Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Magnetization of eugenol to fabricate magnetic-responsive emulsions for targeted delivery of caffeic acid phenethyl ester
Tao Wangabcd,
Ren Wangabc,
Zhengxing Chen *abc and
Qixin Zhong*d
*abc and
Qixin Zhong*d
aState Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, People's Republic of China. E-mail: zxchen_2008@126.com
bNational Engineering Laboratory for Cereal Fermentation Technology, Jiangnan University, Wuxi 214122, People's Republic of China
cSchool of Food Science and Technology, Jiangnan University, Wuxi 214122, People's Republic of China
dDepartment of Food Science and Technology, University of Tennessee, Knoxville, TN 37996-4539, USA. E-mail: qzhong@utk.edu
First published on 8th September 2017
Abstract
Fabrication of manipulative colloidal vehicles with appreciable stability under an eco-friendly, cross-linkage free environment has long been one of the major challenges in targeted delivery for curative, acoustic, and imaging purposes. A new targeted delivery system was developed in this paper based on magnetization of eugenol with magnetic Fe3O4 nanoparticles by simply evaporating water from the 3-phase system prior to a one-step coating of the oil using rice proteins to form core/shell structured composites. The droplets, examined as well-defined spheres with a mean hydrodynamic diameter of 204 nm, were metastable against long-term storage for up to 4 months, thermal treatment for up to 90 °C and UV (365 nm) radiation for up to 24 h. Furthermore, upon an external manipulation, the magnetic emulsions enhanced the anti-proliferation effects of encapsulated CAPE, an anti-cancer drug, on HCT-116 cells by over 20% compared to CAPE encapsulated in normal emulsions at dosages of 0.2 and 2 μg mL−1. In principle, a wide variety of o/w emulsions can be magnetized using this approach. As a first step towards magnetic delivery, magnetization of oil may become a new targeted delivery strategy in the development of future site-specific diagnosis and treatment.
Introduction
Nanotechnology provides incredible opportunities for developing micro carriers encapsulating drugs,1 nutraceuticals,2 bioactives,3 etc., for their protective delivery pathways. Optimized drug delivery practices entail successful fabrication of biocompatible carriers and effective delivery of curative and bioactive compounds suffering from various drawbacks such as low solubility, poor absorption in the digestive tract, and low bioavailability, poor stability at the target sites.4 Since vegetable proteins are among the biocompatible, biodegradable materials and are extensively explored as a potential in vitro drug carrier medium, a combination of such protein-based drug carriers and targeted delivery is therefore particularly appealing.5 Superparamagnetic iron oxide nanoparticles (SPIONs) have their metabolic pathways in human blood with high biocompatibility and biodegradability.6 The combination of SPIONs with protein-based carriers allows for the deposition and accumulation of drugs from outside the patient's body using suitable force fields in a highly site-specific fashion.Despite pioneering efforts regarding carrier fabrication,7 magnetization,8 and targeted delivery,9 the motive to improved formulations, strategies, and specialized approaches is still imperative and challenging. SPIONs anchoring is among the most critical steps towards magnetization of drug carriers. One sort of techniques is to incorporate SPIONs into protein shells through covalent binding between carboxyl groups (negative charge) and amino groups (positive charge). In a practical scenario, SPIONs are brought to reaction by surface modification10 or by dissolving in cyclohexane,11 chloroform12 or dichloromethane,13 and subsequent synthesis generally involves the usages of unwelcome cross-link agents like polyvinyl alcohol,13 poly(allylamine hydrochloride), and sodium poly(styrene-4-sulfonate)14 for desired grafting. However, these methods yielded microspheres with short storage life, low stability, and high toxicity.11 Another technique for magnetization is by well suspending SPIONs in oil phase, followed by emulsification to form magnetic-responsive hybrid composites.15 Due to a lack of affinity between oil and SPIONs, this approach is forwarded using solid oil as a disperse phase. The resultant carriers revealed no significant, extremely slow release of encapsulated drugs, thus, envisaged as of low practical interests for therapeutic purposes.
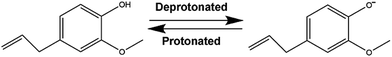
Suspensions of SPIONs in a liquid-oil phase without contributions from intermolecular linkages are intriguing with the aim of engineering a carrier filled with a magnetic, nontoxic core. Eugenol is slightly soluble in water with a solubility of 2.46 mg mL−1 (ref. 16) and a pKa of 10.19 (ref. 17) at 25 °C. By carrying a hydroxyl on its phenolic ring, eugenol can be deprotonated or protonated following the chemical environment of solutions, as showed in Scheme 1. On the other hand, solid oxide particles immersed in aqueous electrolyte solutions can develop surface electrical charges by adsorption or desorption of potential-determining ions like OH− or H+ (ref. 18). Some reports confirmed a negatively charged state of SPIONs under acidic conditions.19,20 Therefore, it is a comparatively simple step for charge transfer from eugenol to water and, subsequently, from water to SPIONs. We therefore hypothesized that the positively charged SPIONs not only create self-repulsion but supply counterions for deprotonated eugenol.
Earlier studies from our group have shown that by adjusting emulsion pH from 9.0 to neutral-adjacent range, modified rice proteins (MRPs) can preferentially precipitate around soybean oil droplets, and resulted emulsions demonstrated well-defined core/shell structures at a final pH of 6.6 (ref. 21). Here, we present a new, robust method for magnetization of eugenol, followed by deposition of rice proteins to fabricate magnetized-core protein-sell composites for targeted delivery. The strategy was based on mixing aqueous SPIONs solutions with eugenol (oil) under mild heat (80 °C) and extensive evaporation of moistures from the three-phase system. Specifically, the first objective was to characterize SPIONs suspensions from a mechanistic perspective. The secondary objective was to investigate the characteristics of emulsions, including morphologies, magnetism, and stabilities. The third objective was to study targeted delivery properties of the magnetic emulsions by investigating the viabilities of HCT-116 cells treated with caffeic acid phenethyl ester (CAPE) encapsulated in emulsions with or without SPIONs loading under external magnetic field.
Experimental section
Materials
Rice protein isolate powder containing 90.14 wt% protein, determined by the Kjeldahl method,22 was purchased from Jinnong Biotechnology Ltd. (Yichun, Jiangxi, China). Eugenol, FeSO4·7H2O, FeCl3·6H2O, caffeic acid phenethyl ester (CAPE), dimethyl sulfoxide (DMSO), and fetal bovine serum (FBS) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Human colon cancer cell line HCT-116 were purchased from American Type Culture Collection (Manassas, VA, USA) and were cultured in McCoy's media (Mediatech, Herdon, VA, USA). All other chemicals were of an analytical grade and were used without further purification.Preparation of MRPs
MRPs were prepared according to our previous study.23 In brief, rice proteins were suspended in distilled water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, w/v) containing 0.03 M NaOH, incubated overnight at −20 °C, and directly milled using an impact mill (model XFB-500, Zhongcheng Mechanical Co., Changsha, China). The milled suspension after warming to room temperature (25 °C) was adjusted to pH 7.0 and centrifuged at 7000g for 10 min. The supernatant was transferred and desalted by dialysis against distilled water using a Fisherbrand membrane (Thermo Scientific Co., Marietta, OH, USA) with a molecular-weight-cut-off (MWCO) of 1000 Da, followed by freeze-drying to prepare lyophilized powder as the MRPs.
30, w/v) containing 0.03 M NaOH, incubated overnight at −20 °C, and directly milled using an impact mill (model XFB-500, Zhongcheng Mechanical Co., Changsha, China). The milled suspension after warming to room temperature (25 °C) was adjusted to pH 7.0 and centrifuged at 7000g for 10 min. The supernatant was transferred and desalted by dialysis against distilled water using a Fisherbrand membrane (Thermo Scientific Co., Marietta, OH, USA) with a molecular-weight-cut-off (MWCO) of 1000 Da, followed by freeze-drying to prepare lyophilized powder as the MRPs.
Synthesis of magnetic Fe3O4 nanoparticles (SPIONs)
The Fe3O4 SPIONs were prepared through an improved chemical co-precipitation method.20 Briefly, a mixture of FeCl3·6H2O and FeSO4·7H2O was dissolved in water by a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (100 mL degassed by bubbling N2 prior to use) and heated to 80 °C under N2 protection along with vigorously agitation under mechanical stirring. Meanwhile, aqueous NH3·H2O (25%, 10 mL) was added by a syringe for maintaining the pH approximately at 10 and heated for an additional 30 min. The reaction mixture was cooled to room temperature under continuous nitrogen protection. The supernatant was while the SPIONs were kept in the reaction flask using a magnet, and then the SPIONs were rinsed with distilled water for dozens of times. Finally, the SPIONs were air dried for further analysis.
1 (100 mL degassed by bubbling N2 prior to use) and heated to 80 °C under N2 protection along with vigorously agitation under mechanical stirring. Meanwhile, aqueous NH3·H2O (25%, 10 mL) was added by a syringe for maintaining the pH approximately at 10 and heated for an additional 30 min. The reaction mixture was cooled to room temperature under continuous nitrogen protection. The supernatant was while the SPIONs were kept in the reaction flask using a magnet, and then the SPIONs were rinsed with distilled water for dozens of times. Finally, the SPIONs were air dried for further analysis.
Preparation of SPIONs suspensions in eugenol
SPIONs were mixed with distilled water by a solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio of 1
liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. 10 mL of eugenol was added into equal volume of aqueous SPIONs under vigorous mechanic stirring accompanied by mild heating at 80 °C to allow evaporation of moisture. After 24 h stirring, aqueous water was fully removed, and the SPIONs suspensions were allowed to cool at room temperature. The resulting suspensions were centrifuged at 5000g to remove unstable Fe3O4 aggregates.
20. 10 mL of eugenol was added into equal volume of aqueous SPIONs under vigorous mechanic stirring accompanied by mild heating at 80 °C to allow evaporation of moisture. After 24 h stirring, aqueous water was fully removed, and the SPIONs suspensions were allowed to cool at room temperature. The resulting suspensions were centrifuged at 5000g to remove unstable Fe3O4 aggregates.
Preparation of magnetic emulsions (Mag-emulsions)
Stock aqueous solutions were prepared with 1% w/v MRPs in distilled water and adjusted to pH 9.0 with 0.1 M NaOH. SPIONs loaded eugenol was mixed with the protein stock solution at a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 using a mixer (model T18BS25, IKA, Wilmington, NC, USA) at gear 5 for 60 s to obtain a pre-emulsion that was constantly stirred to suspend oil drops. To coat oil droplets with MRPs, 0.01 M HCl was drop-wise added to the pre-emulsion at room temperature until it reached a final pH of 6.6. The resulted emulsions were used for characterization as magnetic emulsions (Mag-emulsions). For comparison, pure eugenol was also emulsified and coated with MRPs at the same condition mentioned above to prepare a control (Con-emulsions).
100 using a mixer (model T18BS25, IKA, Wilmington, NC, USA) at gear 5 for 60 s to obtain a pre-emulsion that was constantly stirred to suspend oil drops. To coat oil droplets with MRPs, 0.01 M HCl was drop-wise added to the pre-emulsion at room temperature until it reached a final pH of 6.6. The resulted emulsions were used for characterization as magnetic emulsions (Mag-emulsions). For comparison, pure eugenol was also emulsified and coated with MRPs at the same condition mentioned above to prepare a control (Con-emulsions).
Characterization of SPIONs suspension
Microscopic observations
Size and zeta-potential
Droplet size and distributions and zeta-potential were determined at room temperature. Emulsions were diluted by 100 times using distilled water before testing on a Malvern Nano ZS (Malvern Instrument Ltd, Worcestershire, UK).Superconductive quantum interference device (SQUID)
A magnetometer from Quantum Design MPMS5XL was used to perform magnetization measurements. About 3 mg of SPIONs or spray-dried Mag-emulsions were inserted into the sample holder. Magnetization curves were measured from −3000 to 3000 Oe at 5 K upon zero field cooling (ZFC). The remnant magnetization value of samples was divided by the weight of the analyzed powder (emu g−1). Subsequently, the remnant magnetization per gram of spray-dried Mag-emulsions divided by that of SPIONs gave the average amount of magnetic material inside a droplet.Interfacial tension
The surface tension between eugenol and water (γe–w) and eugenol and MRP stock solution (γe–m) was tested on a Cahn DCA 322 analyzer (Thermo Scientific Co., Marietta, OH, USA) using a Du Nouy ring as the probe. 25 mL eugenol and an identical volume of water/MRP solutions in a 100 mL beaker were used for each test. The platinum–iridium Du Nouy ring was immersed in the heavier phase under the interface. The force required to pull the ring from heavier to the lighter phase until interface breakdown was monitored in the process to calculate the interfacial tension (γ), which is corrected by a correction factor φ summarized by Zuidema and Waters:25
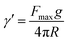 | (1) |
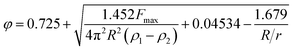 | (2) |
| γ = φγ′ | (3) |
Emulsion stability
After each treatment, the hydrodynamic diameter (Dh) and zeta-potential of Mag-emulsions were analyzed and used as indicators of stability.
Cell proliferation assays
CAPE was separately dissolved in SPIONs suspensions or eugenol at a final concentration of 20 mg mL−1, then emulsified by MRPs as above mentioned. Fresh Mag-emulsions or Con-emulsions with CAPE loading were properly diluted with distilled water to obtain working solutions with a CAPE concentration of 0.8 mg mL−1. For comparison purposes, a CAPE solution (0.8 mg mL−1) dissolved in DMSO was also prepared.Cell proliferation was evaluated by using the method of Pan et al. with slight modifications.26 Human colon cancer cell line HCT-116 cells were cultured in a media supplemented with 10% FBS, 100 unit per mL penicillin and 100 mg mL−1 streptomycin under a humidified atmosphere with 5% CO2 at 37 °C. The anti-cancer activity of CAPE was tested using CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega Corp., Madison, WI, USA). Briefly, HCT-116 cells were seeded in 96-well microtiter plates at a density of 5000 cells per well in a final volume of 100 μL of media, underneath was a magnet to generate manipulation field. The cells were then treated with 0.2, 2 and 20 μg mL−1 of free (dissolved in DMSO) or encapsulated CAPE diluted from the working solutions in the cell media, followed by incubation for another 24 h under the above conditions. Positive controls were cells cultured in the media treated with CAPE-free DMSO or CAPE-free emulsions at the same concentrations as the CAPE-loaded counterparts, and negative control was the pure culture media. After 48 h treatment, 20 μL of CellTiter 96 Aqueous One Solution (Promega, WI, USA) was added to each well and then incubated for 1 h at 37 °C. The absorbance at 490 nm was measured with a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). Normalized cell viability (VN) was expressed using eqn (4):
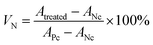 | (4) |
Statistical analysis
The experiments were performed in triplicate, and values were expressed as mean ± standard deviation (SD). Analysis of variance was evaluated using the Duncan's multiple range test.Results and discussion
Characterization of SPIONs suspensions
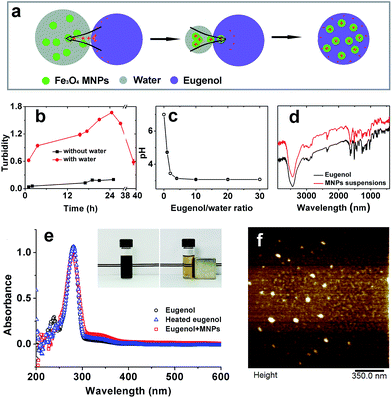
To maximize the accommodation of magnetic responsiveness on a carrier would statistically require increasing magnetic components. As such an increase is not possible under the constraints of limited reaction sites on coated structures, we instead chose incorporating SPIONs into an oil reservoir. The protocol was based on deprotonation of eugenol when brought to contact with water, followed by protonation of SPIONs by absorption of H+, thus incorporating them into oil phase driven by electrostatic attraction (Fig. 1a). As showed by Fig. 1b, the turbidity in oil phase was enhanced by mixing eugenol and aqueous SPIONs along with elapsing time until peaked at 24 h. Further heating caused desorption of H+ from SPIONs and again reduced the turbidity of eugenol. By contrast, simply mixing air-dried SPIONs in eugenol evidenced negligible incorporation. Considering that the pH of distilled water was systematically lowered by addition and stirring of eugenol until reached a final value of 3.05 (Fig. 1c), the comparison addressed an important role played by water that delivered H+ from eugenol to SPIONs. After full evaporation of moisture, SPIONs and eugenol were oppositely charged, and the electrostatic interaction may be responsible for the colloidal stability of the suspensions which could be stable for over three months without visible sedimentation (data no shown).The interaction stabilizing SPIONs was further confirmed by FTIR as being a non-covalent bridging between Fe3O4 and eugenol (Fig. 1d). The UV spectra indicated the same results, where the identical spectra among eugenol, heated eugenol, and SPIONs–eugenol suspensions evidenced the same nature of eugenol (Fig. 1e). AFM observations showed spherical particles of SPIONs with an average diameter of 20 nm (Fig. 1f), and the particles can be recycled under an external magnet field (inset of Fig. 1e).
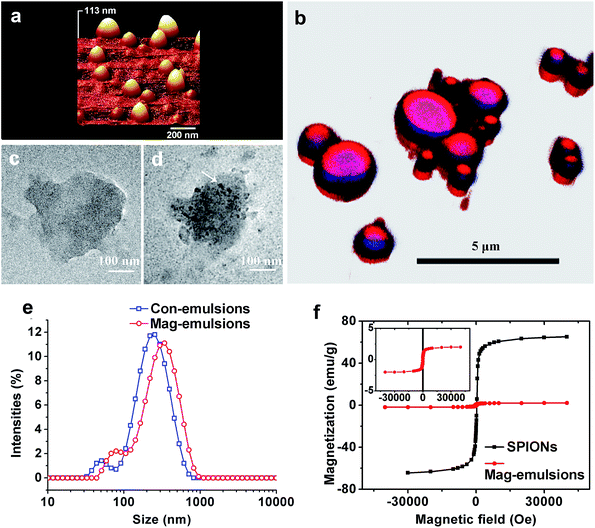
Morphologies and structures of Mag-emulsions
From AFM topographical scans in a three-dimensional mode, it is possible to observe that the SPIONs loaded carriers were of well-defined spherical shapes and presented smooth surface (Fig. 2a), characterized by a mean diameter of 208 ± 40 nm, calculated via software analysis. The carrier structures can be distinguished by respectively scanning of fluorophore-stained components. Since CLSM is an optical instrument of relatively low resolution, only composite carriers larger than 1 μm were well resolved whereas smaller ones, especially those observed in AFM, were distorted or even invisible. Nevertheless, the observations showed that the carriers had obvious core/shell structures with a large size distribution resulted from heterogeneous nature of high speed emulsification (Fig. 2b). Experimental results from STEM illustrated compositional differences between emulsions without (control) or with SPIONs loading. As showed by representative STEM images, a droplet from Con-emulsions was evenly transmitted by electrons (Fig. 2c), whereas SPIONs loaded sample was decorated with dark spots (Fig. 2d) with a mean diameter similar to that observed by AFM, signifying the existence of SPIONs. Of note that both droplets in STEM showed deformed structures, which was probably due to the quick evaporation of eugenol under vacuum observations resulted from a high saturated vapor pressure (0.0221 mm Hg at 25 °C) of the oil model. The above microscopic observations collectively indicated that the carriers were composed of materials characterized by oil-core protein-shell structures with SPIONs embedded inside the lipid core, therefore suggesting successful magnetization of lipid–protein carriers.Size and distributions
Both Con- and Mag-emulsions showed similar broad distributions in a bimodal pattern, with the majority sized between 100–900 nm. The peaks with bigger structures may be assigned to oil droplets with protein shells because the preparation process did not use high shear deformation, while the smaller ones may be assigned to protein-only particles. The polydispersibility index (PDI) calculated from the experiment was up to 0.49 and 0.43 for Con-emulsions and Mag-emulsions, respectively. The results agreed well with large SD in size calculations from AFM and wide size range in CLSM observations. However, droplet size was increased by up to 100 nm when SPIONs were encapsulated, compared to Con-emulsions. By deprotonation of hydroxyl and binding with SPIONs, eugenol may have been partially deprived of miscibility with water and hence, increasing the oil–water interfacial tension during emulsification. The capillary forces maximizing the oil surface area was therefore augmented, and so was the droplet size. Moreover, the increased size for protein-only particles was probably due to aggregation of smaller particles induced by eugenol.27Filippidi and co-authors practiced a protocol similar to our study by lowering the alcohol content so that zein became precipitated on oil droplets, and a large PDI was also observed but a mean diameter of 30–40 μm was reported.28 Apart from the differences in protein structures, the amphiphilic nature of eugenol played a significant role in reducing the droplet size,29 again because of the ability to reduce oil–water interfacial tension. Of note that blood circulation of particles with size greater than 10–15 micrometers could jeopardize the capillaries thanks to infarction or blockage, leading to ischemia or oxygen deprivation and possible tissue death.30
Magnetization measurements
The hysteresis curves of pristine SPIONs and spray-dried Mag-emulsions were similar (Fig. 3b), thus indicating analogous magnetic properties between the two materials. The saturation magnetization at 5 K was 65 emu g−1 for SPIONs, while 2 emu g−1 for the carriers. Further considering that the magnetic moment of SPIONs should be identical with or without encapsulation, we can conclude that the percentage of SPIONs in the dried emulsions is about 3.1 wt%. The results were comparable to magnetic solid–lipid particles emulsified with Tween 80.15 On the other hand, while SPIONs were homogeneously dispersed in the oil matrix (Fig. 2d) and retained superparamagnetic behavior (Fig. 2f) after the encapsulation process, the carriers presented the absence of magnetic dipoles and attractive interactions among oil droplets (Fig. 2a) that would cause embolism during the intravenous administration.13Emulsion stability
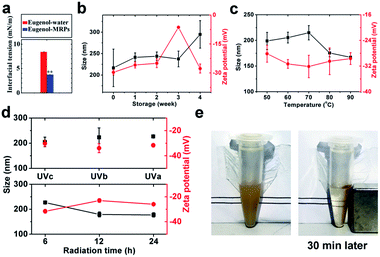
Protein emulsions were believed to be unconditionally instable due to the large interfacial energy moving proteins away from the interface,31 leading to phase separation, which is detrimental to their applications in the food, cosmetic, and pharmaceutical industries. Ostwald ripening and coalescence are the two major factors governing the sedimentation and creaming of oil droplets, following linear and exponential growth of droplet size, respectively.32 The former destabilization relies on the transport of oil to form large droplets at the expense of small ones, while the latter depends on the thinning and disruption of the liquid film that covers the droplets.33,34MRPs was found to effectively reduce the interfacial tension between eugenol and water (Fig. 3a). This on one hand confirmed our former hypothesis about the formation of nanoscale sized droplets; on the other hand, accounted for the thermodynamic stability of the emulsions during 4 week storage (Fig. 3b). Apart from reduced interfacial tension, the competition between Laplace pressure and osmosis pressure can effectively resist Ostwald ripening and coalescence due to considerable water solubility of eugenol.35 However, an appreciable decrease in surface charge occurred at 3rd week, followed by a relatively sharp increase in hydrodynamic diameter (Dh) at 4th week. Upon long-term storage, approaching droplets aggregate and expel water from the hydration layer,36 burying charged groups and increasing Dh at 4th week. Interestingly, the droplets became charged again at 4th week, a plausible assumption is that aggregation was accompanied by osmosis of eugenol among droplets and the released eugenol in turn realigned the protein structures. In addition, the aggregated structure has a lower free energy than the structures in the native state ensemble thus making the folded state metastable.37 The emulsions can be stable for another 3 months without visible sedimentation or creaming.
Thermal treatment can cause aggregation of protein-emulsified emulsions through exposed hydrophobic patches on the denatured proteins.38 Being heated at temperatures from 60 to up to 90 °C, the emulsions maintained considerable colloidal stabilities without significant changes in zeta-potential (ca. −30 mV), assigned to excellent thermal stability of MRPs.39 Then the progressive decrease of Dh from 70 to 90 °C suggested shrinkages of droplets due to release of eugenol.
When radiated by UV, proteins and other macromolecule can generate hydrogen peroxide and superoxide, oxidizing thiols to form disulfide bonds and leading to droplet aggregation.40 As eugenol is a kind of free radical scavenger and suppresses oxidation of thiols on the side chains of proteins, both zeta-potential and Dh were not significantly changed during 6 h UV radiation among all tested wavelengths (top Fig. 3d). By increasing radiation time, however, UVa significantly lowered droplet surface charges but unexpectedly reduced Dh (bottom Fig. 3d). The reason may be that radiation partially removed hydration layer around droplet surface, which accounts for a great part of the droplet size, by oxidizing and packing of protein coatings toward more hydrophobic structures.
Ostwald growth and coalescence are dominating in protein emulsions, only a limited kind of thermodynamic stability is attainable.41 The above results demonstrated a high stability of the SPIONs loaded carriers, and the stability was reached by neither mechanical agitation nor addition of any surfactants as stabilizers. Nevertheless, a robust magnetic response was observed and could readily be used to manipulate and collect the carriers with an external magnetic field (Fig. 3e).
Improved bioavailability of encapsulated bioactives
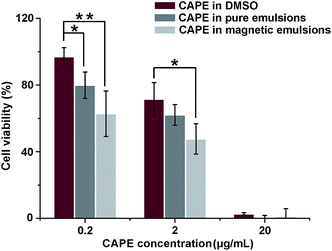
CAPE is a kind of plant phenolic compounds suppressing the expression of β-catenin and reducing tumors in APC Min mice.42 In vitro anti-cancer effect of CAPE is illustrated in Fig. 4. By dosed in culture medium, CAPE, either pre-dissolved in DMSO or encapsulated in emulsions, suppressed the viability of HCT-116 cells in a dose-dependent manner from 0.2 to 20 μg mL−1. The cells were mostly or fully killed at a dosage of 20 μg mL−1 in all administrations, confirming that CAPE is effective anti-proliferation medicine. Carrier materials have the potential of improving cellular uptake of encapsulated bioactives.43 CAPE encapsulated in Con-emulsions showed improved anti-proliferation activity when compared to CAPE pre-dissolved in DMSO, although the improvement was statistically insignificant (p > 0.05) at a dosage of 2 μg mL−1.The cell viability was further inhibited when CAPE was encapsulated in Mag-emulsions and manipulated under an external field. Compared to CAPE encapsulated in Con-emulsions, magnetically guided CAPE reduced the viability of HTC-116 cells by 21.4% (p > 0.05) and 23.2% (p < 0.05) at dosages of 0.2 and 2 μg mL−1, respectively. Those values came to 30.3% (p < 0.01) and 33.3% (p < 0.05) as against CAPE pre-dissolved in DMSO at dosages of 0.2 and 2 μg mL−1, respectively. The enhancement was correlated with improved membrane uptake of drug carriers and the accelerated release of drugs was proposed to result from the increased membrane fluidity before drug insertion.43 The significantly enhanced anti-proliferation activity of manipulated CAPE further highlights the excellent potential of the present magnetization technology, i.e., utilizing reactive oils as SPIONs reservoirs in synthesis of magnetic core/shell composites.
Conclusions
By means of a straightforward evaporation in a double-solvent system, we successfully prepared magnetic responsive eugenol loaded with SPIONs. Using a one-step anti-solvation at a neutral-adjacent pH, eugenol-core MRPs-shell composites with magnetism were subsequently fabricated. The emulsion droplets were spherical, with a hydrodynamic diameter of around 204 nm, and can be readily controlled under an external field. Importantly, the Mag-emulsions were metastable against long-term storage, thermal treatment and UV radiation, which enabled their application in medical and pharmaceutical industries without the need of further addition of stabilizers. Also, using an external magnetic field as a governing tool, CAPE encapsulated in Mag-emulsions showed better anti-proliferation effects on HCT-116 cells than encapsulated in Con-emulsions or pre-dissolved in DMSO. The simple, robust, potent, and additive-free magnetization method simplifies magnetic delivery of oil-soluble components toward desired targets, which may become a new targeted delivery strategy in the development of future site-specific diagnosis and treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National High Technology Research Development Program of China (863 Program) (No. 2013AA102204, 2013AA102206), National Natural Science Foundation of China (No. 31201381, 31471616 and 31371874) and Special Fund for Agro-Scientific Research in the Public Interest of China (No. 201303071). T. Wang would like to thank the scholarship provided by the China Scholarship Council. Q. Zhong would like to acknowledge the support from the University of Tennessee and the USDA National Institute of Food and Agriculture Hatch Project 223984.References
- S. Mura, J. Nicolas and P. Couvreur, Stimuli-responsive nanocarriers for drug delivery, Nat. Mater., 2013, 12, 991 CrossRef CAS PubMed.
- L. Chen and M. Subirade, Chitosan/β-lactoglobulin core–shell nanoparticles as nutraceutical carriers, Biomaterials, 2005, 26, 6041 CrossRef CAS PubMed.
- L. Chen and M. Subirade, Alginate – whey protein granular microspheres as oral delivery vehicles for bioactive compounds, Biomaterials, 2006, 27, 4646 CrossRef CAS PubMed.
- G. L. Amidon, H. Lennernäs, V. P. Shah and J. R. Crison, A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability, Pharm. Res., 1995, 12, 413 CrossRef CAS.
- I. J. Joye and D. J. McClements, Biopolymer-based nanoparticles and microparticles: fabrication, characterization, and application, Curr. Opin. Colloid Interface Sci., 2014, 19, 417 CrossRef CAS.
- L. H. Reddy, J. L. Arias, J. Nicolas and P. Couvreur, Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications, Chem. Rev., 2012, 112, 5818 CrossRef CAS PubMed.
- S. Lam, K. P. Velikov and O. D. Velev, Pickering stabilization of foams and emulsions with particles of biological origin, Curr. Opin. Colloid Interface Sci., 2014, 19, 490 CrossRef CAS.
- J. Dobson, Magnetic nanoparticles for drug delivery, Drug Dev. Res., 2006, 67, 55 CrossRef CAS.
- S. Ganta, H. Devalapally, A. Shahiwala and M. Amiji, A review of stimuli-responsive nanocarriers for drug and gene delivery, J. Controlled Release, 2008, 126, 187 CrossRef CAS PubMed.
- D. Vlaskou, O. Mykhaylyk, F. Krötz, N. Hellwig, R. Renner, U. Schillinger, B. Gleich, A. Heidsieck, G. Schmitz and K. Hensel, Magnetic and acoustically active lipospheres for magnetically targeted nucleic acid delivery, Adv. Funct. Mater., 2010, 20, 3881 CrossRef CAS.
- W. Jiang, Z. Sun, F. Li, K. Chen, T. Liu, J. Liu, T. Zhou and R. Guo, A novel approach to preparing magnetic protein microspheres with core–shell structure, J. Magn. Magn. Mater., 2011, 323, 435 CrossRef CAS.
- H. Y. Koo, S. T. Chang, W. S. Choi, J.-H. Park, D.-Y. Kim and O. D. Velev, Emulsion-based synthesis of reversibly swellable, magnetic nanoparticle-embedded polymer microcapsules, Chem. Mater., 2006, 18, 3308 CrossRef CAS.
- E. Carenza, O. Jordan, P. Martínez-San Segundo, R. Jiřík, Z. Starčuk Jr, G. Borchard, A. Rosell and A. Roig, Encapsulation of VEGF 165 into magnetic PLGA nanocapsules for potential local delivery and bioactivity in human brain endothelial cells, J. Mater. Chem. B, 2015, 3, 2538 RSC.
- Y. Han, D. Radziuk, D. Shchukin and H. Moehwald, Sonochemical synthesis of magnetic protein container for targeted delivery, Macromol. Rapid Commun., 2008, 29, 1203 CrossRef CAS.
- A. Grillone, E. R. Riva, A. Mondini, C. Forte, L. Calucci, C. Innocenti, C. de Julian Fernandez, V. Cappello, M. Gemmi and S. Moscato, Active Targeting of Sorafenib: Preparation, Characterization, and In Vitro Testing of Drug-Loaded Magnetic Solid Lipid Nanoparticles, Adv. Healthcare Mater., 2015, 4, 1681 CrossRef CAS PubMed.
- S. H. Yalkowsky, Y. He and P. Jain, Handbook of Aqueous Solubility Data, CRC Press, 2016 Search PubMed.
- G. Kortüm, W. Vogel and K. Andrussow, Dissociation constants of organic acids in aqueous solution, Pure Appl. Chem., 1960, 1, 187 CrossRef.
- R. Atkinson, A. Posner and J. P. Quirk, Adsorption of potential-determining ions at the ferric oxide–aqueous electrolyte interface, J. Phys. Chem., 1967, 71, 550 CrossRef CAS.
- A. Dong, S. Lan, J. Huang, T. Wang, T. Zhao, L. Xiao, W. Wang, X. Zheng, F. Liu and G. Gao, Modifying Fe3O4-functionalized nanoparticles with N-halamine and their magnetic/antibacterial properties, ACS Appl. Mater. Interfaces, 2011, 3, 4228 CAS.
- Z. Lu, Y. Qin, J. Fang, J. Sun, J. Li, F. Liu and W. Yang, Monodisperse magnetizable silica composite particles from heteroaggregate of carboxylic polystyrene latex and Fe3O4 nanoparticles, Nanotechnology, 2008, 19, 055602 CrossRef PubMed.
- T. Wang, R. Wang, Z. Chen and Q. Zhong, Coating oil droplets with rice proteins to control the release rate of encapsulated beta-carotene during in vitro digestion, RSC Adv., 2016, 6, 73627 RSC.
- A. G. Gornall, C. J. Bardawill and M. M. David, Determination of serum proteins by means of the biuret reaction, J. Biol. Chem., 1949, 177, 751 CAS.
- T. Wang, H. Zhang, L. Wang, R. Wang and Z. Chen, Mechanistic insights into solubilization of rice protein isolates by freeze–milling combined with alkali pretreatment, Food Chem., 2015, 178, 82 CrossRef CAS PubMed.
- M. Bachar, A. Mandelbaum, I. Portnaya, H. Perlstein, S. Even-Chen, Y. Barenholz and D. Danino, Development and characterization of a novel drug nanocarrier for oral delivery, based on self-assembled β-casein micelles, J. Controlled Release, 2012, 160, 164 CrossRef CAS PubMed.
- H. Zuidema and G. Waters, Ring method for the determination of interfacial tension, Ind. Eng. Chem., 1941, 13, 312 CAS.
- K. Pan, Y. Luo, Y. Gan, S. J. Baek and Q. Zhong, pH-driven encapsulation of curcumin in self-assembled casein nanoparticles for enhanced dispersibility and bioactivity, Soft Matter, 2014, 10, 6820 RSC.
- R. A. Frazier, A. Papadopoulou and R. J. Green, Isothermal titration calorimetry study of epicatechin binding to serum albumin, J. Pharm. Biomed. Anal., 2006, 41, 1602 CrossRef CAS PubMed.
- E. Filippidi, A. R. Patel, E. Bouwens, P. Voudouris and K. P. Velikov, All-Natural Oil-Filled Microcapsules from Water-Insoluble Proteins, Adv. Funct. Mater., 2014, 24, 5962 CrossRef CAS.
- Y. He, E. Heine, N. Keusgen, H. Keul and M. Möller, Synthesis and characterization of amphiphilic monodisperse compounds and poly(ethylene imine)s: influence of their microstructures on the antimicrobial properties, Biomacromolecules, 2013, 13, 612 CrossRef PubMed.
- N. P. Desai, P. Soon-Shiong, P. A. Sandford, M. W. Grinstaff and K. S. Suslick, Methods for in vivo delivery of substantially water insoluble pharmacologically active agents and compositions useful therefor, Utility Patent, US5439686, 1995.
- S. Melle, M. Lask and G. G. Fuller, Pickering emulsions with controllable stability, Langmuir, 2015, 21, 2158 CrossRef PubMed.
- A. Kabalnov, A. Pertzov and E. Shchukin, Ostwald ripening in emulsions: I. Direct observations of Ostwald ripening in emulsions, J. Colloid Interface Sci., 1987, 118, 590 CrossRef CAS.
- N. L. Sitnikova, R. Sprik, G. Wegdam and E. Eiser, Spontaneously formed trans-anethol/water/alcohol emulsions: mechanism of formation and stability, Langmuir, 2005, 21, 7083 CrossRef CAS PubMed.
- B. Binks, W. Cho, P. Fletcher and D. Petsev, Stability of oil-in-water emulsions in a low interfacial tension system, Langmuir, 2000, 16, 1025 CrossRef CAS.
- A. Webster and M. Cates, Stabilization of emulsions by trapped species, Langmuir, 1998, 14, 2068 CrossRef CAS.
- D. Thirumalai, G. Reddy and J. E. Straub, Role of water in protein aggregation and amyloid polymorphism, Acc. Chem. Res., 2011, 45, 83 CrossRef PubMed.
- I. V. Baskakov, G. Legname, M. A. Baldwin, S. B. Prusiner and F. E. Cohen, Pathway complexity of prion protein assembly into amyloid, J. Biol. Chem., 2002, 277, 21140 CrossRef CAS PubMed.
- C. M. Bryant and D. J. McClements, Molecular basis of protein functionality with special consideration of cold-set gels derived from heat-denatured whey, Trends Food Sci. Technol., 1998, 9, 143 CrossRef CAS.
- T. Wang, F. Liu, R. Wang, L. Wang, H. Zhang and Z. Chen, Solubilization by freeze-milling of water-insoluble subunits in rice proteins, Food Funct., 2015, 6, 423 CAS.
- C. Pourzand and R. M. Tyrrell, Apoptosis, the role of oxidative stress and the example of solar UV radiation, Photochem. Photobiol., 1999, 70, 380 CrossRef CAS PubMed.
- P. Taylor, Ostwald ripening in emulsions, Adv. Colloid Interface Sci., 1998, 75, 107 CrossRef CAS.
- N. N. Mahmoud, A. M. Carothers, D. Grunberger, R. T. Bilinski, M. R. Churchill, C. Martucci, H. L. Newmark and M. M. Bertagnolli, Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis, Carcinogenesis, 2000, 21, 921 CrossRef CAS PubMed.
- J. Wang, Y. Wang and W. Liang, Delivery of drugs to cell membranes by encapsulation in PEG–PE micelles, J. Controlled Release, 2012, 160, 637 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |