Open Access Article
Open Access ArticleBiodistribution of upconversion/magnetic silica-coated NaGdF4:Yb3+/Er3+ nanoparticles in mouse models†
Uliana Kostiva,
Lenka Rajsiglováb,
Dominika Luptákováb,
Tomáš Pluháčekb,
Luca Vannucci*b,
Vladimír Havlíčekb,
Hana Engstováb,
Daniel Jirákcd,
Miroslav Šlouf a,
Peter Makovickye,
Radislav Sedláčeke and
Daniel Horák
a,
Peter Makovickye,
Radislav Sedláčeke and
Daniel Horák *a
*a
aInstitute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Heyrovského nám. 2, 162 06 Prague 6, Czech Republic
bInstitute of Microbiology, Academy of Sciences of the Czech Republic, Vídeňská 1083, 142 20 Prague 4, Czech Republic
cRadiodiagnostic and Interventional Radiology Department, Institute for Clinical and Experimental Medicine, Vídeňská 1958/9, 140 21 Prague, Czech Republic
dDepartment of Biophysics, Institute of Biophysics and Informatics, First Faculty of Medicine, Charles University, Salmovská 1, 120 00 Prague 2, Czech Republic
eCzech Centre for Phenogenomics, Institute of Molecular Genetics, Academy of Sciences of the Czech Republic, 252 50 Vestec, Czech Republic
First published on 27th September 2017
Abstract
Nanoparticles are constructs that can be used for cellular interventions and targeted drug delivery. They are useful for overcoming the dose-related toxic effects of drugs or diagnostic preparations by predominant or selective accumulation in the pathologic tissues. Gadolinium(III) compounds are largely used as contrast agents in magnetic resonance imaging (MRI) but may have toxic effects, especially in nephropathic patients, due to the dose required for use in MRI. Here, we describe the preparation of new multifunctional NaGdF4:Yb3+/Er3+ nanoparticles, their characteristic properties, and some preliminary data about their effect on cell viability and tissue localization. Hexagonal-phase NaGdF4 nanocrystals that were doped with optically active Yb3+ and Er3+ ions, were synthesized by coprecipitation of lanthanide chlorides in octadec-1-ene at high temperature, stabilized by oleic acid, and subsequently coated with a thin silica layer. The morphology, elemental composition, crystalline structure, and SiO2 coating of the prepared NaGdF4:Yb3+/Er3+@SiO2 nanoparticles were characterized in detail by transmission electron microscopy (TEM) combined with energy-dispersive spectroscopy (TEM/EDX) and selected area electron diffraction (TEM/SAED) and attenuated total reflection Fourier transform infrared (ATR FTIR) spectroscopy. The upconversion and paramagnetic properties of the particles were measured using confocal microscopy and MRI, respectively. The biocompatibility of the NaGdF4:Yb3+/Er3+@SiO2 nanoparticles was tested in vitro using mouse 3T3 fibroblasts and B16F10 melanoma cells. Particle localization was evaluated ex vivo in tumor, liver, and brain tissues of B16F10 melanoma bearing mice after intravenous administration. The NaGdF4:Yb3+/Er3+@SiO2 particles proved to be non-toxic at moderate concentrations. Particle localization within the organs was demonstrated by analysis of the tissues using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and showed vascular localization.
1. Introduction
Lanthanide-based nanocrystals have gained considerable attention for various biological applications due to their unique luminescent and magnetic properties, which can be easily controlled by varying the lanthanide ion dopants.1–3 In particular, upconversion/magnetic NaGdF4:Yb3+/Er3+ nanoparticles have great potential as promising multimodal probes for in vitro and in vivo bioimaging,4 targeted drug delivery,5 and image-guided surgery.6 Due to the presence of optically active Yb3+/Er3+ ion pair in the Gd3+-containing host lattice, the particles show not only a strong near-infrared (NIR) to visible upconversion luminescence but also a short longitudinal relaxation time T1 that allows simultaneous optical and magnetic resonance (MR) images of healthy and diseased tissues.6–8 Moreover, the maximum light absorption of the NaGdF4:Yb3+/Er3+ nanoparticles falls within the “optical window” of the biological tissues. The advantages of the particles include a large anti-Stokes shift of ∼500 nm, absence of photobleaching and photoblinking, weak autofluorescence, deep light penetration, and reduced photodamage to living organisms.9,10 Integration of the magnetic and upconversion features in one particle provides more detailed and accurate non-invasive imaging information than using each method alone, facilitating diagnoses and follow-up therapeutic responses over long time periods.Biocompatibility, dispersibility in water, and uniform particle size are prerequisite conditions to meet the demands of biomedical applications. Currently, NaGdF4:Yb3+/Er3+ nanoparticles are generally synthetized by high-temperature coprecipitation of lanthanide chlorides in an organic solvent, e.g., octadec-1-ene, and stabilized by oleic acid.11 However, these particles are hydrophobic and lack functional groups, which presents an obstacle for further surface conjugation of targeting ligands and therapeutic agents. Surface chemistry thus remains a key issue to minimize nonspecific uptake by the reticuloendothelial system, prolong blood circulation time, and improve diagnostic efficiency. Strategies to design water-dispersible particles include ligand exchange12 or oxidation,13 and encapsulation with SiO2 or polymers.14,15
Gd is a paramagnetic lanthanide that was used more than 30 years ago in the formulation of contrast agents to enhance contrast in magnetic resonance imaging (MRI) due to its large magnetic moment and short relaxation time.16 Once in the circulation after intravenous administration, it demarks the vascularization of an organ and is widely used for magnetic resonance angiography. However, compromised kidney function is a contra-indication to its administration because Gd filtration is inefficient. Subsequent systemic toxicity is possible, even leading to fatal nephrogenic systemic fibrosis.17
Studies during the last twenty years have demonstrated the importance of the tumor microenvironment and of therapeutic interventions specifically directed toward cancer cells, infiltrating and surrounding immune cells, stroma cells, stromal structures, vessels, nerves and soluble products.18 A targeted therapy is not only critical to achieve highly efficient anticancer effects but also necessary to reduce unwanted side reactions in the rest of the organism. At present, the development of nanotherapeutics allows a selective targeting of the cancer cells and the tumor microenvironment. The availability of diagnostic tracers, e.g., gold, iron oxide, gadolinium,19,20 or human ferritin loaded with maghemite21,22 as carriers of therapeutic agents has opened the field of theranostics, in which the dual functions of diagnosis and therapy are combined in a single nanoconstruct.23 Moreover, a selective targeting of tumors can be obtained through the enhanced permeability and retention effect (EPR) due to endothelial leakages in the tumor neovascularization24 and the reduced lymphatic circulation in the tumor microenvironment with possibility to reach concentrations 5–10 times higher than in normal tissue within 1–2 days.25 Imaging of tumor vascularization and identification of the sites of EPR can help in deciding the type of tumor targeting, as well as the opportunity for anti-angiogenic therapy.
In our study, we synthesized NaGdF4:Yb3+/Er3+@SiO2 nanoparticles and evaluated them in the context of achieving a new system that, while reducing the general toxic effects by lowering the diagnostic dose, may maintain an efficient vascular localization. This might lead to targeted treatment of cancer or even other pathologies with aggressive neo-vascularization, such as neo-vascular eye diseases.26 For a preliminary evaluation, three main tissues were analyzed (tumor, liver, and brain) as examples of different vascular conditions: neo-angiogenesis, regular and abundant vascularization associated with reticular-endothelial cells, and vascularization with restricted permeability due to the brain–blood barrier. Biocompatibility, i.e., cell viability, was also tested in vitro on tumor and non-tumor mouse cell lines and on immune cells.
2. Experimental
2.1 Chemicals
Lanthanide chlorides, such as anhydrous gadolinium(III), ytterbium(III), and erbium(III) chloride (99%), octadec-1-ene (90%), ammonium fluoride, tetramethoxysilane (TMOS), and Igepal CO-520 [polyoxyethylene (5) nonylphenylether], were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium hydroxide was acquired from Lach-Ner (Neratovice, Czech Republic), and oleic acid, methanol, ethanol, and acetone were acquired from Lachema (Brno, Czech Republic). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was purchased from Serva Electrophoresis GmbH (Heidelberg, Germany). Ultrapure Q-water (pH 7; resistivity 18.2 MΩ cm) produced using ultrafiltration on a Milli-Q Gradient A10 system (Millipore; Molsheim, France) was used in all experiments.2.2 Synthesis of NaGdF4:Yb3+/Er3+ nanoparticles
NaGdF4:Yb3+/Er3+ nanoparticles were prepared according to previously described procedure with some modifications.27,28 In a typical synthesis, 1 mmol of lanthanide chloride (0.78 mmol GdCl3, 0.2 mmol YbCl3, and 0.02 mmol ErCl3) was placed in a 100 ml three-neck flask to which octadec-1-ene (15 ml) and oleic acid stabilizer (6 ml) were added. The flask was heated to 160 °C for 30 min to obtain a homogenous solution and cooled to room temperature (RT), after which a methanolic solution (5 ml) containing NH4F (4 mmol) and NaOH (4 mmol) was added dropwise. The resulting mixture was slowly heated to 70 °C until all the methanol evaporated, stirred under a gentle flow of argon, and heated to 300 °C for 90 min. After cooling to RT, the resulting NaGdF4:Yb3+/Er3+ nanoparticles were precipitated in acetone (30 ml), separated using a Sorvall Legend X1 centrifuge (ThermoFisher; Osterode, Germany), washed three times with ethanol, separated by centrifugation, and stored in hexane.2.3 Synthesis of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles
The surfaces of the NaGdF4:Yb3+/Er3+ nanoparticles were coated with silica using a microemulsion technique.29 NaGdF4:Yb3+/Er3+ nanoparticles (30 mg) were dispersed in hexane (10 ml) and Igepal CO-520 (0.5 ml) and 25% aqueous ammonia (80 μl) were added. After sonication for 20 min, a stable emulsion was formed; TMOS (20 μl) was added and the mixture was stirred (600 rpm) at RT for 2 days. The resulting NaGdF4:Yb3+/Er3+@SiO2 particles were precipitated with acetone (5 ml), washed three times with water/ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), and stored in water.
1 v/v), and stored in water.
2.4 Viability test of cells incubated with NaGdF4:Yb3+/Er3+@SiO2 nanoparticles
Murine B16F10 melanoma and fetal 3T3 fibroblast cell lines (ATCC; Manassas, VA, USA) were used to evaluate the possible cytotoxic effects and biocompatibility of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles using the MTT cell viability assay. The 3T3 cells were used not only as a common model of non-tumor cells, but also because fibroblasts are important component of the tumor microenvironment.30 The cells were cultured at 37 °C under a 5% CO2 atmosphere in Dulbecco's modified Eagle medium (DMEM; Institute of Molecular Genetics; Prague, Czech Republic) supplemented with 2 mM L-glutamine, 0.05 mg gentamycin, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol (Sigma-Aldrich) and 10% heat-inactivated fetal calf serum (FCS; Gibco; Grand Island, NY, USA).Male Balb/c mice (Institute of Physiology, Prague, Czech Republic) were used. After adaptation to the conditions of local animal facility (Institute of Microbiology, Prague, Czech Republic), the spleen from a Balb/c mouse was harvested in sterile conditions into HMEM-d culture medium (Institute of Molecular Genetics). After mashing through a nylon net, half of the splenocytes was separated on the Ficoll-PaqueTM 1085 (GE HealthCare; Stockholm, Sweden) using gradient density centrifugation (500 g/30 min at 18 °C) to isolate the mononuclear cells; the other half was used unseparated to include myeloid cells. Both types of splenocyte suspensions were then transferred to DMEM for culture.
The original colloid (2 mg of NaGdF4:Yb3+/Er3+@SiO2/ml) was diluted 50-fold in a phosphate-buffered solution (PBS) to avoid aggregation of the particles and sterilized using UV light. Further dilutions were obtained from this stock dispersion to evaluate the effect of the NaGdF4:Yb3+/Er3+@SiO2 particles at the different concentrations. Cells were seeded on 96-well plates in 150 μl of media at concentrations ranging from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 30
000 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well. In the next step, the cells were incubated in the presence of 4.4, 22 or 44 ng of particles per well (equivalent to 0.03, 0.15 and 0.3 mg ml−1), or 2.2, 22 or 45 μg of nanoparticles per well (corresponding to 0.015, 0.15 and 0.35 mg ml−1). The cell viability was compared to control group in the absence of the nanoparticles. The tests were performed in triplicate, and 12 wells with medium alone were used as a blank. The cells were cultured in the incubator at 37 °C for 48 and/or 72 h under a 5% CO2 atmosphere. The cell viability was evaluated by adding MTT (10 μl; 5 mg ml−1) to each well containing the particles or control and to 6 wells containing only the medium. The plates were kept in the dark and incubated for 1 h longer. After formation of the formazan crystals, the color of which turned to violet, the medium (80 μl) was carefully removed using a multichannel pipette, and dimethylsulfoxide (130 μl) was added to each well to dissolve the formazan crystals. The optical densities were obtained using a plate-reading spectrophotometer at 530 nm. The mean value of the blank (medium and MTT) was subtracted from each sample; the means and standard deviations for each group were determined using a Student's t-test; statistically significant results at p ≥ 0.05 are shown in the graphs.
000 cells per well. In the next step, the cells were incubated in the presence of 4.4, 22 or 44 ng of particles per well (equivalent to 0.03, 0.15 and 0.3 mg ml−1), or 2.2, 22 or 45 μg of nanoparticles per well (corresponding to 0.015, 0.15 and 0.35 mg ml−1). The cell viability was compared to control group in the absence of the nanoparticles. The tests were performed in triplicate, and 12 wells with medium alone were used as a blank. The cells were cultured in the incubator at 37 °C for 48 and/or 72 h under a 5% CO2 atmosphere. The cell viability was evaluated by adding MTT (10 μl; 5 mg ml−1) to each well containing the particles or control and to 6 wells containing only the medium. The plates were kept in the dark and incubated for 1 h longer. After formation of the formazan crystals, the color of which turned to violet, the medium (80 μl) was carefully removed using a multichannel pipette, and dimethylsulfoxide (130 μl) was added to each well to dissolve the formazan crystals. The optical densities were obtained using a plate-reading spectrophotometer at 530 nm. The mean value of the blank (medium and MTT) was subtracted from each sample; the means and standard deviations for each group were determined using a Student's t-test; statistically significant results at p ≥ 0.05 are shown in the graphs.
2.5 Animal model and localization of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles in mouse organs
B16F10 melanoma cells (1 × 106) were subcutaneously inoculated into the lower back of syngeneic C57BL/6 mice (AnLab; Prague, Czech Republic), and the tumours were allowed to develop until they reached 10 mm in diameter. All experiments were approved by the Animal Care and Use Committee at the Institute of Microbiology (ID 64/2015). The animals were sacrificed 24 h after 3 intravenous injections (one per day every 24 h) of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles (2 mg ml−1 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 dilution in sterile PBS). The volume of each administered dose was 100 μl.
50 dilution in sterile PBS). The volume of each administered dose was 100 μl.
Both tumor (melanoma) and healthy tissues (liver and brain) were snap-frozen immediately after harvesting and preserved at −80 °C. The Gd localization and amount in the samples was determined by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). The deeply frozen tissues were allowed to warm up to −13 °C prior to the sectioning with a Leica cryomicrotome CM1950 (Leica Biosystems; Wetzlar, Germany). The tissues were cut to 30 μm-thick slices for the quantitative LA-ICP-MS study of Gd and Fe distributions in the tissues, which were conducted using an Analyte G2 laser ablation unit (Photon Machines; Bozeman, MT, USA) equipped with an ArF excimer nanosecond laser (193 nm) coupled to a 7700× ORS-ICP-MS spectrometer (Agilent Technologies; Tokyo, Japan). An octupole reaction system working in helium mode was used to eliminate spectral interferences observed mainly on 56Fe. The raw data (csv files) were transformed to the elemental distribution images using ImageLab multisensor imaging software (v. 2.18, Epina; Pressbaum, Austria). The elemental quantitative data were achieved by the analysis of a set of dried droplet calibration standards with the increasing concentration of both elements: 0, 5, 50, 100, 250, 500, and 1000 μg g−1 for Fe and 0, 0.1, 1, 10, 100, 500, and 1000 μg g−1 for Gd, respectively. The linear relationships were established with the correlation coefficients >0.993 for all calibration curves (Fig. S1 in ESI†).
The resulting images showing the NaGdF4:Yb3+/Er3+@SiO2 particle localization and accumulation in the tissue were compared with classical histology after haematoxylin and eosin staining performed on sequential sections. The Gd accumulations were expressed as a colorimetric gradient scale corresponding to the μg of accumulated Gd/g.
The tissue samples used in this analysis were evaluated to demonstrate the vascular net distribution by immunohistochemistry detection of endothelia. The samples were fixed in 4% formalin solution, stored at RT for 24 h, placed in a Leica ASP6025 automated tissue processor (Wetzlar, Germany), and embedded in paraffin blocks using a Leica EG 1150H paraffin embedding station. Slices (2–3 μm thick) were cut from each sample using a Leica RM2255 microtome and mounted on special glass slides. The first slices were stained with haematoxylin-eosin (DiaPath; Martinengo, Italy). The second set, for detection of the endothelial cells by immunohistochemistry methods, was performed using the anti-mouse CD31 (PECAM-1) antibody (Zytomed Systems; Berlin, Germany). 3,3′-Diaminobenzidine chromogen (Dako/Agilent; Glostrup, Denmark) was used for visualization of the detected vessels. The prepared slides were evaluated as light-microscopic images using a Carl Zeiss Axio Scope A1 (Oberkochen, Germany) and a Zeiss Axio Scan.Z1 slide scanner.
2.6 Characterization of the nanoparticles
| Average diameter = 1/2 × (MaxFeret + MinFeret), | (1) |
| Ellipticity = MaxFeret/MinFeret, | (2) |
| Dn = ∑niDi/∑ni, | (3) |
| Dw = ∑niDi4/∑niDi3, | (4) |
The MR relaxometry was performed for different concentrations of the particles at magnetic field B0 = 0.5 T and 20 °C.
3. Results and discussion
3.1 Morphology, structure, phase, and composition of NaGdF4:Yb3+/Er3+ and NaGdF4:Yb3+/Er3+@SiO2 nanoparticles
Upconversion/magnetic nanoparticles composed of NaGdF4 host lattice doped with Yb3+ and Er3+ ions and stabilized with oleic acid were obtained by the high-temperature coprecipitation of lanthanide chlorides. The morphology and particle size were evaluated using the TEM microscopy (Fig. 1a). The particles exhibited an ellipsoidal shape with a mean size Dn = 27 nm and a reasonably narrow particle size distribution (PDI = 1.20). Size distribution histograms of all synthesized particles were presented in ESI (Fig. S2†). The hydrodynamic diameter of the NaGdF4:Yb3+/Er3+ nanoparticles in hexane was Dh = 35 nm with polydispersity PI = 0.28, which confirmed the relatively narrow size distribution. The difference between Dn and Dh of the NaGdF4:Yb3+/Er3+ particles can be explained by the presence of the adsorbed steric stabilizer (oleic acid) on the nanoparticle surface, which is not visible on the TEM micrographs of the particles in the dry state due to low contrast of oleic acid compared to the lanthanide particles. | ||
| Fig. 1 TEM micrographs of (a) starting NaGdF4:Yb3+/Er3+ and (b, c) NaGdF4:Yb3+/Er3+@SiO2 nanoparticles; (b) 20 μl and (c) 40 μl TMOS in the feed. | ||
Freshly synthesized NaGdF4:Yb3+/Er3+ particles are hydrophobic in nature; hence they are dispersible only in organic solvents (e.g., hexane), but not in water, which is required for all biological experiments. To render the particles dispersible in aqueous media, their surface was modified with a silica shell using the reverse microemulsion technique.28 It is worth mentioning that the nanoparticle surface must be completely covered by a homogeneous silica shell without the formation of any additional pure silica particles without a core. In this report, the shell thickness of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles was controlled by varying TMOS amount added to the mixture under otherwise constant reaction conditions, including the concentrations of the nanoparticles, NH4OH, and Igepal CO-520. When 20 μl of TMOS was added to the reaction mixture, the nanoparticles reached 36 nm in size (PDI = 1.18), i.e., they were covered by a 5 nm-thick silica shell (Fig. 1b). In contrast, the addition of a greater quantity of TMOS (40 μl) increased the particle size and shell thickness to 41 and 7 nm, respectively. However, under the latter conditions, additional pure silica particles ∼10 nm in size were formed (Fig. 1c). Therefore, further experiments were performed using the optimal amount, 20 μl of the TMOS precursor to achieve complete coverage of the nanoparticle surface with a homogeneous silica shell while avoiding the production of neat silica. The DLS measurements (Dh, PI, and ζ-potential) in water confirmed successful modification of the NaGdF4:Yb3+/Er3+ particle surface with silica. The Dh of the NaGdF4:Yb3+/Er3+@SiO2 particles was 65 nm (PI = 0.3), and the particles exhibited a good colloidal stability due to a total negative surface charge (−30 mV) that originated from the silanol groups.34
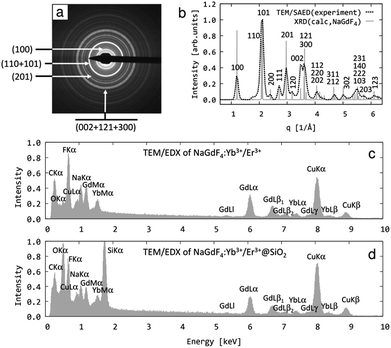
The crystal structure and elemental composition of the prepared nanoparticles were verified by TEM microscopy using selected area electron diffraction (TEM/SAED) and energy-dispersive analysis of X-rays (TEM/EDX; Fig. 2). Regardless of the SiO2 shell, the SAED diffractograms of the nanoparticles were identical; only one representative diffraction pattern of uncoated NaGdF4:Yb3+/Er3+ nanocrystals is therefore shown on Fig. 2a. A comparison of the experimental SAED with theoretically calculated X-ray diffractograms (XRD) of known crystal structures from crystallographic databases33 showed very good agreement between the experiment and the hexagonal phase of NaGdF4 (Fig. 2b). Consequently, the most intense diffraction rings could be described (Fig. 2a) and the diffraction positions and intensities in the recalculated 1D-diffractogram were analyzed (Fig. 2b). The diffraction positions of SAED and XRD showed a perfect fit, which unambiguously confirmed that the prepared nanoparticles were the hexagonal phase of NaGdF4. The diffraction intensities of the SAED and XRD exhibited certain differences that could be attributed to the quite complex preferred orientation (texture) of the nanocrystals lying on the flat, electron-transparent carbon film. The strongest SAED diffraction (hkl) was (101), and two additional SAED diffractions, (002) and (111), were remarkably stronger than the corresponding XRD diffractions (Fig. 2b). The first pair of strong SAED diffractions, (101) and (002), defined the first zone of strongly diffracting lattice planes that was described by the zone axis [uvw] = [0,1,0] and corresponded to the first preferred orientation of nanocrystals on the carbon film. The second pair of strong SAED diffractions, (101) and (111), defined the second zone of strongly diffracting lattice planes that was described by the zone axis [uvw] = [1,0,−1] and corresponded to the second preferred orientation of the nanocrystals on the carbon film (calculation of zone axes from the strong diffractions was described elsewhere35). As a result, the strongest experimentally observed diffractions associated with the above two zone axes should obey the Weiss Zone Law (WZL): hu + kv + lw = 0, where (h, k, l) are the diffraction indices of the planes, and [u, v, w] are the indices of the zone axis.35 For the first zone axis [uvw] = [0,1,0], the WZL takes the form k = 0, and consequently, the strongest SAED diffractions should have indices of the type (h0l). For the second zone axis [uvw] = [1,0,−1], the WZL takes the form h − l = 0, i.e., h = l, which means that the strongest SAED diffractions should have indices of the type (hkh), i.e., the first and the last diffraction indices are equal. Indeed, the four strongest SAED diffraction rings (Fig. 2a) were formed by a combination of (h0l) and/or (hkh) diffractions (with just one exception of strong low-angle diffraction (110)), which proved that the above analysis of preferred orientation of nanocrystals deposited on the carbon film was correct. Moreover, the stronger higher-angle diffractions were usually (h0l) and/or (hkh) (Fig. 2b).
Embedding of the NaGdF4:Yb3+/Er3+ nanoparticles in the SiO2 shell was verified using TEM/EDX (Fig. 2c and d). The EDX spectrum of uncoated nanoparticles (Fig. 2c) showed only the peaks from the standard TEM support (carbon coated copper grid; peaks of C and Cu) and the peaks of the prepared particles (Na, Gd, and F, including the substituting Yb but lacking any of the substituting Er ions, whose concentration was below the detection limit). The very weak oxygen peak probably resulted from the slight oxidation of the carbon film. The EDX spectrum of the NaGdF4:Yb3+/Er3+@SiO2 nanoparticles exhibited the same peaks as described above plus strong peaks of the enveloping silica (Si and O; Fig. 2d).
Modification of NaGdF4:Yb3+/Er3+ nanoparticles with the silica shell was also confirmed by ATR FTIR spectroscopy (Fig. 3). The peak at 1560 cm−1 corresponding to the COO− band and peaks at 2850 and 2920 cm−1 belonging to the symmetric and asymmetric CH2 stretching vibrations, respectively, confirmed the presence of oleic acid on the nanoparticle surface. After the addition of the silica shell to the NaGdF4:Yb3+/Er3+ nanoparticles, the latter bands disappeared and characteristic SiO2 peaks were recorded in the spectrum of the NaGdF4:Yb3+/Er3+@SiO2 particles. The symmetric and asymmetric vibrations of Si–O–Si and Si–OH appeared at 795 and 950 cm−1, respectively. The peak at 1080 cm−1 was attributed to the Si–O–Si stretching vibration, and the intense broad peaks at 1630 and ∼3420 cm−1 in the spectrum of NaGdF4:Yb3+/Er3+@SiO2 were induced by the bending and stretching vibrations of the OH groups of adsorbed water molecules, respectively.
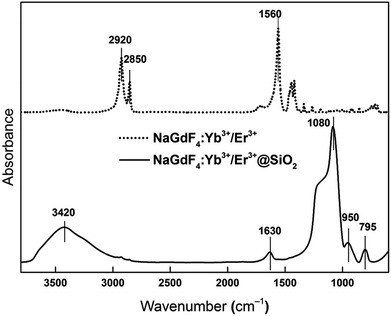 | ||
| Fig. 3 ATR FTIR spectra of NaGdF4:Yb3+/Er3+ (full line) and NaGdF4:Yb3+/Er3+@SiO2 nanoparticles (dashed line). | ||
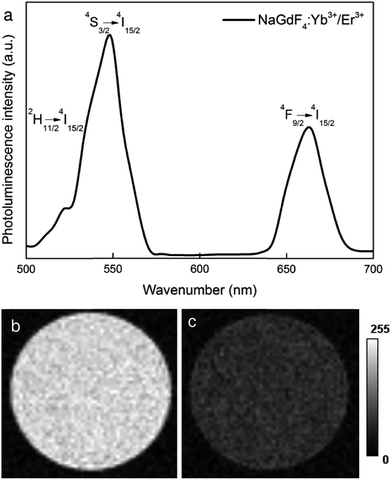
NaGdF4 nanoparticles that were doped with the optically active Yb3+/Er3+ ion pair exhibited a characteristic photoluminescence (Fig. 4a) that emitted high-energy visible photons after being excited by two low-energy NIR photons via the energy transfer upconversion mechanism. Under NIR irradiation at 970 nm, the NaGdF4:Yb3+/Er3+ nanoparticles emitted intense green (520 and 550 nm) and red light (660 nm), which can be ascribed to 2H11/2 → 4I15/2, 4S3/2 → 4I15/2 and 4F9/2 → 4I15/2 radiative transitions of Er3+ ions, respectively, which originated from energy transfer between the Yb3+ and Er3+ ions. T1-weighted MR images of NaGdF4:Yb3+/Er3+ particles showed a noticeable higher MR signal (3.5×) compared to that obtained from water phantoms (Fig. 4b). The SNR in the phantom containing NaGdF4:Yb3+/Er3+ nanoparticles (0.5 mg ml−1) was 22.5 (the control water phantom had an SNR = 6.4). This result suggested suitable paramagnetic properties that would allow use of the NaGdF4:Yb3+/Er3+nanoparticles as a contrast agent for magnetic resonance imaging. This was supported by the relaxometry measurements, where significant shortening of the relaxation time was observed even at low particle concentrations (Table S1†).
3.2 Viability of cells incubated with NaGdF4:Yb3+/Er3+@SiO2 nanoparticles assessed using the MTT assay
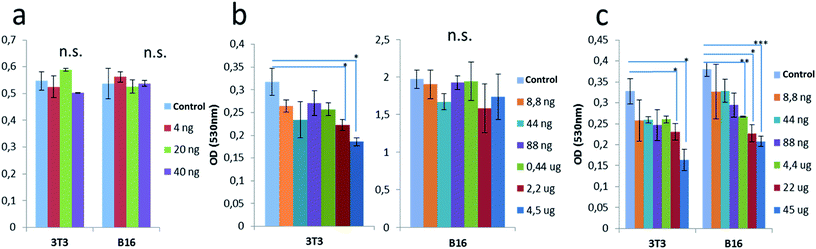
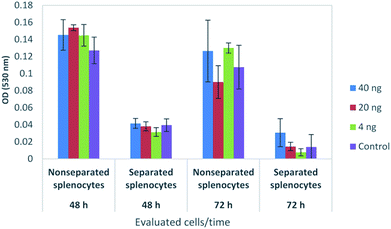
The viability of tumor and non-tumor mouse cell lines (B16F10 melanoma cells and 3T3 fibroblasts, respectively) was repeatedly investigated in vitro using MTT assay after 48 h of incubation with 4, 20, and 40 ng of the NaGdF4:Yb3+/Er3+@SiO2 particles per well containing 150 μl of cultivation medium (corresponding to 27, 133, and 267 ng ml−1, respectively; Fig. 5a). None of these particle concentrations induced significant cytotoxicity. In further experiments, the 3T3 and B16F10 cells were incubated with progressively higher concentrations of the nanoparticles for 48 h (Fig. 5b). At concentrations ≤4.5 μg per well (corresponding to 30 μg ml−1), the colloid did not cause any toxicity in the tumor cells, whereas in the range of 8.8 ng to 0.44 μg per well (corresponding to 0.06–3 μg ml−1) the non-tumor cells (3T3) showed a slight reduction of viability and a progressive cytotoxic effect at 2.2 and 4.5 μg of particles per well (corresponding to 15 and 30 μg ml−1). The cytotoxic effect was evident and progressive with further increases in the particle concentrations in μg scale, up to 45 μg per well corresponding to 0.35 mg ml−1; (Fig. 5c). In this case, both kinds of cells presented cytotoxic effect when the particle concentration was >4.4 μg per well (corresponding to 0.03 mg ml−1). Interestingly, the toxic effect was more relevant on tumor cells.Additional experiments were performed to evaluate the effects of various NaGdF4:Yb3+/Er3+@SiO2 nanoparticle concentrations on the viability of immune cells after 48 and 72 h of cultivation. Immune cells were obtained from the spleens of healthy C57BL/6 mice after spleen homogenization. The complete suspension consisting of lymphocytes and myeloid cells was compared with a purified lymphocyte suspension obtained by density gradient separation with Ficoll-Paque™. This was performed because lymphocytes co-cultivated with myeloid cells are more resistant than those after separation. Therefore, the separated lymphocyte suspension was expected to be more sensitive to possible cytotoxic agents. The incubation with NaGdF4:Yb3+/Er3+@SiO2 nanoparticles at concentrations 4–40 ng per well (27–267 ng ml−1) did not produce a significant change in the cell viability in either preparations even after 72 h of culture. As expected, the viability of the isolated lymphocytes was lower than that of the non-separated cells, but in neither case did the doses of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles induce cytotoxicity (Fig. 6).
3.3 Localization of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles in mouse organs
The distribution of the NaGdF4:Yb3+/Er3+@SiO2 nanoparticles in the tumor and body tissues was evaluated using LA-ICP-MS, which is a powerful analytical technique for highly sensitive direct examination of the content of elements, in the present case Gd, in biological tissues. The Fe present in the hemoglobin was evaluated for comparison. The experiments were performed in the tumor tissue (melanoma) and healthy tissue (brain and liver) in a mouse model after intravenous administration of a standard concentration of the NaGdF4:Yb3+/Er3+@SiO2 nanoparticles. First, the tumor localization was evaluated. The NaGdF4:Yb3+/Er3+@SiO2 nanoparticles (100 μl of a colloid; 2 mg of particles per ml diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
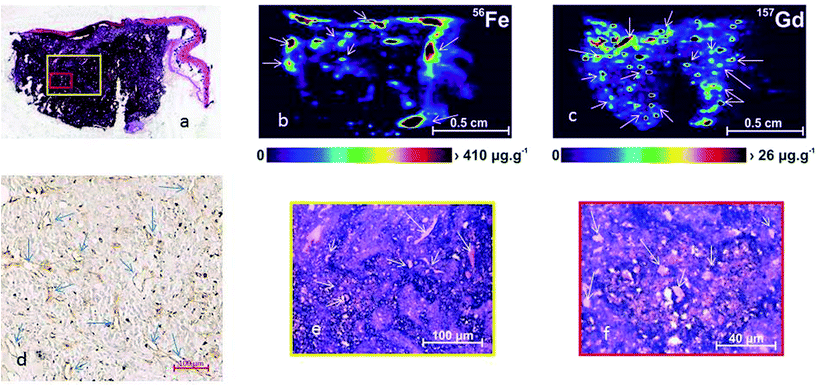
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 in PBS) were injected into animals harboring murine B16F10 melanoma cells. As described in the Experimental section, sequential sections of frozen samples were prepared and analyzed to permit comparison of the histological picture (Fig. 7a, d and e) with the LA-ICP-MS analysis. The LA-ICP-MS images showed that the Fe was predominantly associated with large blood vessels due to high amounts of Fe present in hemoglobin (Fig. 7b) and a high background. In contrast, the Gd accumulation better defined the vascular component of the tumor tissue. The Gd signal was apparent at a much lower particle concentration (26 μg g−1) than the Fe signal (410 μg g−1; Fig. 7b and c). Many blood vessels with accumulated NaGdF4:Yb3+/Er3+@SiO2 particles can be seen in Fig. 7d. These nanoparticles have thus the substantial advantages of having a very low background and highly sensitive demonstration of blood vasculature as long as 48 h after the injection.
50 in PBS) were injected into animals harboring murine B16F10 melanoma cells. As described in the Experimental section, sequential sections of frozen samples were prepared and analyzed to permit comparison of the histological picture (Fig. 7a, d and e) with the LA-ICP-MS analysis. The LA-ICP-MS images showed that the Fe was predominantly associated with large blood vessels due to high amounts of Fe present in hemoglobin (Fig. 7b) and a high background. In contrast, the Gd accumulation better defined the vascular component of the tumor tissue. The Gd signal was apparent at a much lower particle concentration (26 μg g−1) than the Fe signal (410 μg g−1; Fig. 7b and c). Many blood vessels with accumulated NaGdF4:Yb3+/Er3+@SiO2 particles can be seen in Fig. 7d. These nanoparticles have thus the substantial advantages of having a very low background and highly sensitive demonstration of blood vasculature as long as 48 h after the injection.
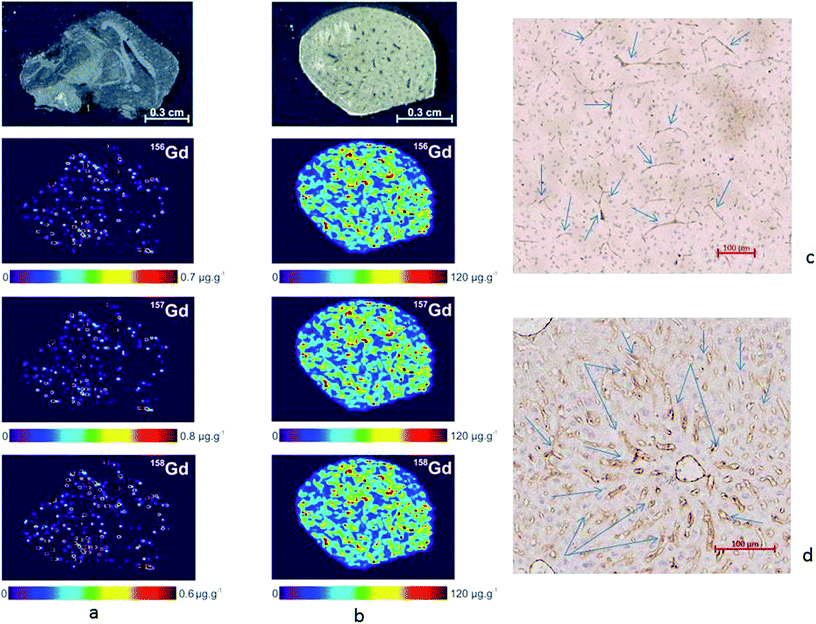
Second, the brain and liver tissues were analyzed, and the NaGdF4:Yb3+/Er3+@SiO2 particles were distinctly detected in the vasculature net (Fig. 8). In the brain, the NaGdF4:Yb3+/Er3+@SiO2 particles were present only in the blood vessels (Fig. 8a). In contrast, very high accumulation of the NaGdF4:Yb3+/Er3+@SiO2 nanoparticles was observed in the vascular and perivascular spaces of the liver, as indicated by the presence of some background (Fig. 8b). This was expected because it is known that the Kupffer cells can easily take up circulating particles.36 Chronic accumulation of the particles may represent a problem, especially within the kidneys, on the basis that Gd contrast agents, which are widely used in MRI studies, have been often associated with a risk of systemic toxicity and fibrosis in patients with reduced renal function.37 However, the nanoparticle formulation may take advantage of the liver detoxification ability, implying that the particles are eventually removed and engulfed by macrophages.
4. Conclusions
Rare-earth upconversion nanocrystals with a thin silica coating layer, NaGdF4:Yb3+/Er3+@SiO2 particles, have been successfully prepared. Their morphology, composition, structure, and coating were verified by detailed TEM analysis, including energy dispersive spectroscopy (EDX) and electron diffraction (SAED). The comparison of experimental SAED diffraction patterns with theoretically calculated XRD patterns proved unambiguously that the prepared nanocrystals had a crystalline structure of hexagonal NaGdF4. The upconversion property in combination with an immobilized photosensitizer, namely, the emission of red/green light after irradiation with deep-tissue penetrating NIR light, is very important for future applications of the particles in photodynamic tumor therapy. Moreover, Gd renders the particles, when accumulated by efficient EPR effect, with contrast in MRI, which may permit non-invasive detection of NaGdF4:Yb3+/Er3+@SiO2 particle-engulfed cells of pathological sites in the organism.Biological experiments confirmed that the cytotoxicity of NaGdF4:Yb3+/Er3+@SiO2 nanoparticles was dose-related with a threshold above 0.5 μg of particles per well (3 μg ml−1) and clearly present at concentrations of 2.2 μg per well (15 μg ml−1). Lower concentrations did not affect the viability of non-tumor, tumor, or immune cells. The LA-ICP-MS method proved to be very effective for the direct determination of the NaGdF4:Yb3+/Er3+@SiO2 particle distribution within the biological tissues. This technique enables visualization of even very small quantities of the particles (pico- to femtograms), thus facilitating highly sensitive (parts per billion) monitoring of the nanoparticle distribution inside a tissue. In this manner, the distribution of blood vessels containing NaGdF4:Yb3+/Er3+@SiO2 nanoparticles within the tumor was easily visualized. Even a very small quantity of accumulated particles was detectable, which is beneficial in terms of injection of very low particle doses. This might be applicable even in biopsies of investigated organs or tumor tissues, allowing extensive evaluation of the nanoparticle distribution in the organism to improve planning of highly precise theranostic interventions. A huge difference in the nanoparticle accumulation was found between brain and liver according to their vascular characteristics. The LA-ICP-MSI method may also be suitable for studies of the bio-distribution of the nanoparticles. In fact, the nanoparticles are actively investigated not only for therapeutic purposes38 but also in terms of undesirable particle penetration into the organs, such as the lung and brain, due to the environmental pollution, as well as particle accumulation/elimination by the reticulo-endothelial system.39,40
Our investigation provides the first useful information on the biocompatibility of the newly synthetized NaGdF4:Yb3+/Er3+@SiO2 nanoparticles and their vascular localization in the evaluated organs even 24 h after the administration. This is important for prospective applications of Gd compounds not only for MRI, but also for efficient targeting of pathological neoangiogenesis (cancer, diabetic retinopathy, etc.) and microenvironmental components of cancers. Moreover, NaGdF4:Yb3+/Er3+@SiO2 particles can be proposed for bi-modal upconversion luminescence/MR imaging and photodynamic therapy41 and, after functionalization or coupling with other molecules, as radiosensitizers for enhanced radiotherapy,42 or neutron capture therapy,43 as well as therapeutic carriers.
Conflicts of interest
There are no conflicts to declare.Ethical statement
All procedures were conducted in strict accordance with the European Convention for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee at the Institute of Microbiology, AS CR, approval ID: 64/2015.Acknowledgements
The financial support of the Czech Science Foundation (15-01897S) is gratefully acknowledged. The biological experiments were supported by RVO 61388971 Institutional Grant and MŠMT COST CZ LD 15135, in the frame of TD1301 MiMed COST Action. Support for the electron microscopy experiments by the Technology Agency of the Czech Republic (TE01020118) is also acknowledged. The MR experiment was supported by the Ministry of Health of the Czech Republic (Institute for Clinical and Experimental Medicine, project IN 00023001).References
- B. Zhou, B. Shi, D. Jin and X. Liu, Nat. Nanotechnol., 2015, 10, 924 CrossRef CAS PubMed.
- Y. Zhang, W. Wei, G. K. Das and T. T. Y. Tan, J. Photochem. Photobiol., C, 2014, 20, 71 CrossRef CAS.
- B. R. Smith and S. S. Gambhir, Chem. Rev., 2017, 117, 901 CrossRef CAS PubMed.
- R. Naccache, P. Chevallier, J. Lagueux, Y. Gossuin, S. Laurent, L. Vander Elst, C. Chilian, J. A. Capobianco and M. A. Fortin, Adv. Healthcare Mater., 2013, 2, 1478 CrossRef CAS PubMed.
- L. Zhou, X. Zheng, Z. Gu, W. Yin, X. Zhang, L. Ruan, Y. Yang, Z. Hu and Y. Zhao, Biomaterials, 2014, 35, 7666 CrossRef CAS PubMed.
- J. Key and J. F. Leary, Int. J. Nanomed., 2014, 9, 711 Search PubMed.
- Y. I. Park, J. H. Kim, K. T. Lee, K. S. Jeon, H. B. Na, J. H. Yu, H. M. Kim, N. Lee, S. H. Choi, S. I. Baik, H. Kim, S. P. Park, B. J. Park, Y. W. Kim, S. H. Lee, S. Y. Yoon, I. C. Song, W. K. Moon, Y. D. Suh and T. Hyeon, Adv. Mater., 2009, 21, 4467 CrossRef CAS.
- C. Liu, Y. Hou and M. Gao, Adv. Mater., 2014, 26, 6922 CrossRef CAS PubMed.
- F. Zhang, Photon Upconversion Nanomaterials, Springer, 2015 Search PubMed.
- F. Wang, Y. Han, C. S. Lim, Y. H. Lu, J. Wang, J. Xu, H. Y. Chen, C. Zhang, M. H. Hong and X. G. Liu, Nature, 2010, 463, 1061 CrossRef CAS PubMed.
- A. Sedlmeiera and H. H. Gorris, Chem. Soc. Rev., 2015, 44, 1526 RSC.
- J. C. Boyer, M. P. Manseau, J. I. Murray and F. van Veggel, Langmuir, 2010, 26, 1157 CrossRef CAS PubMed.
- H. Hu, M. X. Yu, F. Y. Li, Z. G. Chen, X. Gao, L. Q. Xiong and C. H. Huang, Chem. Mater., 2008, 20, 7003 CrossRef CAS.
- H. S. Qian, H. C. Guo, P. C. L. Ho, R. Mahendran and Y. Zhang, Small, 2009, 5, 2285 CrossRef CAS PubMed.
- R. Naccache, F. Vetrone, V. Mahalingam, L. A. Cuccia and J. A. Capobianco, Chem. Mater., 2009, 21, 717 CrossRef CAS.
- L. W. Yang, Y. Y. Zhang, J. J. Li, Y. Li, J. X. Zhong and P. K. Chu, Nanoscale, 2010, 2, 2805 RSC.
- T. A. Rose Jr and J. W. Choi, Am. J. Med., 2015, 128, 943 CrossRef PubMed.
- A. K. Alshememry, S. S. El-Tokhy and L. D. Unsworth, Curr. Pharm. Des., 2017, 23 DOI:10.2174/1381612823666170522100545.
- C. Xu, Y. Wang, C. Zhang, Y. Jia, Y. Luo and X. Gao, Nanoscale, 2017, 9, 4620 RSC.
- A. C. Anselmo and S. Mitragotri, AAPS J., 2015, 17, 1041 CrossRef CAS PubMed.
- L. Vannucci, E. Falvo, M. Fornara, P. Di Micco, O. Benada, J. Krizan, J. Svoboda, K. Hulikova-Capkova, V. Morea, A. Boffi and P. Ceci, Int. J. Nanomed., 2012, 7, 1489 CAS.
- L. Vannucci, E. Falvo, C. M. Failla, M. Carbo, M. Fornara, R. Canese, S. Cecchetti, L. Rajsiglova, D. Stakheev, J. Krizan, A. Boffi, G. Carpinelli, V. Morea and P. Ceci, J. Biomed. Nanotechnol., 2015, 11, 81 CrossRef CAS PubMed.
- J. Elgqvist, Int. J. Mol. Sci., 2017, 18, 1102 CrossRef PubMed.
- H. Hashizume, P. Baluk, S. Morikawa, J. W. McLean, G. Thurston, S. Roberge, R. K. Jain and D. M. McDonald, Am. J. Pathol., 2000, 156, 1363 CrossRef CAS PubMed.
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18 CrossRef CAS PubMed.
- H. H. Shen, E. C. Chan, J. H. Lee, Y. S. Bee, T. W. Lin, G. J. Dusting and G. S. Liu, Nanomedicine, 2015, 10, 2093 CrossRef CAS PubMed.
- U. Kostiv, O. Janoušková, M. Šlouf, N. Kotov, H. Engstová, K. Smolková, P. Ježek and D. Horák, Nanoscale, 2015, 7, 18096 RSC.
- U. Kostiv, I. Kotelnikov, V. Proks, M. Šlouf, J. Kučka, H. Engstová, P. Ježek and D. Horák, ACS Appl. Mater. Interfaces, 2016, 8, 20422 CAS.
- U. Kostiv, V. Patsula, M. Šlouf, I. Pongrac, S. Škokić, M. Dobrivojević Radmilović, I. Pavičić, I. Vinković Vrček, S. Gajović and D. Horák, RSC Adv., 2017, 7, 8786 RSC.
- L. Miao and L. Huang, Cancer Treat. Res., 2015, 166, 193 CrossRef CAS PubMed.
- J. L. Lábár, Ultramicroscopy, 2005, 103, 237 CrossRef PubMed.
- W. Kraus and G. Nolze, J. Appl. Crystallogr., 1996, 29, 301 CrossRef CAS.
- L. Glasser, J. Chem. Educ., 2016, 93, 542 CrossRef CAS.
- D. Fairhurst and R. W. Lee, Drug Dev. Delivery, 2011, 11, 60 CAS.
- K. W. Andrews, D. J. Dyson and S. R. Keown, Interpretation of Electron Diffraction Patterns, Plenum Press, 1967 Search PubMed.
- E. Sadauskas, H. Wallin, M. Stoltenberg, U. Vogel, P. Doering, A. Larsen and G. Danscher, Part. Fibre Toxicol., 2007, 4, 10 CrossRef PubMed.
- D. J. Todd and J. Kay, Annu. Rev. Med., 2016, 67, 273 CrossRef CAS PubMed.
- P. Muralidharan, M. Malapit, E. Mallory, D. Hayes Jr and H. M. Mansour, Nanomedicine, 2015, 11, 1189 CrossRef CAS PubMed.
- J. Morton, E. Tan and S. K. Suvarna, J. Trace Elem. Med. Biol., 2016, 43, 63 Search PubMed.
- E. Principi, R. Girardello, A. Bruno, I. Manni, E. Gini, A. Pagani, A. Grimaldi, F. Ivaldi, T. Congiu, D. De Stefano, G. Piaggio, M. de Eguileor, D. M. Noonan and A. Albini, Int. J. Nanomed., 2016, 11, 4299 CrossRef CAS PubMed.
- J. Liu, L. Huang, X. Tian, X. Chen, Y. Shao, F. Xie, D. Chen and L. Li, Int. J. Nanomed., 2016, 12, 1 CrossRef PubMed.
- S. Kotb, A. Detappe, F. Lux, F. Appaix, E. L. Barbier, V. L. Tran, M. Plissonneau, H. Gehan, F. Lefranc, C. Rodriguez-Lafrasse, C. Verry, R. Berbeco, O. Tillement and L. Sancey, Theranostics, 2016, 6, 418 CrossRef CAS PubMed.
- N. Protti, S. Geninatti-Crich, D. Alberti, S. Lanzardo, A. Deagostino, A. Toppino, S. Aime, F. Ballarini, S. Bortolussi, P. Bruschi, I. Postuma, S. Altieri and H. Nikjoo, Radiat. Prot. Dosim., 2015, 166, 369 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: T1 relaxometry (Table S1), calibration curves (Fig. S1), and size distribution histograms (Fig. S2). See DOI: 10.1039/c7ra08712h |
| This journal is © The Royal Society of Chemistry 2017 |