu and D. Lu, Vet. Immunol. Immunopathol., 2012, 145, 134–142 Search PubMed.
- J. Fan, G. Li, L. Wu, S. Tao, W. Wang, Z. Sheng and Q. Meng, Nutrition, 2015, 31, 766–774 Search PubMed.
- S.-M. Chen, C.-P. Lin, J.-D. Tsai, Y.-H. Chao and J.-N. Sheu, Pediatrics & Neonatology, 2014, 55, 120–126 Search PubMed.
- E. Teixeira-Lemos, J. Oliveira, L. P. Teixeira-Lemos, M. J. Reis-Lima and J. P. Pinheiro, in Nutraceuticals, Academic Press, 2016, pp. 669–714, DOI:10.1016/B978-0-12-804305-9.00017-8.
| This journal is © The Royal Society of Chemistry 2017 |