DOI:
10.1039/C7RA08887F
(Paper)
RSC Adv., 2017,
7, 47148-47163
Synthesis, characterization and corrosion inhibition potential of two novel Schiff bases on mild steel in acidic medium†
Received
11th August 2017
, Accepted 25th September 2017
First published on 5th October 2017
Abstract
The present study deals with the synthesis of two novel Schiff bases (SBs) namely; 5-((4-chloro-3-nitrophenylimino)methyl)-2-methoxyphenol (SB-1) and 2-(4-hydroxy-3-methoxybenzylidineamino)-4-nitrophenol (SB-2) using a solid–liquid phase equilibrium diagram and their characterization via 1H and 13C NMR, FT-IR and UV-vis spectral analyses. Differential scanning calorimetric (DSC) thermographs were also obtained to further support the synthesis and identify the melting temperatures of SB-1 and SB-2. The XRD technique was employed to study the atomic packing, crystal structure and space group of the grown crystals of SB-1. Results showed that powder X-ray diffraction patterns of both SBs are entirely different than those of their corresponding starting compounds. The inhibition properties of the investigated SBs were evaluated for mild steel corrosion in 1 M HCl using electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP) and computational (DFT) methods. Electrochemical and DFT studies revealed that the tested SBs act as good inhibitors for acidic corrosion of mild steel and their efficiency increases (η%) on increasing their concentrations. Electrochemical results revealed that SB-1 and SB-2 showed maximum inhibition efficiencies of 95.58% and 96.80%, respectively, at a concentration as low as 0.327 mM. Results from a polarization study suggest that the studied SBs act as mixed type corrosion inhibitors with some cathodic predominance. The DFT based parameters such as EHOMO, ELUMO, ΔE, electronegativity (χ), hardness (η), softness (σ) and the fraction of electron transfer (ΔN) were calculated to corroborate the experimentally obtained results. Both experimental and DFT results were in good agreement.
1. Introduction
Mild steel is a widely employed construction material owing to its high mechanical strength and exceptionally low cost.1–3 However, it rapidly undergoes corrosion through chemical and electrochemical reactions with the components of surrounding environments. Corrosion is an extremely damaging phenomenon and adversely affects the global economy of the world. A study of the National Association of Corrosion Engineers (NACE; 2002) revealed that in 1998, the total direct cost of corrosion was US $276 billion, which constitutes about 3.4% of the world's GDP.4 In the United States (U. S.), corrosion resulted in a loss of more than US $2.2 trillion in 2011. According to the 1st Global Corrosion Summit held in New Delhi, India in 2011, India losses around Rs. 2 lakh crores (US $45 billion) every year because of corrosion.5 The most cited cost study data of NACE suggests that recent annual cost of corrosion (worldwide) is about US $2.5 trillion which results nearly 3.4% of the global GDP.6,7 In India and South Africa the total annual cost of corrosion is around US $100-billion and US $ 9.6 billion, respectively.6,7 However, several attempt such as coating, alloying, de-alloying and use of synthetic corrosion inhibitors etc. being made to reduce this cost of corrosion by implementing them the cost of corrosion can be reduced from 15 (US $ 375 billion) to 35% (US $ 875 billion).
Among the available methods of corrosion protection, use of synthetic inhibitors is one of the most popular and effective methods due to their cost effective nature and ease of application.1–3,8–10 These inhibitors adsorb on the metallic surface through their non-bonding and pi-electrons and form protective film which isolates the metals from the corrosive environment and protects from corrosion.8–10 The adsorption of these inhibitors depends upon numerous factors such as nature of metal and electrolyte, electronic structure of the inhibitor, temperature, exposure time etc.11,12 Recently, widespread studies of organic compounds show that these compounds exhibit various attractive linear and non-linear optical properties.13,14 Schiff bases are generally synthesized by condensation reaction between aromatic aldehyde and primary amine (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). They have been extensively used for variety of biological applications in the field of pharmaceuticals.15–18 They have also been used as promising candidates for antimicrobial activity19 such as antibacterial, antifungal, antitumor and anticancer agents.20 They are also being utilized as common and effective intermediate candidates for the synthesis of several organic and coordination compounds.21 Additionally, the Schiff bases are also being utilized as fluorescent probe,13,22–24 non-linear optical (NLO) active,25 catalyst, dyes and inhibitors for metallic corrosion26 etc. Owing to their cost effective nature, high synthetic yield, low toxicity and high chemical purity, Schiff bases (SBs) are ideal candidates to be used for a variety of purposes like agents for antimicrobial and anticorrosive activities. The presence of –CH
N–). They have been extensively used for variety of biological applications in the field of pharmaceuticals.15–18 They have also been used as promising candidates for antimicrobial activity19 such as antibacterial, antifungal, antitumor and anticancer agents.20 They are also being utilized as common and effective intermediate candidates for the synthesis of several organic and coordination compounds.21 Additionally, the Schiff bases are also being utilized as fluorescent probe,13,22–24 non-linear optical (NLO) active,25 catalyst, dyes and inhibitors for metallic corrosion26 etc. Owing to their cost effective nature, high synthetic yield, low toxicity and high chemical purity, Schiff bases (SBs) are ideal candidates to be used for a variety of purposes like agents for antimicrobial and anticorrosive activities. The presence of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group in their molecular structures makes them suitable candidates for several applications in medicinal, agricultural, pharmaceutical and material science. Previously, several Schiff bases have been prepared by condensation reactions of carbonyl and amine compounds. Literature survey reveals that several Schiff's bases have been employed as effective corrosion inhibitors for metals and alloys using experimental and computational methods in different aggressive media.27–29 Their high effectiveness and adsorption tendency on the metallic surfaces mainly attributed to the presence of –CH
N– group in their molecular structures makes them suitable candidates for several applications in medicinal, agricultural, pharmaceutical and material science. Previously, several Schiff bases have been prepared by condensation reactions of carbonyl and amine compounds. Literature survey reveals that several Schiff's bases have been employed as effective corrosion inhibitors for metals and alloys using experimental and computational methods in different aggressive media.27–29 Their high effectiveness and adsorption tendency on the metallic surfaces mainly attributed to the presence of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (imine) group through which they can adsorb effectively i.e. –CH
N– (imine) group through which they can adsorb effectively i.e. –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group acts as an adsorption canter during meta-inhibitor interactions.27–29 A comparison of inhibitive strengths of 2-amino-6-(4-methoxybenzelideneamino)hexanoic acid (SB-2-M) and 2-amino-6-((4-dimethylamino)benzylideneamino)hexanoic acid (SB-4-D) having p-OCH3 and p-N(CH3)2, respectively showed that SB-4-D is a better inhibitor than SB-2-M.27 The higher inhibition efficiency of SB-4-D is attributed due to more electron releasing nature of –NMe2 as compared to –OCH3 group of SB-2-M. 4-Hydroxy-3-methoxybenzaldehyde (vanillin) is the major chemical ingredient of vanilla and is generally used as flavor in sweet, beverages, pharmaceuticals, food and perfumery industries because of its desirable flavor and fragrance properties.30 Schiff's bases obtained from vanillin have been verified to take many functional groups that act as chelating legends31 and potential as antibacterial agents against some bacterial strains.32 4-Chloro-3-nitroaniline is used as Schiff bases synthesis as well as conformers in co-crystals.33 2-Amino-4-nitrophenol derived Schiff base known for anion sensor,34 fluorescent chemosensor35 and related metal complexes for the cytotoxic agent.36 3-Hydroxy-4-methoxybenzaldehyde Schiff base metal complexes are reported for biological activity.37
N– group acts as an adsorption canter during meta-inhibitor interactions.27–29 A comparison of inhibitive strengths of 2-amino-6-(4-methoxybenzelideneamino)hexanoic acid (SB-2-M) and 2-amino-6-((4-dimethylamino)benzylideneamino)hexanoic acid (SB-4-D) having p-OCH3 and p-N(CH3)2, respectively showed that SB-4-D is a better inhibitor than SB-2-M.27 The higher inhibition efficiency of SB-4-D is attributed due to more electron releasing nature of –NMe2 as compared to –OCH3 group of SB-2-M. 4-Hydroxy-3-methoxybenzaldehyde (vanillin) is the major chemical ingredient of vanilla and is generally used as flavor in sweet, beverages, pharmaceuticals, food and perfumery industries because of its desirable flavor and fragrance properties.30 Schiff's bases obtained from vanillin have been verified to take many functional groups that act as chelating legends31 and potential as antibacterial agents against some bacterial strains.32 4-Chloro-3-nitroaniline is used as Schiff bases synthesis as well as conformers in co-crystals.33 2-Amino-4-nitrophenol derived Schiff base known for anion sensor,34 fluorescent chemosensor35 and related metal complexes for the cytotoxic agent.36 3-Hydroxy-4-methoxybenzaldehyde Schiff base metal complexes are reported for biological activity.37
Herein, considering the different applications and importance of Schiff bases, we report for the first time in literature synthesis and characterization of two novel Schiff bases based on 3-hydroxy-4-methoxybenzaldehyde, 4-chloro-3-nitroaniline, 4-hydroxy-3-methoxybenzaldehyde (vanillin) and 2-amino-4-nitroaniline namely, 5-((4-chloro-3-nitrophenylimino)methyl)-2-methoxyphenol (SB-1) and 2-(4-hydroxy-3-methoxybenzylidineamino)-4-nitrophenol (SB-2) via solid–liquid phase equilibrium diagram study. Literature survey reveals that previously some of the Schiff bases have been evaluated as corrosion inhibitors for metals and alloys in different electrolytic media which showed the inhibition efficiency of 70–98% at as high as 350–2700 ppm concentrations.38–40 In view of this observation, in the present study we tested the inhibition performance of investigated SBs and observed that both the SBs showed more than 95% efficiency at 0.327 mM concentration. The synthesized SBs were characterized by spectral techniques such as 1H and 13C NMR, and FT-IR analysis. Optical properties were investigated by UV-vis absorption and fluorescence in methanol. Thermal properties, melting temperature, stability and decomposition study of the SB-1, 3-hydroxy-4-methoxybenzaldehyde, 4-chloro-3-nitroaniline, SB-2, 4-hydroxy-3-methoxybenzaldehyde and 2-amino-4-nitrophenol compounds have been studied using DSC and TGA. The effect of the synthesized SBs concentration on mild steel corrosion in 1 M HCl has been investigated using experimental and DFT approaches.
2. Experimental section
2.1. Materials and methods
3-Hydroxy-4-methoxybenzaldhyde (HMB) (99%), 4-chloro-3-nitroaniline (CNA) (≥97%), 2-amino-4-nitrophenol (ANP) (≥98%) and 4-hydroxy-3-methoxybenzaldehyde (vanillin, V) (99%) were obtained from Sigma-Aldrich. All chemicals were used for the synthesis of Schiff bases, without further purification.
2.2. Phase diagram
Solid–liquid phase equilibrium diagram of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems have been studied by thaw–melt method as earlier reported.41–43 Melting temperatures of synthesized materials were determined by using a melting point (Toshniwal melting point) apparatus.
For electrochemical measurements, mild steel specimens having chemical composition (wt%): C (0.076), Mn (0.192), P (0.012), Si (0.026), Cr (0.050), Al (0.023), and Fe (balance; 99.62) was used as test material. The 1 M HCl was used as electrolytic medium for electrochemical measurements of the investigated SBs. The metallic specimens were abraded with emery paper of different grades in order to clean their surface followed by their washing with double deionized water, ultrasonic cleaning in ethanol and finally degreasing with acetone. The cleaned metallic specimens were dried under hot air blower and stored in desiccators before use.
2.3. DSC and TGA analyses
Melting temperature and heat of fusion of both the SBs and eutectics as well as their starting components have been determined using differential scanning calorimetry (DSC) (Mettler DSC-4000 system). Before starting the experiment, DSC unit was calibrated using the indium metal as a standard. DSC and TGA experiments were performed under gaseous environment of nitrogen and the gas flow rate was maintained throughout the measurements. Test sample (4–6 mg) was carefully measured in aluminum pan and further sealed which was heated at rate of 10 °C min−1. Enthalpy of fusion was obtained and reproducible within ±0.01 kJ mol−1. Thermal stability of both the SBs was demonstrated by thermo gravimetric analysis. TGA was performed using a Perkin-Elmer STA 6000 thermal analyzer in the temperature range 303 to 800 K at heating rate 10 °C min−1. The samples were purged with a stream of flowing nitrogen throughout the experiments.
2.4. Spectral studies
1H and 13C NMR spectra of both the Schiff's bases and starting materials were recorded in CDCl3 using JOEL AL300 MHz spectrometer using tetra-methyl silane (TMS) as internal reference. FT-IR analysis of both the SBs was carried out using Perkin Elmer FT-IR Spectrum, 1000 Infrared spectrometer. During FT-IR experiment, samples were pelletized with KBr. FT-IR spectra of SBs and starting materials were recorded at 300 K in 4000–400 cm−1 region.
2.5. Powder X-ray diffraction (PXRD)
Crystalline behaviour and diffraction patterns of the SBs were determined using the powder X-ray diffraction (PXRD). The X-ray diffraction (XRD) technique has been utilized for the identification of the nature of eutectics shown in the phase diagrams. The PXRD patterns of eutectics of the synthesized SBs and their starting chemicals were recorded using an 18 kW rotating (Cu) anode based Rigaku powder diffractometer fitted with a graphite monochromator in the diffracted beam. The very fine powdered samples were scanned from 10° to 70° with at the scan rate of 4° min−1.
2.6. Single crystal XRD
Structural analysis of SBs undertaken in present study was also carried out using single crystal XRD by grown single crystals of SB-1. The saturated solution of SB-1 was prepared in mixed (methanol and water) solvent at room temperature and slow evaporation method was used for single crystals growth. After few days fine needle shaped (yellowish) single crystals were harvested. We have made several attempts in order to find the single crystals for SB-2 but unlike to SB-1, it gives very poor crystalline behaviour at room temperature. The single crystal X-ray diffraction pattern of SB-1 was recorded using the X Caliber Oxford CCD diffractometer. The data detection was carried out using Chrysalis Pro software. The structure solution and refinement were studied utilizing SHELXS and SHLEXL-97.44
2.7. UV-vis absorption and emission studies
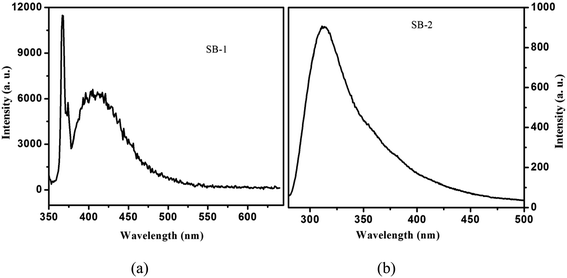
UV-vis absorption of SB-1, SB-2 compounds and 3-hydroxy-4-methoxybenzaldehyde, 4-chloro-3-nitroaniline, 4-hydroxy-3-methoxybenzaldehyde and 2-amino-4-nitrophenol have been recorded in methanol at 1 × 10−5 M concentration using JASCO V-670 absorption spectrophotometer in 200–900 nm range. The fluorescence spectra of SBs have been measured in methanol at 1 × 10−5 M concentration using Varian Cary Eclipse fluorescence spectrophotometer.
2.8. Electrochemical measurements
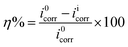
The electrochemical behaviour of synthesized Schiff's bases was investigated using GamryPotentiostat/Galvanostat (Model G-300) instrument embedded with Analyst 5.0 software. A conventional three electrodes cell containing saturated calomel (reference electrode; RE), graphite rod (counter electrode; CE) and mild steel specimens (working electrode; WE) were used for all electrochemical measurements. Potentiodynamic polarization studies were done by changing the WE potential from −250 mV to +250 mV with respect to the potential of RE at a perpetual sweep rate of 1.0 mV s−1. The current density (icorr) was derived by extrapolation of Tafel curves through which inhibition efficiency was derived using following equation:9,12| |
 | (1) |
where, i0corr and iicorr are corrosion current densities in the absence and presence of inhibitors, respectively. EIS studies were performed at the open circuit potential (OCP) in frequency range of 100 kHz to 0.01 Hz through AC current input having amplitude 10 mV peak to peak. The inhibition efficiency was calculated from polarization resistance (Rp; obtained from Nyquist plots) using following relationship:9,12,45| |
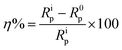 | (2) |
where, R0p and Rip are polarization resistances in the absence and presence of inhibitors, respectively. DFT studies were performed using Gaussian 09 software as described earlier.12,43,44 Several outputs and descriptors such as energies of highest and lowest unoccupied molecular orbitals (EHOMO, ELUMO), energy band gap (ΔE), electronegativity (χ), global hardness (η), softness (σ), and fraction of electron transfer (ΔN) have been calculated using following relationships:12,46–48| |
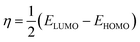 | (4) |
| |
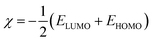 | (5) |
| |
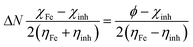 | (6) |
where, χFe and ηinh denote the electronegativity and hardness of iron and inhibitor, respectively. A value of 0.2572 hartree was used for the χFe, while ηFe was taken as 0 hartree for bulk Fe atom in accordance with the Pearson's electronegativity scale.12,46–48 In the present study, value of χFe is replaced by its work function (ϕ) as described earlier.49–53 The values of ϕ derived from DFT calculations which are 0.1436, 0.1771 and 0.1425 hartree for the Fe (100), Fe (110) and Fe (111), respectively. In present study we chosen Fe (110) surface because of its high stabilization energy and highly packed structure.
3. Results and discussion
3.1. Phase diagram
Solid–liquid phase equilibrium diagram of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems are reported in the term of melting temperature-composition curves (Fig. 1a and b). In each case, SBs formation occurs in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of their starting chemicals with congruent melting temperature and two eutectics E1 and E2 as depicted in the phase diagram. In the case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, eutectic E1 and E2 were found at 0.155 and 0.860 mole fraction of component (1), respectively. The melting temperature of SB-1, E1 and E2 were obtained 439, 377 and 367 K, respectively (Fig. 1a). In the diagram, melting temperature of starting compound, 3-hydroxy-4-methoxybenzaldehyde decrease with addition of 4-chloro-3-nitroaniline and reaches to the minimum melting point which is the first eutectic point (E1) and further addition of second mixture component CNA melting temperature increases and reaches up to maximum value of 439 K which is the congruent melting temperature of SB-1. Further increase in the amount of CNA decreases value of melting temperature and finally reaches minimum value of 367 K which is second eutectic point (E2). Similarly, in case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, E1 and E2 was obtained at 0.180, 0.975 mole fraction of vanillin, respectively. Melting temperature for SB-2, E1 and E2 are 474, 399 and 353 K, respectively. Both the synthesized compounds are pure in nature and behave as one of the parent component for both eutectics (E1 and E2).
1 molar ratio of their starting chemicals with congruent melting temperature and two eutectics E1 and E2 as depicted in the phase diagram. In the case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, eutectic E1 and E2 were found at 0.155 and 0.860 mole fraction of component (1), respectively. The melting temperature of SB-1, E1 and E2 were obtained 439, 377 and 367 K, respectively (Fig. 1a). In the diagram, melting temperature of starting compound, 3-hydroxy-4-methoxybenzaldehyde decrease with addition of 4-chloro-3-nitroaniline and reaches to the minimum melting point which is the first eutectic point (E1) and further addition of second mixture component CNA melting temperature increases and reaches up to maximum value of 439 K which is the congruent melting temperature of SB-1. Further increase in the amount of CNA decreases value of melting temperature and finally reaches minimum value of 367 K which is second eutectic point (E2). Similarly, in case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, E1 and E2 was obtained at 0.180, 0.975 mole fraction of vanillin, respectively. Melting temperature for SB-2, E1 and E2 are 474, 399 and 353 K, respectively. Both the synthesized compounds are pure in nature and behave as one of the parent component for both eutectics (E1 and E2).
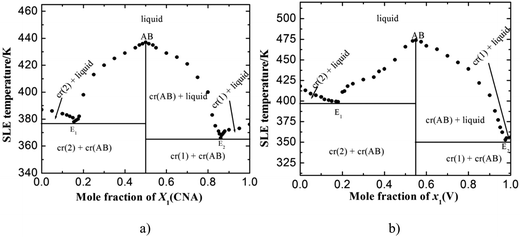 |
| | Fig. 1 Solid–liquid phase equilibrium diagram of (a) 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and (b) 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems. | |
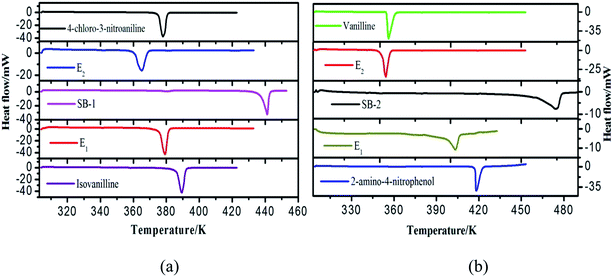
3.2. Differential scanning calorimetry (DSC)
Melting temperatures and heat of fusion for the SB-1 and SB-2 and eutectics were determined with the help of DSC. DSC curves of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems are shown in Fig. 2a and b. The endothermic peaks in DSC indicate that the melting temperature of the SB-1, SB-2 and eutectics. DSC studies provide the information about the phase transitions, in both the cases SB-1, SB-2 and relative eutectics not show additional phase transitions in DSC curves. DSC study provide precise and clear melting point of SB-1 and SB-2 compounds and eutectics, sharp endotherm indicates that the purity of compounds. Furthermore, the various thermal parameters have been computed using DSC data such as enthalpy of fusion, heat of mixing, entropy of fusion and roughness parameters using mixture law and are presented in Table 1. The enthalpy of mixing was found negative (Table 1) in each eutectic of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems suggesting that clustering of appropriate molecules.54 The excess thermodynamics function such as excess free energy (gE), excess enthalpy (hE) and excess entropy (sE) for eutectic composition were computed by using eqn (7)–(9) as reported earlier,50| |
gE = RT[x1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ11 + x2 γ11 + x2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ12] γ12]
| (7) |
| |
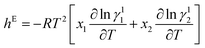 | (8) |
| |
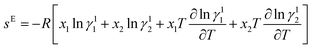 | (9) |
where, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ1i, xi and
γ1i, xi and 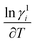 respectively represent the activity coefficients in the liquid state, the mole fraction and the variation of log of activity coefficient in liquid state as a function of temperature for the component i. Activity coefficient (γli) can be evaluated using the equation below,50
respectively represent the activity coefficients in the liquid state, the mole fraction and the variation of log of activity coefficient in liquid state as a function of temperature for the component i. Activity coefficient (γli) can be evaluated using the equation below,50| |
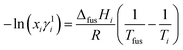 | (10) |
where, xi, ΔfusHi, Ti and Tfus are mole fraction, enthalpy of fusion, melting temperature of component i and melting temperature of eutectics, respectively. The variation of activity coefficient with temperature can be computed using differentiating the eqn (11) given below:| |
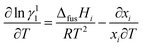 | (11) |
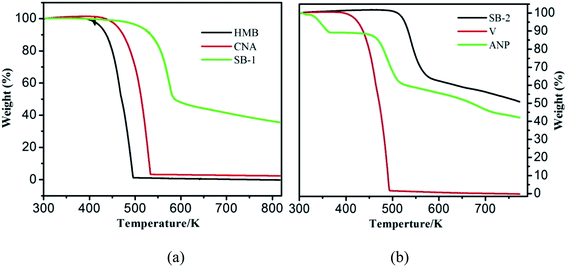
where, value of ∂xi/∂T in the above equation can be evaluated by allowing for two nearest points of eutectic composition. In case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitroaniline (2) system, positive value of excess free energy was found at both the eutectics indicating that an association between like molecules are stronger than that of unlike molecules. In the case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, negative value of excess free energy was observed at each eutectic which indicates that stronger association between unlike molecules. The excess thermodynamic functions of eutectics are reported in Table 2. The roughness parameters were also calculated using earlier reported equations.50 The values of roughness parameters was found to be α > 2 (ref. 42 and 55) in all the cases which indicate that the interface is quite smooth and the crystal developed with a faceted morphology those values are presented in Table 1. Thermal stability of SB-1 and SB-2 are also studied by TGA method. TGA plots of SB-1 and SB-2 are shown in Fig. 3a and b, respectively. The TGA plot of SB-1 showed that it is thermally stable up to 500 K and shows stability after its melting. Fig. 3a indicates that SB-1 is more stable than that of 3-hydroxy-4-methoxybenzaldehyde (HMB) and 4-chloro-3-nitroaniline (CNA). It is clear from Fig. 3b, SB-2 is stable up to 505 K and thermally more stable than that of starting compound 4-hydroxy-3-methoxybenzaldehyde and 2-amino-4-nitrophenol.
 |
| | Fig. 2 DSC curves of (a) 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and (b) 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems. | |
Table 1 Heat of fusion, entropy of fusion and roughness parameter of starting components, complexes (SB-1 and SB-2) and their eutectics
| Materials |
Enthalpy of fusion (kJ mol−1) |
Heat of mixing (kJ mol−1) |
Entropy of fusion (kJ mol−1 K−1) |
Roughness parameter |
| HMB |
26.70 |
|
0.069 |
8.299 |
| CNA |
34.03 |
|
0.090 |
10.825 |
| E1 |
(Exp.) |
27.98 |
−2.29 |
0.074 |
8.900 |
| (Cal.) |
30.27 |
| E2 |
(Exp.) |
23.47 |
−11.73 |
0.064 |
7.697 |
| (Cal.) |
35.20 |
| SB-1 |
(Exp.) |
38.21 |
7.85 |
0.087 |
10.464 |
| (Cal.) |
30.36 |
| ANP |
23.42 |
|
0.056 |
6.7356 |
| V |
24.00 |
|
0.067 |
8.0587 |
| E1 |
(Exp.) |
12.18 |
−13.06 |
0.030 |
3.6084 |
| (Cal.) |
25.24 |
| E2 |
(Exp.) |
21.74 |
−2.48 |
0.061 |
7.3370 |
| (Cal.) |
24.22 |
| SB-2 |
(Exp.) |
28.47 |
4.76 |
0.060 |
7.2167 |
| (Cal.) |
23.71 |
Table 2 Excess free energy, excess enthalpy and excess entropy eutectics of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitroaniline (2) systems
| System |
gE (kJ mol−1) |
hE (kJ mol−1) |
sE (kJ mol−1 K−1) |
| (CNA (1)–HMB (2)) |
| E1 |
−0.2078 |
224.28 |
0.5953 |
| E2 |
−0.5315 |
−22.40 |
−0.0596 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| (V (1)–ANP (2)) |
| E1 |
0.0146 |
−5.777 |
−0.014 |
| E2 |
0.0948 |
−16.045 |
−0.046 |
 |
| | Fig. 3 TGA curves of (a) SB-1, HMB and CNA (b) SB-2, ANP and vanillin (V). | |
3.3. Spectral analyses
3.3.1. 1H and 13C analysis. 1H NMR spectrum of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, a signal appears at 9.87 ppm (1H, s) which is attributed to the presence of carbonyl (〉C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) proton of –CHO group. The 4-chloro-3-nitroaniline shows the signal at 3.99 ppm (2H, s) for –NH2 protons while in SB-1, 1H NMR spectrum, a new signal at 8.33 ppm (1H, s) confirmed that the presence of imine (–CH
O) proton of –CHO group. The 4-chloro-3-nitroaniline shows the signal at 3.99 ppm (2H, s) for –NH2 protons while in SB-1, 1H NMR spectrum, a new signal at 8.33 ppm (1H, s) confirmed that the presence of imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) proton. In 13C NMR spectrum of 4-chloro-3-nitroaniline, a NMR signal at 132 ppm which is attributed to carbon attached to –NH2 group and 3-hydroxy-4-methoxybenzaldehyde shows a NMR signal at 190 ppm which is due to carbonyl (–CHO) carbon. These both signals disappeared in 13C NMR spectra of synthesized Schiff's bases and a new NMR signal appeared at 162 ppm due to presence of imine (–CH
N–) proton. In 13C NMR spectrum of 4-chloro-3-nitroaniline, a NMR signal at 132 ppm which is attributed to carbon attached to –NH2 group and 3-hydroxy-4-methoxybenzaldehyde shows a NMR signal at 190 ppm which is due to carbonyl (–CHO) carbon. These both signals disappeared in 13C NMR spectra of synthesized Schiff's bases and a new NMR signal appeared at 162 ppm due to presence of imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) carbon. This finding indicates that the –NH2 group of 4-chloro-3-nitroaniline reacts with the carbonyl (–CHO) group of 3-hydroxy-4-methoxybenzaldehydeand forms new moiety (–CH
N–) carbon. This finding indicates that the –NH2 group of 4-chloro-3-nitroaniline reacts with the carbonyl (–CHO) group of 3-hydroxy-4-methoxybenzaldehydeand forms new moiety (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) by chemical association. 1H and 13C NMR spectra of SB-1 are shown in Fig. S1a and b.†
N–) by chemical association. 1H and 13C NMR spectra of SB-1 are shown in Fig. S1a and b.†In case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, 1H and 13C NMR spectra of SB-2 have been shown in Fig. S1c and d,† 1H NMR spectrum of 4-hydroxy-3-methoxybenzaldehydeshows NMR signal for –CHO proton at 9.82 ppm (1H, s) and 1H NMR spectrum of 2-amino-4-nitrophenol shows a NMR signal at 5.20 ppm (2H, s) for –NH2 protons. 1H NMR spectrum of SB-2 furnished a signal at 8.61 ppm (1H, s) for imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) proton. Presence of new 1H NMR signal at 8.61 ppm (1H, s) in SB-2 and absence of NMR signals for –CHO and –NH2 protons conformed that the formation of SB-2. In 13C NMR spectrum of 4-hydroxy-3-methoxybenzaldehyde, signal at 190 ppm appeared for –CHO carbon and in 2-amino-4-nitrophenol spectrum signal at 137 ppm appeared for carbon attached to the –NH2 group. The 13C NMR spectrum of SB-2 showed a signal at 162 ppm which is attributed to due to imine (–CH
N–) proton. Presence of new 1H NMR signal at 8.61 ppm (1H, s) in SB-2 and absence of NMR signals for –CHO and –NH2 protons conformed that the formation of SB-2. In 13C NMR spectrum of 4-hydroxy-3-methoxybenzaldehyde, signal at 190 ppm appeared for –CHO carbon and in 2-amino-4-nitrophenol spectrum signal at 137 ppm appeared for carbon attached to the –NH2 group. The 13C NMR spectrum of SB-2 showed a signal at 162 ppm which is attributed to due to imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) carbon (Fig. S1d†). These results confirm that the formation of Schiff bases (SB-1 and SB-2). Chemical structure, IUPAC name, molecular formula, m.p. and NMR data of the synthesized SB-1 and SB-2 are reported in Table 3.
N–) carbon (Fig. S1d†). These results confirm that the formation of Schiff bases (SB-1 and SB-2). Chemical structure, IUPAC name, molecular formula, m.p. and NMR data of the synthesized SB-1 and SB-2 are reported in Table 3.
Table 3 Chemical structure, IUPAC name, molecular formula, m.p., IR and NMR data of SB-1 and SB-2
| S. no. |
IUPAC name |
Chemical structure |
Molecular formula, mp and IR and NMR data |
| 1 |
4-((4-Chloro-3-nitrophenylimino)methyl)-2-methoxyphenol (SB-1) |
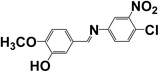 |
C14H11ClN2O4 (mol. wt 306.70), mp – 439 K, IR (KBr) – 1623 cm−1, 1H NMR (300 MHz, CDCl3), δ (ppm); 8.33 (1H, s) |
| 2 |
2-(4-Hydroxy-3-methoxybenzylideneamino)-4-nitrophenol (SB-2) |
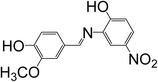 |
C14H12N2O5 (mol. wt 288), mp – 474 K, IR (KBr) – 1629 cm−1, 1H NMR (300 MHz, CDCl3), δ (ppm); 8.61 (1H, s) |
3.3.2. FT-IR analysis. In FT-IR spectrum, the 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, 4-chloro-3-nitroaniline show two bands at 3225 and 3407 cm−1 which is attributed to the symmetric and asymmetric stretching of –NH2. The 3-hydroxy-4-methoxybenzaldehyde shows band at 1672 cm−1 which is due to 〉C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of –CHO. Spectrum of SB-1, shows a band at 1623 cm−1 due to the imine (–CH
O of –CHO. Spectrum of SB-1, shows a band at 1623 cm−1 due to the imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). In 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, the spectrum of 2-amino-4-nitrophenol show two bands at 3291 and 3352 cm−1 which is attributed to the symmetric and asymmetric stretching of –NH2 group. The spectrum of 4-hydroxy-3-methoxybenzaldehyde shows a band at 1666 cm−1 due to carbonyl of –CHO group. In the spectrum of SB-2 these bands are disappeared and one new band appears at 1629 cm−1 due to imine (–CH
N–). In 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, the spectrum of 2-amino-4-nitrophenol show two bands at 3291 and 3352 cm−1 which is attributed to the symmetric and asymmetric stretching of –NH2 group. The spectrum of 4-hydroxy-3-methoxybenzaldehyde shows a band at 1666 cm−1 due to carbonyl of –CHO group. In the spectrum of SB-2 these bands are disappeared and one new band appears at 1629 cm−1 due to imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). The IR analysis confirmed that the formation of both SBs.
N–). The IR analysis confirmed that the formation of both SBs.
3.3.3. Single crystal X-ray diffraction (SCXRD). In case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, Schiff base formation occurs in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the parent components. Single crystals of the SB-1 were grown from the mixed (methanol and water) solvent due to moderate solubility of the SB-1 in this solvent mixture. Saturated solution of SB-1 was prepared in the mixed solvent and kept solution for the slow evaporation, within a weak small size and hexagonal shape crystals were harvested. The single crystal XRD pattern indicates that SB-1 crystallizes in monoclinic crystal system with P21/c space group and chemical formula is C14H11ClN2O4. The nature of the bonding has been confirmed by the SCXRD data. The ORTEP and numbering scheme of the SB-1 is shown in Fig. 4a and unit cell packing shown in Fig. 4b. The hydrogen bonding growth pattern of SB-1 along ‘b’ axis has been shown in Fig. 5a in which SB-1 molecules are showing inter molecular hydrogen bonding. The imine (–CH
1 molar ratio of the parent components. Single crystals of the SB-1 were grown from the mixed (methanol and water) solvent due to moderate solubility of the SB-1 in this solvent mixture. Saturated solution of SB-1 was prepared in the mixed solvent and kept solution for the slow evaporation, within a weak small size and hexagonal shape crystals were harvested. The single crystal XRD pattern indicates that SB-1 crystallizes in monoclinic crystal system with P21/c space group and chemical formula is C14H11ClN2O4. The nature of the bonding has been confirmed by the SCXRD data. The ORTEP and numbering scheme of the SB-1 is shown in Fig. 4a and unit cell packing shown in Fig. 4b. The hydrogen bonding growth pattern of SB-1 along ‘b’ axis has been shown in Fig. 5a in which SB-1 molecules are showing inter molecular hydrogen bonding. The imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) nitrogen of one SB-1 molecule shows inter molecular hydrogen bonding with the hydroxyl proton of another SB-1 molecule which has been shown in Fig. 5b, this provides the extra stability to the SB-1. The crystallographic data and details of refinement of the crystal are reported in the Table 4. The hydrogen bond parameters are reported in Table 5. The bond lengths and bond angles of the SB-1 are reported in Tables 6 and 7, respectively.
N–) nitrogen of one SB-1 molecule shows inter molecular hydrogen bonding with the hydroxyl proton of another SB-1 molecule which has been shown in Fig. 5b, this provides the extra stability to the SB-1. The crystallographic data and details of refinement of the crystal are reported in the Table 4. The hydrogen bond parameters are reported in Table 5. The bond lengths and bond angles of the SB-1 are reported in Tables 6 and 7, respectively.
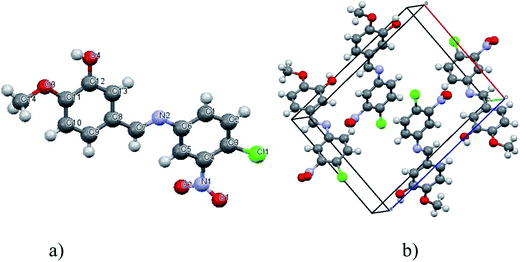 |
| | Fig. 4 (a) ORTEP view and numbering scheme and (b) molecular crystal packing in unit cell of SB-1. | |
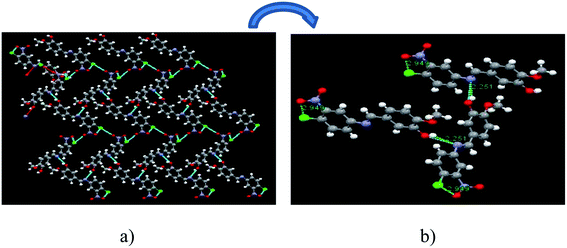 |
| | Fig. 5 (a) Pattern growth of SB-1 along ‘b’ axis and (b) hydrogen bonding interaction between two SB-1 molecules. | |
Table 4 Crystallographic data and details of refinement of SB-1
| Formula |
C14H11ClN2O4 |
| Fw |
306.70 |
| Crystal system |
Monoclinic |
| Space group |
P21/c |
| a [Å] |
10.5441(2) |
| b [Å] |
9.6460(2) |
| c [Å] |
13.7613(3) |
| α [1] |
90 |
| β [1] |
93.6636 |
| γ [1] |
90° |
| V [Å3] |
1396.78(5) |
| Z |
4 |
| Dcalcd [g cm−3] |
1.458 g cm−3 |
| μ (MoKα) [mm−1] |
0.291 |
| F(000) |
632 |
| hkl range |
12, 11, 16 |
| T [K] |
296 |
| Reflections measured |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081 081 |
| Reflections unique |
2459 |
| Rint |
0.09310 |
| GoF (F2) |
1.062 |
Table 5 Hydrogen bond parameters ([Å] and [α°]) in the crystal of SB-1
| D–H⋯A |
D–H |
H⋯A |
D⋯A |
∠DHA |
| O2–H2⋯O5 |
0.820 |
1.772 |
2.591 |
175.98 |
Table 6 Bond lengths [Å] of the SB-1 crystal
| C1–C2 |
1.370(4) |
C1–C6 |
1.390(3) |
| C3–Cl1 |
1.727(3) |
C2–C3 |
1.380(4) |
| C4–N1 |
1.461(3) |
C3–C4 |
1.388(4) |
| C7–N2 |
1.272(3) |
C4–C5 |
1.374(4) |
| C8–C13 |
1.400(3) |
C5–C6 |
1.389(3) |
| C11–C12 |
1.406(3) |
C6–N2 |
1.416(3) |
| C12–C13 |
1.363(3) |
C7–C8 |
1.453(3) |
| C14–O3 |
1.417(3) |
C8–C9 |
1.387(3) |
| N1–O1 |
1.180(4) |
C9–C10 |
1.382(4) |
| C11–O3 |
1.365(3) |
C10–C11 |
1.375(3) |
| C12–O4 |
1.363(3) |
N1–O2 |
1.202(4) |
Table 7 Bond angles [α°] of the SB-1 crystal
| C2–C1–C6 |
121.6(2) |
C7–N2–C6 |
120.8(2) |
| C2–C3–C4 |
118.0(2) |
C1–C2–C3 |
120.6(3) |
| C4–C3–Cl1 |
122.8(2) |
C2–C3–Cl1 |
119.2(2) |
| C5–C4–N1 |
117.0(2) |
C5–C4–C3 |
121.8(2) |
| C4–C5–C6 |
120.0(2) |
C3–C4–N1 |
121.2(2) |
| C1–C6–N2 |
116.3(2) |
C1–C6–C5 |
118.0(2) |
| N2–C7–C8 |
122.9(2) |
C5–C6–N2 |
125.7(2) |
| C9–C8–C7 |
119.7(2) |
C9–C8–C13 |
119.0(2) |
| C10–C9–C8 |
120.9(2) |
C13–C8–C7 |
121.3(2) |
| O3–C11–C10 |
126.5(2) |
C11–C10–C9 |
119.8(2) |
| C10–C11–C12 |
119.8(2) |
O3–C11–C12 |
113.6(2) |
| O4–C12–C11 |
120.9(2) |
O4–C12–C13 |
119.0(2) |
| C12–C13–C8 |
120.3(2) |
C13–C12–C11 |
120.2(2) |
| O1–N1–C4 |
119.7(3) |
O1–N1–O2 |
122.6(3) |
| O2–N1–C4 |
117.7(3) |
C11–O3–C14 |
118.1(2) |
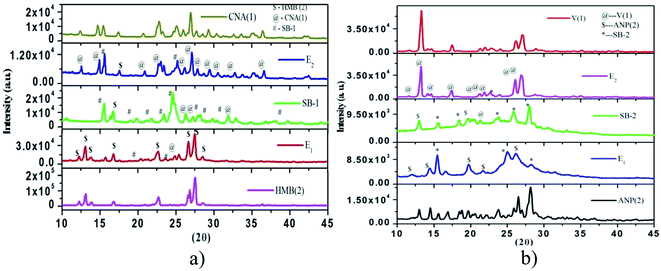
3.3.4. Powder X-ray diffraction (PXRD). The PXRD pattern of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system is shown in Fig. 6a. The Bragg's peaks of the 3-hydroxy-4-methoxybenzaldehyde, 4-chloro-3-nitroaniline (parent components) and SB-1 are assigned by using the symbols ($), (@) and (#), respectively. New Bragg's diffraction peaks at 19.00, 19.74, 21.69, 23.37, 28.16 appeared in the PXRD pattern of SB-1 apart from these the variation in the intensity of the parents and few diffraction peaks of parent components completely disappear. This indicates that the compound is entirely different from its constituents. In the PXRD pattern of E1, peaks of 3-hydroxy-4-methoxybenzaldehyde and few peaks of compound SB-1 has appeared while in case of E2, peaks of 4-chloro-3-nitroaniline as well as SB-1 are observed this indicates that molecular complex is different compound and behaves as one of the parent component for eutectics.
 |
| | Fig. 6 Powder XRD plots of (a) 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) and (b) 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) systems. | |
The PXRD pattern of the systems vanillin (1)–2-amino-4-nitrophenol (2), is shown in Fig. 6b. The Braggs' peaks of vanillin, 2-amino-4-nitrophenol and SB-2 assigned by symbol (@, $ and *), respectively. Results show that the pattern of SB-2 is different from their parent components, new peaks 15.55, 18.40, 23.70, 25.90, 27.98 appear at 2θ value as well as several peaks of parent components disappear. This indicates that SB-2 is new entity and behaves as one of the parent component for both eutectics E1 and E2.
3.4. Corrosion inhibition study
3.4.1. Electrochemical studies.
3.4.1.1. Open circuit potential. Potential developed over the surface of working electrode (mild steel) with respect to the potential of saturated calomel (reference electrode) without applying any external current is known as open circuit potential (OCP). Fig. 9 depicts the change in the open circuit potential (OCP) of the working electrode in the acidic solution of in 1 M HCl with and without tested SB molecules at their optimum concentration of 0.327 mM. It can be seen from the figure that after 30 minutes immersion time the OCP vs. time plots, in the presence of tested Schiff's bases shifted towards more negative direction without changing common features of the OCP vs. time plots. This finding reveals that the surface layer of oxides (Fe2O3, Fe3O4) has been completely removed and inhibitive layer of inhibitors have been formed.57,58
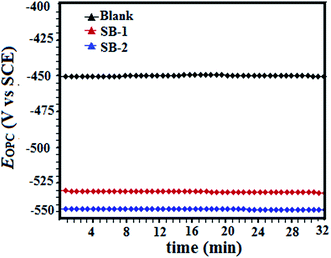 |
| | Fig. 9 Variation of open circuit potential for mild steel dissolution in the absence and presence of tested inhibitors with time (minutes). | |
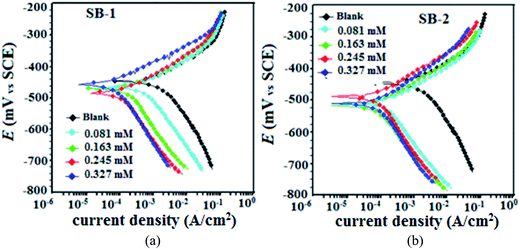 |
| | Fig. 10 Potentiodynamic polarization curves for mild in the absence and presence of different concentrations of the studied inhibitors (a) SB-1 and (b) SB-2. | |
Table 8 Tafel polarization parameters for mild steel in 1 M HCl in the absence and presence of different concentrations of SB-1 and SB-2
| Inhibitor |
Conc. (mM) |
Ecorr (mV per SCE) |
βa (mV dec−1) |
−βc (mV dec−1) |
icorr (μA cm−2) |
η% |
| Blank |
— |
−445 |
70.5 |
114.6 |
1150 |
— |
| SB-1 |
0.081 |
−469 |
62.4 |
195.2 |
432 |
62.43 |
| 0.163 |
−483 |
66.4 |
219.3 |
146 |
87.30 |
| 0.245 |
−482 |
58.9 |
141.9 |
82.3 |
92.84 |
| 0.327 |
−455 |
55.1 |
105.8 |
50.8 |
95.58 |
| SB-2 |
0.081 |
−489 |
80.6 |
109.1 |
286 |
75.13 |
| 0.163 |
−511 |
85.8 |
189.9 |
110 |
90.43 |
| 0.245 |
−489 |
75.6 |
178.8 |
73.4 |
93.61 |
| 0.327 |
−518 |
69.8 |
150.7 |
36.8 |
96.80 |
3.4.1.3. EIS study. The Nyquist and Bode plots for the mild steel corrosion in the absence and presence of studied concentrations of SB-1 and SB-2 are shown in Fig. 11(a and b) and 12(a and b), respectively. The inhibited and uninhibited Nyquist plots depict a single semicircle loop suggesting that corrosion of mild steel in acid solution is a charge transfer phenomenon which is further supported by single maxima in the Bode plots. Furthermore, Nyquist plots show some inductive loop in the low frequency regions which is attributed to the relaxation adsorbed inhibitor molecules on the metallic surface.59,60 In present investigation all the EIS data were analyzed using an equivalent circuit shown in Fig. 11c. This circuit contains polarization resistance (Rp), solution resistance (Rs) and a constant phase element (CPE). In present analysis difference in the real impedance at lower and higher frequencies depicted as polarization resistance (Rp) rather than more commonly used charge transfer resistance (Rct). The criterion behind using Rp is based on the fact that the metal corroding in aqueous solution is generally associated with other types of resistances such as diffusion resistance (Rd), accumulation resistance (Ra) and film resistance (Rf) along with charge transfer resistance (Rct) i.e. Rp = Rd + Ra + Rf + Rct.61–63 From Fig. 11(a and b) it is apparent that diameter of the Nyquist plots in presence of studied SBs are higher than in their absence indicating that inhibition of metallic corrosion occurs via formation of protective film at metal/electrolyte interfaces.46–63 Several electrochemical impedance parameters such as Rs (solution resistance), polarization resistance (Rp), and phase shift (n) double layer capacitance (Cdl) were derived using equivalent circuit and presented in Table 9. The inhibition efficiencies at different studied concentration have been evaluated using eqn (2) and are also summarized in the same table. In general, for a metal corroding system, CPE is being utilized rather than pure capacitor due to the presence of surface in homogeneity. Impedance of the CPE (ZCPE) can be represented by following equation:46,47| |
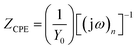 | (12) |
where, Y0, n, ω and j represent CPE constant, phase shift, angular frequency and imaginary number, respectively. Higher value of phase shift is associated with high surface smoothness and vice versa. In addition, to the surface in homogeneity value of n can also be employed for studied the nature of impedance spectra as value of n = 0, 1, −1, and 0.5 represent resistance, capacitance, inductance and Warburg impedance, respectively.12,46–48 In present study, values of n in the presence and absence of studied SBs varies from 0.827 to 0.871 and are slightly deviated from unity (ideal capacitor). This type of deviation from ideal capacitive behaviour is attributed to the surface in homogeneity caused from structural and interfacial origin.12,43–45 In the present study, values of double layer capacitance (Cdl) are derived using following relationship:46–48where, ωmax denotes frequency at which imaginary part of impedance is maximum (rad s−1). The careful examination of results depicted in Table 9 it shows that values of Rp increased and values of Cdl decreased in the presence of SBs particularly at higher SBs concentrations. The increase in values of Rp and decrease in value of Cdl indicate that both SBs adsorbed at the metal/electrolyte interfaces and thereby increased thickness of electric double layer and inhibit acidic mild steel corrosion.12,46–63
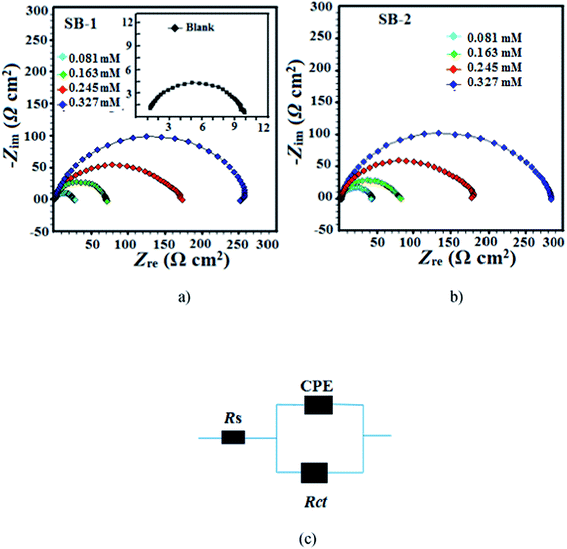 |
| | Fig. 11 Nyquist plot for mild steel in 1 M HCl in the absence and presence of different concentrations of (a) SB-1 and (b) SB-2. (c) Equivalent circuit used for the analysis of the EIS data. | |
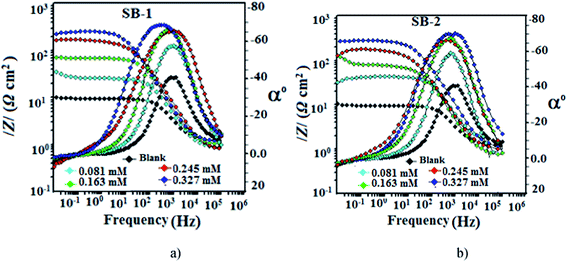 |
| | Fig. 12 Bode plots for mild steel in 1 M HCl in the absence and presence of different concentrations of (a) SB-1 and (b) SB-2. | |
Table 9 EIS parameters for mild steel in 1 M HCl in the absence and presence of different concentrations of SBs
| Inhibitor |
Conc. |
Rs (Ω cm2) |
Rp (Ω cm2) |
Y0 |
n |
Cdl (μF cm−2) |
η% |
| Blank |
— |
1.12 |
9.58 |
|
0.827 |
106.21 |
— |
| SB-1 |
0.081 |
0.844 |
26.08 |
156.0 |
0.837 |
56.43 |
63.30 |
| 0.163 |
1.000 |
68.95 |
96.7 |
0.868 |
41.94 |
86.10 |
| 0.245 |
0.716 |
164.3 |
136.2 |
0.844 |
38.05 |
91.93 |
| 0.327 |
1.121 |
250.9 |
102.3 |
0.832 |
35.90 |
96.18 |
| SB-2 |
0.081 |
1.133 |
40.23 |
87.6 |
0.871 |
38.95 |
76.18 |
| 0.163 |
0.838 |
81.80 |
96.3 |
0.837 |
34.83 |
88.28 |
| 0.245 |
0.957 |
166.3 |
89.6 |
0.838 |
32.60 |
94.23 |
| 0.327 |
1.034 |
276.3 |
61.2 |
0.847 |
23.50 |
96.53 |
The adsorption of the SBs on the metal/electrolyte interfaces and non-ideal capacitive behaviour of impedance spectra have also been supported by Bode plots (Fig. 12a and b). An ideal capacitor is characterized by a constant slope value of −1 and phase angle of 90°.12,46–48 However, in present investigation, slight deviation is observed from the ideal capacitive behaviour as values of slopes and phase angles are not equal to their corresponding ideal values. Deviation from the ideal capacitor is again attributed to surface roughness. However, it can be seen that values of phase angles increased significantly in the presence of SBs suggesting that surface morphology of inhibited specimens have been smoothened remarkably due to adsorption of SBs on metallic surface.12,14,46–48
3.4.2. Adsorption isotherm. Generally, organic inhibitors act by adsorbing on the interfaces of metal and electrolyte that result into formation of protective barrier for corrosion process. Behaviour and mode of adsorption can be determined through several commonly used isotherms such as Temkin, Frumkin, Freundlich and Langmuir adsorption isotherms. In present study, out of several tested isotherms, Langmuir adsorption isotherm represents the best fit with value of regression coefficient (R2) close to one (Fig. 13). The Langmuir adsorption isotherm can be presented as:12| |
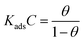 | (14) |
where, θ is the surface coverage, Kads is the equilibrium constant of adsorption–desorption processes taking place over metallic surface and C is the equilibrium inhibitor concentration. Values of the Kads were in the order of 104 suggesting that these inhibitor molecules have strong interaction with the metal in acid solution. Free energy of adsorption (ΔGads) for mild steel corrosion was calculated using the following expression:12| |
ΔGads = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(55.5Kads) ln(55.5Kads)
| (15) |
where, Kads is the equilibrium binding constant, T is temperature and R is gas constant. Generally a higher value of Kads results into the stronger adsorption. The negative value of Gibb's free energy of adsorption in the present case indicates that adsorption of studied SBs on mild steel is a spontaneous process. In general, values of ΔGads ≤ 20 kJ mol−1 is resulted in to the electrostatic interaction (physical adsorption) between charged inhibitor molecules and metal whereas values of ΔGads ≤ 40 kJ mol−1 involve transfer charges from the inhibitor molecules to mild steel surface in order to form covalent bond (chemisorption). In present study, the ΔGads values ranged from −34 kJ mol−1 to −38 kJ mol−1. Since, the values of ΔGads are more close to −40 kJ mol−1 (chemisorption) as compared to the −20 kJ mol−1 (physisorption) and therefore it is concluded that adsorption of the tested compounds on the mild steel surface in 1 M HCl follows predominantly chemisorption mechanism.64,65
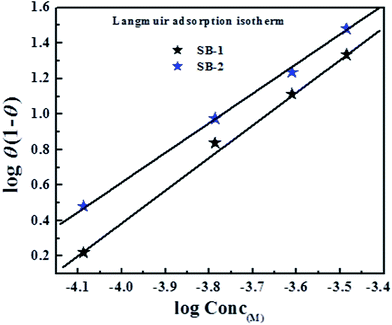 |
| | Fig. 13 Langmuir adsorption isotherm plots for adsorption of investigated Schiff's bases (SBs) on mild steel surface in 1 M HCl solution. | |
3.4.3. DFT analysis. DFT based quantum chemical calculations were also performed on the studied SBs in order to derive some more insight in to their inhibition and adsorption behaviour. The optimized structures of both SBs and their frontier molecular electron (HOMO and LUMO) distribution are shown in Fig. 14(a and b) and 15(a and b), respectively. From Fig. 14, it is observed that frontier electron distributions of HOMO and LUMO are localized almost on entire part of the molecules indicating that entire part of molecules is involved in electron transfer (HOMO) and acceptance (LUMO).66–68 Several theoretical parameters have also been derived and listed in Table 10. In general, value of EHOMO and ELUMO related with electron donating and accepting tendency of the molecule, respectively. A higher value of EHOMO and lower value of ELUMO is consistent with high inhibition efficiency. In our present study, both the studied inhibitors obeyed the order of EHOMO and ELUMO. It is reported that an organic species with high chemical reactivity i.e. lower energy band gap (ΔE; ELUMO – EHOMO) is associated with high inhibition efficiency. In present study, values of ΔE followed the order: SB-2 > SB-1, which is consistent with order of inhibition efficiency obtained by electrochemical measurements.12,46–48 The electronegativity (χ) is another important parameter which can be directly correlated with the inhibition efficiency of inhibitors. High value of χ indicates lower electron donating ability and ultimately lower inhibition efficiency and vice versa. SB-2 has lower value of χ (0.237535) as compared to SB-1 (0.243485) suggesting that SB-2 more readily transfers its electrons to d-orbital of the surface Fe atoms as compared to SB-1 resulting in high inhibition efficiency of SB-2.12,46–48,66–68 The electron transfer ability of the organic compounds also depends upon the values of global hardness (η) and softness (σ). A chemical species with lower value of η or higher value of σ is related with high chemical reactivity and thereby high inhibition efficiency.12,46–48,66–68 Values of η and σ, in present study obey the experimental order of inhibition efficiency. Lastly, values of fraction of electron transfer (ΔN) for both inhibitor molecules have been derived in order to corroborate the experimental results. It is important to mention that adsorption of organic inhibitors on metallic surface involves electron transfer and therefore, higher value of ΔN suggest high electron transfer and thereby high adsorption tendency. Values of ΔN follow the order: SB-2 (1.427238) > SB-1 (0.555966), which supports the order of experimental inhibition efficiency.12,46–48,66–68
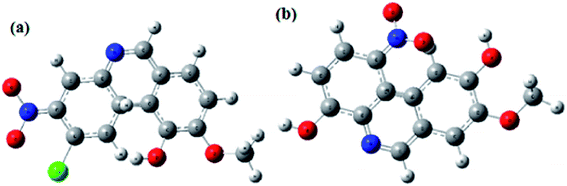 |
| | Fig. 14 Optimized molecular structures of (a) SB-1 and (b) SB-2 at B3LYP/6-31+(d,p) level of theory. | |
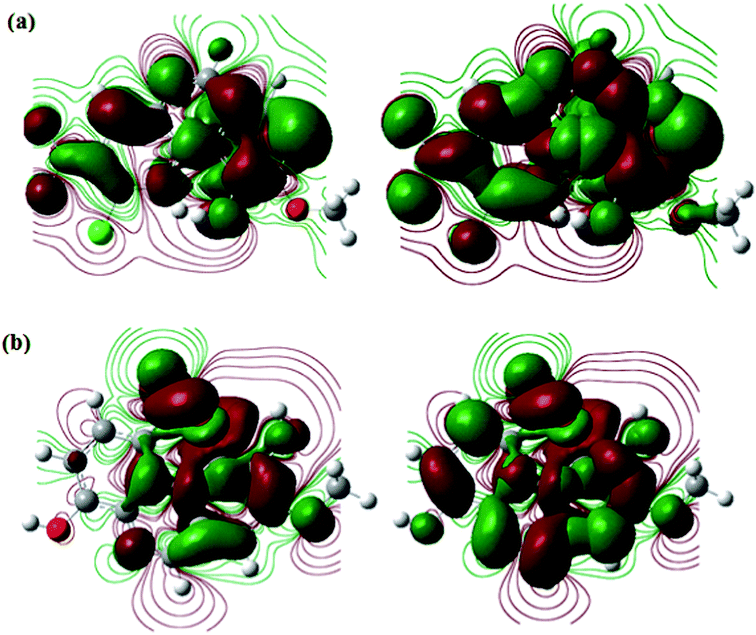 |
| | Fig. 15 The frontier molecular orbital (left-hand side: HOMO; and right-hand side: LUMO) of the studied SBs (a) SB-1 and (b) SB-2. | |
Table 10 DFT based quantum chemical calculations parameters derived for neutral form of the inhibitors
| Inhibitors |
Parameters |
| EHOMO (hartree) |
ELUMO (hartree) |
ΔE (hartree) |
χ |
η |
σ |
ΔN |
| SB-1 |
−0.25586 |
−0.23111 |
0.02475 |
0.243485 |
0.012375 |
80.80808 |
2.6951 |
| SB-2 |
−0.24444 |
−0.23063 |
0.01381 |
0.237535 |
0.006905 |
144.8226 |
4.3768 |
4. Conclusions
Two novel Schiff bases (SBs) namely; 5-((4-chloro-3-nitrophenylimino)methyl)-2-methoxyphenol (SB-1) and 2-(4-hydroxy-3-methoxybenzylidineamino)-4-nitrophenol (SB-2). Solid–liquid phase have been synthesized and investigated as effective corrosion inhibitors for mild steel in 1 M HCl. Investigated SBs were characterized by their spectral analyses (1H and 13C NMR and FT-IR). Single crystal XRD study of SB-1 reveals that it crystallizes in monoclinic unit cell with P21/c space group. The DSC and TGA study show SB-1 and SB-2 are highly stable than that of their starting compounds. DSC curves of SB-1, SB-2 show the no other phase transition occurs. TGA curve of SB-1 and SB-2 indicates that both are stable after their melting temperature and information about the decomposition behavior. Results showed that SB-2 shows better fluorescence property as compared to the SB-1. The EIS and PDP studies suggest that investigated SBs act as efficient inhibitors for mild steel corrosion in 1 M HCl and showed maximum efficiencies of 95.58% and 96.80% for SB-1 and SB-2, respectively at very low concentration of 0.327 mM. Polarization results showed investigated SBs act as mixed corrosion inhibitors with slight cathodic dominance. Several DFT based quantum chemical parameters supported the order of inhibition efficiency obtained by experimental means. Both experimental and DFT results well complemented each other.
Conflicts of interest
The authors declare no conflict of interest of any form.
Acknowledgements
Authors would like grateful thanks to the UGC New Delhi for financial support through BSR-RFSMS scheme and Head, Department of Chemistry, B.H.U., Varanasi, for providing the infrastructure facilities.
References
- S. M. A. Hosseini, M. Salari, E. Jamalizadeh, S. Khezripoor and M. Seif, Mater. Chem. Phys., 2010, 119, 100–105 CrossRef CAS.
- M. A. Quraishi, A. Singh, V. K. Singh, D. K. Yadav and A. K. Singh, Mater. Chem. Phys., 2010, 122, 114–122 CrossRef CAS.
- I. B. Obot and N. O. Obi-Egbedi, Corros. Sci., 2010, 52, 282–285 CrossRef CAS.
- Special Issue, NACE, Materials Performance, Houston, Texas, USA, July 2002 Search PubMed.
- Confederation of Indian Industry, 1st Global Corrosion Summit, New Delhi, India, 2011 Search PubMed.
- R. K. Gupta, M. Malviya, C. Verma and M. A. Quraishi, Mater. Chem. Phys., 2017, 198, 360–373 CrossRef CAS.
- C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2017, 233, 403–414 CrossRef CAS.
- O. Olivares-Xometl, N. V. Likhanova, M. A. Domínguez-Aguilar, J. M. Hallen and E. Arce, Appl. Surf. Sci., 2006, 252, 2139–2152 CrossRef CAS.
- C. Verma, L. O. Olasunkanmi, E. E. Ebenso, M. A. Quraishi and I. B. Obot, J. Phys. Chem. C, 2016, 120, 11598–11611 CAS.
- O. Olivares-Xomet, N. V. Likhanova, M. A. Domınguez-Aguilar, E. Arcec, H. Dorantes and P. Arellanes-Lozada, Mater. Chem. Phys., 2008, 110, 344–351 CrossRef.
- O. Olivares, N. V. Likhanova, B. Gomez, J. Navarrete, M. E. Llanos-Serrano, E. Arce and J. M. Hallen, Appl. Surf. Sci., 2006, 252, 2894–2909 CrossRef CAS.
- C. Verma, M. A. Quraishi and A. Singh, J. Taibah Univ. Sci., 2016, 10, 718–733 CrossRef.
- M. Rodriguez, G. R. Ortiz, J. L. Maldonado, V. M. H. Ambriz, O. Dominguez, R. Santillan, N. Farfan and K. Nakatani, Spectrochim. Acta, Part A, 2011, 79, 1757–1761 CrossRef CAS PubMed.
- S. Vijayalakshmi and S. Kalyanaraman, Opt. Mater., 2013, 35, 440–443 CrossRef CAS.
- D. Sinha, A. K. Tiwari, S. Singh, G. Shukla, P. Mishra, H. Chandra and A. K. Mishra, Eur. J. Med. Chem., 2008, 43, 160–165 CrossRef CAS PubMed.
- C. M. da Silva, D. L. da Silva, L. V. Modolo, R. B. Alves, M. A. de Resende, C. V. B. Martins and A. de Fatima, J. Adv. Res., 2011, 2, 1–8 CrossRef.
- N. S. Gwaram and P. Hassandarvish, J. Appl. Pharm. Sci., 2014, 10, 75–80 Search PubMed.
- S. Chatterjee and S. Bhattacharyya, Asian J. Biochem. Pharm. Res., 2015, 4, 2231–2560 Search PubMed.
- L. Shi, H. M. Ge, S. H. Tan, H. Q. Li, Y. C. Song, H. L. Zhu and R. X. Tan, Eur. J. Med. Chem., 2007, 42, 558–564 CrossRef CAS PubMed.
- M. M. Abd-Elzaher, A. A. Labib, H. A. Mousa, S. A. Moustafa, M. M. Ali and A. A. El-Rashedy, J. Basic Appl. Sci., 2016, 5, 85–96 Search PubMed.
- H. Q. Chang, L. Jia, J. Xu, T. F. Zhu, Z. Q. Xu, R. H. Chen, T. L. Ma, Y. Wang and W. N. Wu, J. Mol. Struct., 2016, 1106, 366–372 CrossRef CAS.
- Y. Zhou, H. Zhou, T. Ma, J. Zhang and J. Niu, Spectrochim. Acta, Part A, 2012, 88, 56–59 CrossRef CAS PubMed.
- G. Ceyhan, M. Tumer, M. Kose, V. McKee and S. Akar, J. Lumin., 2012, 132, 2917–2928 CrossRef CAS.
- Y. Zhou, H. Zhou, T. Ma, J. Zhang and J. Niu, Spectrochim. Acta, Part A, 2012, 88, 56–59 CrossRef CAS PubMed.
- M. Rodrigueza, G. R. Ortiza, J. L. Maldonadoa, V. M. H. Ambriza, O. Dominguezb, R. Santillanb, N. Farfanc and K. Nakatani, Spectrochim. Acta, Part A, 2011, 79, 1757–1761 CrossRef PubMed.
- N. K. Gupta, C. Verma, M. A. Quraishi and A. K. Mukherjee, J. Mol. Liq., 2016, 215, 47–57 CrossRef CAS.
- K. C. Emregül and O. Atakol, Mater. Chem. Phys., 2003, 82, 188–193 CrossRef.
- T. Sethi, A. Chaturvedi, R. K. Upadhyay and S. P. Mathur, J. Chil. Chem. Soc., 2007, 52, 1206–1213 CAS.
- N. Soltani, M. Behpour, S. M. Ghoreishi and H. Naeimi, Corros. Sci., 2010, 52, 1351–1361 CrossRef CAS.
- D. Zheng, C. Hu, T. Gan, X. Dang and S. Hu, Sens. Actuators, B, 2010, 148, 247–252 CrossRef CAS.
- M. W. Sabaa, R. R. Mohamed and E. H. Oraby, Eur. Polym. J., 2009, 45, 3072–3080 CrossRef CAS.
- S. Chigurupati, J. Med. Biol. Eng., 2015, 4(5), 363–366 Search PubMed.
- S. Ghosh and C. M. Reddy, CrystEngComm, 2012, 14, 2444–2453 RSC.
- Y. M. Hijji, B. Barare, A. P. Kennedy and R. Butcher, Sens. Actuators, B, 2009, 136, 297–302 CrossRef CAS.
- L. Yang, W. Zhu, M. Fang, Q. Zhang and C. Li, Spectrochim. Acta, Part A, 2013, 109, 186–192 CrossRef CAS PubMed.
- A. R. Jimenez, R. L. García, A. C. Lozada, S. Hernandez, T. R. Apan, A. N. Camacho and E. Gomez, J. Organomet. Chem., 2013, 738, 10–19 CrossRef.
- R. M. Ahmed, E. I. Yousif and M. J. A. Jeboori, Sci. World J., 2013, 2013, 754868 Search PubMed.
- S. Issaadi, T. Douadi, A. Zouaoui, S. Chafaa, M. A. Khan and G. Bouet, Corros. Sci., 2011, 53, 1484–1488 CrossRef CAS.
- K. C. Emregul and O. Atakol, Mater. Chem. Phys., 2003, 82, 188–193 CrossRef CAS.
- M. A. Hegazy, Corros. Sci., 2009, 51, 2610–2618 CrossRef CAS.
- N. B. Singh, S. S. Das, P. Gupta and M. K. Dwivedi, J. Cryst. Growth, 2008, 311, 118–122 CrossRef CAS.
- R. K. Gupta and R. A. Singh, J. Cryst. Growth, 2004, 267, 340–347 CrossRef CAS.
- T. Agrawal, P. Gupta, S. S. Das, A. Gupta and N. B. Singh, J. Chem. Eng. Data, 2010, 55, 4206–4210 CrossRef CAS.
- G. M. Sheldirck, Shelex-97, Program for Crystal Structure Refinement from Diffraction Data, University of Gottingen, Gottingen, 1997 Search PubMed.
- J. Haque, V. Srivastava, C. Verma and M. A. Quraishi, J. Mol. Liq., 2017, 225, 848–855 CrossRef CAS.
- C. Verma, L. O. Olasunkanmi, I. B. Obot, E. E. Ebenso and M. A. Quraishi, RSC Adv., 2016, 6, 53933–53948 RSC.
- N. K. Gupta, C. Verma, M. A. Quraishi and A. K. Mukherjee, J. Mol. Liq., 2016, 215, 47–57 CrossRef CAS.
- H. Keles, D. M. Emir and M. Keles, Corros. Sci., 2015, 101, 19–31 CrossRef CAS.
- Z. Cao, Y. Tang, H. Cang, J. Xu, G. Lu and W. Jing, Corros. Sci., 2014, 83, 292–298 CrossRef CAS.
- S. K. Saha, A. Dutta, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2016, 18, 17898–17911 RSC.
- A. Kokalj, Chem. Phys., 2012, 393, 1–12 CrossRef CAS.
- S. K. Saha, M. Murmu, N. C. Murmu and P. Banerjee, J. Mol. Liq., 2016, 224, 629–638 CrossRef CAS.
- S. K. Saha and P. Banerjee, RSC Adv., 2015, 5, 71120–71130 RSC.
- R. S. B. Reddi, V. S. A. K. Satuluri, U. S. Rai and R. N. Rai, J. Therm. Anal. Calorim., 2012, 107, 377–385 CrossRef CAS.
- N. B. Singh, S. S. Das, P. Gupta and A. Gupta, J. Cryst. Growth, 2008, 311, 118–122 CrossRef CAS.
- P. Gupta, T. Agrawal, S. S. Das and N. B. Singh, J. Chem. Thermodyn., 2012, 48, 291–299 CrossRef CAS.
- P. S. Kalsi, Spectroscopy of Organic Compounds, New Age Publication, India, 6th edn, 2005 Search PubMed.
- X. Li, S. Deng and X. Xie, Corros. Sci., 2014, 81, 162–175 CrossRef CAS.
- G. Karthik and M. Sundaravadivelu, Egypt. J. Pet., 2016, 25, 183–191 CrossRef.
- F. Bentissa, M. Traisnelb and M. Lagrenee, Corros. Sci., 2000, 42, 127–146 CrossRef.
- R. Solmaza, G. Kardas, M. Çulha, B. Yazıcı and M. Erbil, Electrochim. Acta, 2008, 53, 5941–5952 CrossRef.
- R. Solmaz, Corros. Sci., 2010, 52, 3321–3330 CrossRef CAS.
- C. Verma, M. A. Quraishi, E. E. Ebenso, I. B. Obot and A. El Assyry, J. Mol. Liq., 2015, 219, 647–660 CrossRef.
- R. Solmaz, Corros. Sci., 2014, 81, 75–84 CrossRef CAS.
- R. Solmaz, Corros. Sci., 2014, 79, 169–176 CrossRef CAS.
- G. Gece, Corros. Sci., 2008, 50, 2981–2992 CrossRef CAS.
- I. B. Obot, S. Kaya, C. Kaya and B. Tuzun, Phys. E, 2016, 80, 82–90 CrossRef CAS.
- S. Kaya, L. Guo, C. Kaya, B. Tüzüna, I. B. Obot, R. Touir and N. Islam, J. Taiwan Inst. Chem. Eng., 2016, 65, 522–529 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra08887f |
|
| This journal is © The Royal Society of Chemistry 2017 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *bc
*bc
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). They have been extensively used for variety of biological applications in the field of pharmaceuticals.15–18 They have also been used as promising candidates for antimicrobial activity19 such as antibacterial, antifungal, antitumor and anticancer agents.20 They are also being utilized as common and effective intermediate candidates for the synthesis of several organic and coordination compounds.21 Additionally, the Schiff bases are also being utilized as fluorescent probe,13,22–24 non-linear optical (NLO) active,25 catalyst, dyes and inhibitors for metallic corrosion26 etc. Owing to their cost effective nature, high synthetic yield, low toxicity and high chemical purity, Schiff bases (SBs) are ideal candidates to be used for a variety of purposes like agents for antimicrobial and anticorrosive activities. The presence of –CH
N–). They have been extensively used for variety of biological applications in the field of pharmaceuticals.15–18 They have also been used as promising candidates for antimicrobial activity19 such as antibacterial, antifungal, antitumor and anticancer agents.20 They are also being utilized as common and effective intermediate candidates for the synthesis of several organic and coordination compounds.21 Additionally, the Schiff bases are also being utilized as fluorescent probe,13,22–24 non-linear optical (NLO) active,25 catalyst, dyes and inhibitors for metallic corrosion26 etc. Owing to their cost effective nature, high synthetic yield, low toxicity and high chemical purity, Schiff bases (SBs) are ideal candidates to be used for a variety of purposes like agents for antimicrobial and anticorrosive activities. The presence of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group in their molecular structures makes them suitable candidates for several applications in medicinal, agricultural, pharmaceutical and material science. Previously, several Schiff bases have been prepared by condensation reactions of carbonyl and amine compounds. Literature survey reveals that several Schiff's bases have been employed as effective corrosion inhibitors for metals and alloys using experimental and computational methods in different aggressive media.27–29 Their high effectiveness and adsorption tendency on the metallic surfaces mainly attributed to the presence of –CH
N– group in their molecular structures makes them suitable candidates for several applications in medicinal, agricultural, pharmaceutical and material science. Previously, several Schiff bases have been prepared by condensation reactions of carbonyl and amine compounds. Literature survey reveals that several Schiff's bases have been employed as effective corrosion inhibitors for metals and alloys using experimental and computational methods in different aggressive media.27–29 Their high effectiveness and adsorption tendency on the metallic surfaces mainly attributed to the presence of –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– (imine) group through which they can adsorb effectively i.e. –CH
N– (imine) group through which they can adsorb effectively i.e. –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group acts as an adsorption canter during meta-inhibitor interactions.27–29 A comparison of inhibitive strengths of 2-amino-6-(4-methoxybenzelideneamino)hexanoic acid (SB-2-M) and 2-amino-6-((4-dimethylamino)benzylideneamino)hexanoic acid (SB-4-D) having p-OCH3 and p-N(CH3)2, respectively showed that SB-4-D is a better inhibitor than SB-2-M.27 The higher inhibition efficiency of SB-4-D is attributed due to more electron releasing nature of –NMe2 as compared to –OCH3 group of SB-2-M. 4-Hydroxy-3-methoxybenzaldehyde (vanillin) is the major chemical ingredient of vanilla and is generally used as flavor in sweet, beverages, pharmaceuticals, food and perfumery industries because of its desirable flavor and fragrance properties.30 Schiff's bases obtained from vanillin have been verified to take many functional groups that act as chelating legends31 and potential as antibacterial agents against some bacterial strains.32 4-Chloro-3-nitroaniline is used as Schiff bases synthesis as well as conformers in co-crystals.33 2-Amino-4-nitrophenol derived Schiff base known for anion sensor,34 fluorescent chemosensor35 and related metal complexes for the cytotoxic agent.36 3-Hydroxy-4-methoxybenzaldehyde Schiff base metal complexes are reported for biological activity.37
N– group acts as an adsorption canter during meta-inhibitor interactions.27–29 A comparison of inhibitive strengths of 2-amino-6-(4-methoxybenzelideneamino)hexanoic acid (SB-2-M) and 2-amino-6-((4-dimethylamino)benzylideneamino)hexanoic acid (SB-4-D) having p-OCH3 and p-N(CH3)2, respectively showed that SB-4-D is a better inhibitor than SB-2-M.27 The higher inhibition efficiency of SB-4-D is attributed due to more electron releasing nature of –NMe2 as compared to –OCH3 group of SB-2-M. 4-Hydroxy-3-methoxybenzaldehyde (vanillin) is the major chemical ingredient of vanilla and is generally used as flavor in sweet, beverages, pharmaceuticals, food and perfumery industries because of its desirable flavor and fragrance properties.30 Schiff's bases obtained from vanillin have been verified to take many functional groups that act as chelating legends31 and potential as antibacterial agents against some bacterial strains.32 4-Chloro-3-nitroaniline is used as Schiff bases synthesis as well as conformers in co-crystals.33 2-Amino-4-nitrophenol derived Schiff base known for anion sensor,34 fluorescent chemosensor35 and related metal complexes for the cytotoxic agent.36 3-Hydroxy-4-methoxybenzaldehyde Schiff base metal complexes are reported for biological activity.37
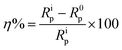
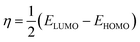
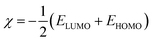
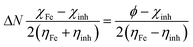
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of their starting chemicals with congruent melting temperature and two eutectics E1 and E2 as depicted in the phase diagram. In the case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, eutectic E1 and E2 were found at 0.155 and 0.860 mole fraction of component (1), respectively. The melting temperature of SB-1, E1 and E2 were obtained 439, 377 and 367 K, respectively (Fig. 1a). In the diagram, melting temperature of starting compound, 3-hydroxy-4-methoxybenzaldehyde decrease with addition of 4-chloro-3-nitroaniline and reaches to the minimum melting point which is the first eutectic point (E1) and further addition of second mixture component CNA melting temperature increases and reaches up to maximum value of 439 K which is the congruent melting temperature of SB-1. Further increase in the amount of CNA decreases value of melting temperature and finally reaches minimum value of 367 K which is second eutectic point (E2). Similarly, in case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, E1 and E2 was obtained at 0.180, 0.975 mole fraction of vanillin, respectively. Melting temperature for SB-2, E1 and E2 are 474, 399 and 353 K, respectively. Both the synthesized compounds are pure in nature and behave as one of the parent component for both eutectics (E1 and E2).
1 molar ratio of their starting chemicals with congruent melting temperature and two eutectics E1 and E2 as depicted in the phase diagram. In the case of 4-chloro-3-nitroaniline (1)–3-hydroxy-4-methoxybenzaldehyde (2) system, eutectic E1 and E2 were found at 0.155 and 0.860 mole fraction of component (1), respectively. The melting temperature of SB-1, E1 and E2 were obtained 439, 377 and 367 K, respectively (Fig. 1a). In the diagram, melting temperature of starting compound, 3-hydroxy-4-methoxybenzaldehyde decrease with addition of 4-chloro-3-nitroaniline and reaches to the minimum melting point which is the first eutectic point (E1) and further addition of second mixture component CNA melting temperature increases and reaches up to maximum value of 439 K which is the congruent melting temperature of SB-1. Further increase in the amount of CNA decreases value of melting temperature and finally reaches minimum value of 367 K which is second eutectic point (E2). Similarly, in case of 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, E1 and E2 was obtained at 0.180, 0.975 mole fraction of vanillin, respectively. Melting temperature for SB-2, E1 and E2 are 474, 399 and 353 K, respectively. Both the synthesized compounds are pure in nature and behave as one of the parent component for both eutectics (E1 and E2).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ11 + x2
γ11 + x2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ12]
γ12]
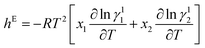
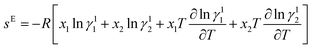
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ1i, xi and
γ1i, xi and 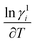 respectively represent the activity coefficients in the liquid state, the mole fraction and the variation of log of activity coefficient in liquid state as a function of temperature for the component i. Activity coefficient (γli) can be evaluated using the equation below,50
respectively represent the activity coefficients in the liquid state, the mole fraction and the variation of log of activity coefficient in liquid state as a function of temperature for the component i. Activity coefficient (γli) can be evaluated using the equation below,50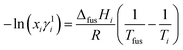
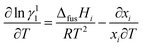

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) proton of –CHO group. The 4-chloro-3-nitroaniline shows the signal at 3.99 ppm (2H, s) for –NH2 protons while in SB-1, 1H NMR spectrum, a new signal at 8.33 ppm (1H, s) confirmed that the presence of imine (–CH
O) proton of –CHO group. The 4-chloro-3-nitroaniline shows the signal at 3.99 ppm (2H, s) for –NH2 protons while in SB-1, 1H NMR spectrum, a new signal at 8.33 ppm (1H, s) confirmed that the presence of imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) proton. In 13C NMR spectrum of 4-chloro-3-nitroaniline, a NMR signal at 132 ppm which is attributed to carbon attached to –NH2 group and 3-hydroxy-4-methoxybenzaldehyde shows a NMR signal at 190 ppm which is due to carbonyl (–CHO) carbon. These both signals disappeared in 13C NMR spectra of synthesized Schiff's bases and a new NMR signal appeared at 162 ppm due to presence of imine (–CH
N–) proton. In 13C NMR spectrum of 4-chloro-3-nitroaniline, a NMR signal at 132 ppm which is attributed to carbon attached to –NH2 group and 3-hydroxy-4-methoxybenzaldehyde shows a NMR signal at 190 ppm which is due to carbonyl (–CHO) carbon. These both signals disappeared in 13C NMR spectra of synthesized Schiff's bases and a new NMR signal appeared at 162 ppm due to presence of imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) carbon. This finding indicates that the –NH2 group of 4-chloro-3-nitroaniline reacts with the carbonyl (–CHO) group of 3-hydroxy-4-methoxybenzaldehydeand forms new moiety (–CH
N–) carbon. This finding indicates that the –NH2 group of 4-chloro-3-nitroaniline reacts with the carbonyl (–CHO) group of 3-hydroxy-4-methoxybenzaldehydeand forms new moiety (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) by chemical association. 1H and 13C NMR spectra of SB-1 are shown in Fig. S1a and b.†
N–) by chemical association. 1H and 13C NMR spectra of SB-1 are shown in Fig. S1a and b.†![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) proton. Presence of new 1H NMR signal at 8.61 ppm (1H, s) in SB-2 and absence of NMR signals for –CHO and –NH2 protons conformed that the formation of SB-2. In 13C NMR spectrum of 4-hydroxy-3-methoxybenzaldehyde, signal at 190 ppm appeared for –CHO carbon and in 2-amino-4-nitrophenol spectrum signal at 137 ppm appeared for carbon attached to the –NH2 group. The 13C NMR spectrum of SB-2 showed a signal at 162 ppm which is attributed to due to imine (–CH
N–) proton. Presence of new 1H NMR signal at 8.61 ppm (1H, s) in SB-2 and absence of NMR signals for –CHO and –NH2 protons conformed that the formation of SB-2. In 13C NMR spectrum of 4-hydroxy-3-methoxybenzaldehyde, signal at 190 ppm appeared for –CHO carbon and in 2-amino-4-nitrophenol spectrum signal at 137 ppm appeared for carbon attached to the –NH2 group. The 13C NMR spectrum of SB-2 showed a signal at 162 ppm which is attributed to due to imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) carbon (Fig. S1d†). These results confirm that the formation of Schiff bases (SB-1 and SB-2). Chemical structure, IUPAC name, molecular formula, m.p. and NMR data of the synthesized SB-1 and SB-2 are reported in Table 3.
N–) carbon (Fig. S1d†). These results confirm that the formation of Schiff bases (SB-1 and SB-2). Chemical structure, IUPAC name, molecular formula, m.p. and NMR data of the synthesized SB-1 and SB-2 are reported in Table 3.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of –CHO. Spectrum of SB-1, shows a band at 1623 cm−1 due to the imine (–CH
O of –CHO. Spectrum of SB-1, shows a band at 1623 cm−1 due to the imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). In 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, the spectrum of 2-amino-4-nitrophenol show two bands at 3291 and 3352 cm−1 which is attributed to the symmetric and asymmetric stretching of –NH2 group. The spectrum of 4-hydroxy-3-methoxybenzaldehyde shows a band at 1666 cm−1 due to carbonyl of –CHO group. In the spectrum of SB-2 these bands are disappeared and one new band appears at 1629 cm−1 due to imine (–CH
N–). In 4-hydroxy-3-methoxybenzaldehyde (1)–2-amino-4-nitrophenol (2) system, the spectrum of 2-amino-4-nitrophenol show two bands at 3291 and 3352 cm−1 which is attributed to the symmetric and asymmetric stretching of –NH2 group. The spectrum of 4-hydroxy-3-methoxybenzaldehyde shows a band at 1666 cm−1 due to carbonyl of –CHO group. In the spectrum of SB-2 these bands are disappeared and one new band appears at 1629 cm−1 due to imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). The IR analysis confirmed that the formation of both SBs.
N–). The IR analysis confirmed that the formation of both SBs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of the parent components. Single crystals of the SB-1 were grown from the mixed (methanol and water) solvent due to moderate solubility of the SB-1 in this solvent mixture. Saturated solution of SB-1 was prepared in the mixed solvent and kept solution for the slow evaporation, within a weak small size and hexagonal shape crystals were harvested. The single crystal XRD pattern indicates that SB-1 crystallizes in monoclinic crystal system with P21/c space group and chemical formula is C14H11ClN2O4. The nature of the bonding has been confirmed by the SCXRD data. The ORTEP and numbering scheme of the SB-1 is shown in Fig. 4a and unit cell packing shown in Fig. 4b. The hydrogen bonding growth pattern of SB-1 along ‘b’ axis has been shown in Fig. 5a in which SB-1 molecules are showing inter molecular hydrogen bonding. The imine (–CH
1 molar ratio of the parent components. Single crystals of the SB-1 were grown from the mixed (methanol and water) solvent due to moderate solubility of the SB-1 in this solvent mixture. Saturated solution of SB-1 was prepared in the mixed solvent and kept solution for the slow evaporation, within a weak small size and hexagonal shape crystals were harvested. The single crystal XRD pattern indicates that SB-1 crystallizes in monoclinic crystal system with P21/c space group and chemical formula is C14H11ClN2O4. The nature of the bonding has been confirmed by the SCXRD data. The ORTEP and numbering scheme of the SB-1 is shown in Fig. 4a and unit cell packing shown in Fig. 4b. The hydrogen bonding growth pattern of SB-1 along ‘b’ axis has been shown in Fig. 5a in which SB-1 molecules are showing inter molecular hydrogen bonding. The imine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) nitrogen of one SB-1 molecule shows inter molecular hydrogen bonding with the hydroxyl proton of another SB-1 molecule which has been shown in Fig. 5b, this provides the extra stability to the SB-1. The crystallographic data and details of refinement of the crystal are reported in the Table 4. The hydrogen bond parameters are reported in Table 5. The bond lengths and bond angles of the SB-1 are reported in Tables 6 and 7, respectively.
N–) nitrogen of one SB-1 molecule shows inter molecular hydrogen bonding with the hydroxyl proton of another SB-1 molecule which has been shown in Fig. 5b, this provides the extra stability to the SB-1. The crystallographic data and details of refinement of the crystal are reported in the Table 4. The hydrogen bond parameters are reported in Table 5. The bond lengths and bond angles of the SB-1 are reported in Tables 6 and 7, respectively.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 081
081
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. In the spectrum of SB-1, two bands appear at 286 and 332 nm, the band at 286 nm is attributed to the π → π* transition of aromatic electrons and at 332 nm is ascribed to the π → π* transition due to formation of imine. The peaks of SB-1 spectrum shows bathochromic as well as hyperchromic shift as compared to HMB parent due to extended conjugation.56
O group. In the spectrum of SB-1, two bands appear at 286 and 332 nm, the band at 286 nm is attributed to the π → π* transition of aromatic electrons and at 332 nm is ascribed to the π → π* transition due to formation of imine. The peaks of SB-1 spectrum shows bathochromic as well as hyperchromic shift as compared to HMB parent due to extended conjugation.56
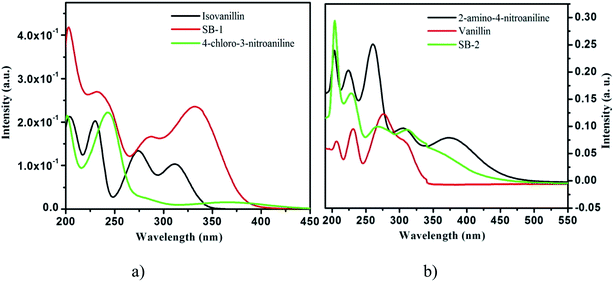
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) n–π* transition. The 308 nm band appeared due to presence of non-bonded electrons of –CHO group. In the spectrum of SB-2, band at 311 nm appears due to n–π* transition. The fluorescence properties of both SB-1 and SB-2 have been studied and shown in Fig. 8a and b. SB-1 shows a weak emission band while SB-2 shows a band at 313 nm.
O) n–π* transition. The 308 nm band appeared due to presence of non-bonded electrons of –CHO group. In the spectrum of SB-2, band at 311 nm appears due to n–π* transition. The fluorescence properties of both SB-1 and SB-2 have been studied and shown in Fig. 8a and b. SB-1 shows a weak emission band while SB-2 shows a band at 313 nm.

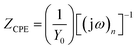


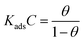
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(55.5Kads)
ln(55.5Kads)