Open Access Article
Open Access ArticlePreparation of Cu(II) porphyrin–TiO2 composite in one-pot method and research on photocatalytic property†
Xin Zhao‡
,
Ying Wang‡,
Wenhua Feng,
Hengtao Lei and
Jun Li *
*
Key Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi 710069, P. R. China. E-mail: junli@nwu.edu.cn
First published on 15th November 2017
Abstract
A promising fabrication strategy used for designing TiO2 photocatalysts, which enhanced the photocatalytic activity by combining TiO2 with a new carboxyl-group-containing Cu(II) porphyrin (CuPp) in a sol–gel processing TiO2 and a one-pot solvothermal condition, has been proposed. Both porphyrins and composite photocatalyst CuPp–TiO2 have been characterized by spectroscopic techniques. Their photocatalytic performances were investigated by testing the photodegradation of 4-nitrophenol (4-NP) in aqueous solution under UV-vis light irradiation. The results reveal that the methodology for the anatase TiO2 photocatalyst is easy to achieve, and the photocatalytic activity measurements illustrate that the Cu(II) porphyrin-based TiO2 photocatalyst we synthesized displays superior photocatalytic activity and good chemical stability for organic pollutant photodegradation due to the strong interactions between Cu(II) porphyrins and TiO2.
1. Introduction
TiO2-based photocatalytic processes can be used in various applications such as waste water treatment, gas purification and environmental protection1–3 due to its nontoxicity, good chemical stability, high photocatalytic activity and versatile properties.4–7 In addition, it has shown a great potential as an inexpensive, environmentally friendly and sustainable treatment technology to remove pollutants from sewage to overcome the shortcomings of the conventional technologies.8,9 However, the utilization for solar energy is limited by the narrow band gap of light absorption region in TiO2,10,11 which impedes its commercialization. To improve the photocatalytic efficiency of TiO2, many techniques12,13 have been carried out with the aim of eliminating the inefficient exploitation of visible light for TiO2. The synthesis of excellent photocatalytic TiO2 materials with heterogeneous structure, which is extensively considered to possess higher photocatalytic activity,14 may have profound implications for organic dye pollutant photodegradation.Porphyrins, as the most promising components, have been widely used in the field of gas sorption, molecular separation, storage, and catalysis.15–17 Their photophysical properties can be easily tuned by metal ion insertion, since porphyrins are able to coordinate with metal ions readily in the central cavity causing stronger and broader photoresponse in the visible region. It is for this reason, that porphyrins are considered to be efficient sensitizers to harvest light on the surface of TiO2.18,19 In particular, ideally designed Cu(II) porphyrins–TiO2 composites were found to be more effective sensitizers in the photodegradation of organics,20,21 which could not only extend the absorption range in the solar spectrum, but also enhance the separation of photogenerated electron–hole pairs, thus increasing activity and stability in the photocatalytic processes of the porphyrin–TiO2 system.
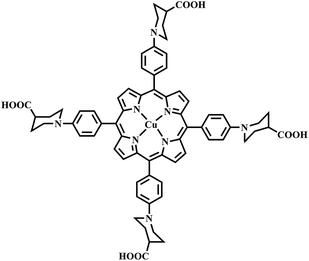
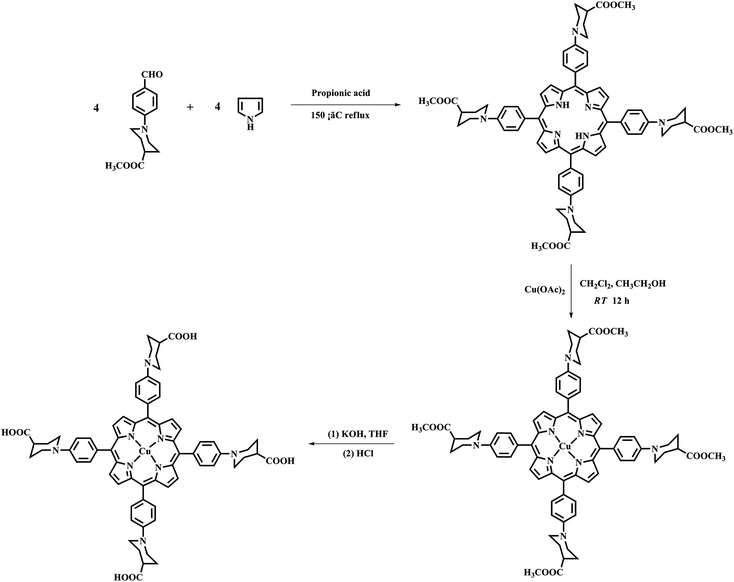
Studies have shown that metal complexes of porphyrins are highly photostable when adsorbed on the surface of TiO2.18,22 The sol–gel process allows the direct introduction of visible light sensitive species like porphyrins inside the TiO2 matrix with ease during the synthesis.23,24 The heterogenous structure formation is advantageous to environmental applications. Herein, we report a route for the synthesis of a novel Cu(II) carboxyl porphyrin (Fig. 1) via a sol–gel process and in solvothermal conditions (Fig. 2), which helps in the easy realization of the efficient porphyrin–TiO2 photocatalyst. The CuPp–TiO2 composite has been characterized with spectroscopic techniques, N2 sorption and 4-nitrophenol (4-NP) degradation, which reveals that the heterogeneous CuPp–TiO2 composite exhibits potential visible photocatalytic activity and higher recyclability than P25, thus, making our synthetic route a prospective method for the preparation of highly efficient and stable porphyrin–TiO2 photocatalysts.
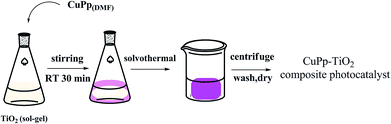 | ||
| Fig. 2 The preparation of CuPp–TiO2 composite photocatalyst with sol–gel procedure and solvothermal conditions. | ||
2. Experimental
2.1 Materials, reagents and equipment
All reagents and solvents used were purchased from commercial sources and used without further purification except pyrrole, which was distilled before use.UV-vis diffuse reflectance spectra (UV-vis-DRS) were recorded on a Shimadzu UV-3100 system using BaSO4 as a reference. Mass spectrometry (MS) analyses were carried out on a matrix assisted laser desorption/ionization time of flight mass spectrometer (MALDI-TOF MS, Krato Analytical Company of Shimadzu Biotech, Manchester, Britain). Elemental analyses (C, H and N) were performed by Vario EL-III CHNOS instrument. The Powder X-ray diffraction (XRD) was examined with a Bruker D8 diffractometer using graphite monochromatic copper radiation (Cu-Kα) at 40 kV and 30 mA over the 2θ range 5–80°. FT-IR spectra were recorded on a BEQUNDX-550 spectrometer on samples embedded in KBr pellets. The surface property of the sample was determined by XPS via Axis Ultra, Kratos (UK) using monochromatic Al K radiation (150 W, 15 kV, 1486.6 eV). Model XPA-VII photocatalytic reactor with a halogen lamp as the light source (Xujiang Electromechanical Plant, Nanjing, China) was employed to evaluate the degradation of 4-NP.
2.2 Synthesis of porphyrins
Yield: 11%. Mp: >250 °C. Anal. calcd (found) for C72H74N8O8 (mol. wt: 1179.41), %: C 73.63 (73.32); H 6.91 (6.32); N 8.97 (9.50); O 10.49 (10.86). MS: m/z: 1180.57 (M + H+) amu. UV-vis (CH2Cl2), λmax/nm, 431 (Soret band), 523, 568, 656 (Q bands). 1HNMR (400 MHz, CDCl3): δ (ppm) = 8.88 (m, 8H, β-H), 8.09 (d, 4H, Ar), 7.29 (d, 4H, Ar), 3.98–3.08 (t, 16H, α-H), 3.78 (s, 12H, –COOCH3), 2.67–2.58 (m, 4H, –CHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–), 2.24–2.01 (m, 16H, β-H), −2.68 (s, 2H, N-H). FT-IR (KBr): υ, cm−1, 3379, 2937, 2803, 1730, 1607, 1515, 1311, 1192, 1042, 922, 804.
O–), 2.24–2.01 (m, 16H, β-H), −2.68 (s, 2H, N-H). FT-IR (KBr): υ, cm−1, 3379, 2937, 2803, 1730, 1607, 1515, 1311, 1192, 1042, 922, 804.
An excess of ten times of Cu(OAc)2 (0.2 g) and H2Pp–OMe (0.1785 g) was dissolved separately in ethanol (20 mL) and CH2Cl2 (20 mL) with stirring at room temperature for 12 h. TLC was checked at the conclusion of the reaction. After removing the unreacted solid salt and solvent, the crude product was chromatographed on a silica column with dichloromethane as the eluent, a purple solid of CuPp–OMe was obtained.
Yield: 86%. Mp: >250 °C. C72H72CuN8O8 (mol. wt: 1240.94), %: C 70.27 (69.69); H 6.01 (5.85); N 9.02 (9.03); O 9.97 (10.31); Cu 4.73 (5.12). MS: m/z 1241.48 (M + H+) amu. UV-vis (CH2Cl2) λmax/nm, 430 (Soret band), 545 (Q band). FT-IR (KBr): υ, cm−1, 2951, 1728, 1600, 1503, 1304, 1187, 1045, 920, 802.
Yield: 86%. Mp: >250 °C. C68H64CuN8O8 (mol. wt: 1184.83), %: C 69.33 (68.93); H 5.36 (5.44); N 9.25 (9.46); O 10.27 (10.80); Cu 5.79 (5.37). MS: m/z 1185.41 (M + H+) amu. UV-vis (DMF) λmax/nm, 432 (Soret band), 547, 591 (Q bands). FT-IR (KBr): υ, cm−1: 3413, 1704, 1602, 1505, 1102, 938, 802.
2.3 Preparation of TiO2 nanometer photocatalyst
The preparation procedure of TiO2 sol nanometer photocatalyst is given below: in the first step, anhydrous ethanol (30 mL) tetrabutyltitanate (10 mL, 29.4 mmol) and acetic acid (2 mL) were mixed together at room temperature under continuous stirring. Then, nitric acid (0.5 mL), H2O (1 mL) and anhydrous ethanol (10 mL) was added dropwise to the above solution for 1.5 h. After that, a translucent TiO2 sol was obtained.Second, 6 drops of water were added to 5 mL of TiO2 sol, and the mixture was stirred for 30 min. Then, the mixture was transferred into a 25 mL Teflon-lined autoclave casing at 90 °C and heated to 150 °C for 24 h. A gel like photocatalyst was obtained after cooling to room temperature. The sample was washed with anhydrous ethanol several times and soaked for 24 h. Finally, TiO2 nanoparticles were fabricated after being dried and ground.
2.4 The synthesis of CuPp–TiO2 photocatalyst
The preparation procedure of mesoporous CuPp–TiO2 was similar to that of TiO2 nanometer photocatalyst synthesis, except that the CuPp load was increased: 0.0175 g amount of as-prepared CuPp was dissolved in 6 mL DMF. TiO2 (5 mL) was added dropwise to the solution of CuPp–DMF (2 mL), and distilled water (0.3 mL) was added to the mixed solution severally. Then, the solvothermal condition was analogous to that mentioned above.2.5 Photocatalytic activity tests
The photoreactivity experiments were carried out using a Model XPA-VII photocatalytic reaction instrument according to the previous report:27 10 mg photocatalysts were added into 50 mL 4-NP (1 × 10−4 mol L−1) solution. The suspension was stirred vigorously with air bubbled when irradiating by 400 W of the central light source. The photocatalysis lasted for 60 min, and every 6 min we took out 3 mL sample of the suspension. The photocatalysts were separated from the solution by centrifugation, and the quantity of 4-NP was measured by its absorption at 317 nm with a Shimadzu UV-1800 UV-vis-NIR system.2.6 Stability of the photocatalyst
The stability test for CuPp–TiO2 photocatalyst was carried out following a procedure similar to that used for the photodegradation of 4-NP. The experimental procedure and test conditions were the same as those mentioned above; the experiments were repeated six times. Catalysts for each test were collected by centrifugation, washed with distilled water, and dried in a vacuum oven after every photocatalytic cycle experiment.2.7 Photocatalytic mechanism investigation
Active species trapping experiment was employed to detect reactive species in photocatalysis process, which was carried out with the same procedure as that for the photodegradation test, except for the addition of selected scavengers. Benzoquinone (BQ, 0.2 mM), ammonium oxalate (AO, 10 mM), and isopropyl alcohol (IPA, 10 mM) were selected as scavengers of superoxide anionic radicals (˙O2−), photoinduced holes (h+) and hydroxyl radicals (˙OH−), respectively.3. Results and discussions
3.1 Morphology analysis
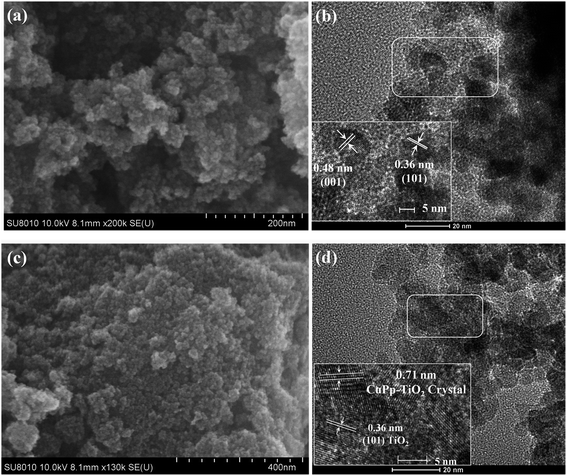
The surface morphology of the TiO2 and CuPp–TiO2 photocatalyst can be seen in Fig. 4a and c from the SEM images. It can be observed that the microsphere of CuPp–TiO2 possesses a similar appearance as the TiO2 we prepared previously. Integral TEM images shown in Fig. S1† show that the composite has a microsphere structure, which indicates that the CuPp molecules on the surface of TiO2 particles have no effect on the size and shape of the TiO2 powders.The high resolution transmission electron microscopy (HRTEM) images of TiO2, which was synthesized with a sol–gel procedure under solvothermal conditions, are shown in Fig. 4b to exhibit the crystal lattice indices of {001} and {101}, which is typical of anatase TiO2.28–31 Therefore, it can be concluded that the crystallization processes, as further confirmed by XRD measurements, occur uniformly via the strategy. CuPp–TiO2 photocatalyst illustrated in Fig. 4d shows a heterojunction structure, which exists as two different types of lattice fringes in the CuPp–TiO2 composite: the narrow fringe spacing (about 0.36 nm) could be assigned to the {101} planes of anatase TiO2. Moreover, a wider lattice spacing of about 0.71 nm is also observed, which may come from the crystal plane of CuPp.32,33 This verified that CuPp deposited well onto the surface of TiO2 nanoparticles and permeated readily into the spaces among the nanoparticles.34 This may be ascribed to the infiltration of CuPp in the process of high-temperature solvothermal synthesis. These observations show that the preservation of CuPp crystals significantly modified the crystal structure of the TiO2. Furthermore, we created massively intrinsic channels for photoproduced holes and electrons. Thereby, CuPp–TiO2 composite photocatalyst with a heterojunction structure is capable of displaying superior photocatalytic activity, which is in a good agreement with the photocatalytic experimental results.
3.2 XRD analysis
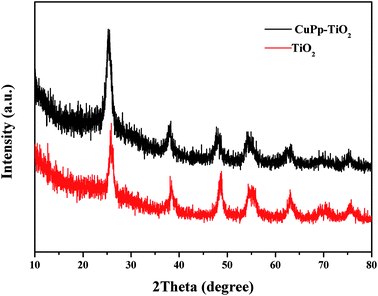
X-ray diffraction (XRD) measurements were conducted to investigate the crystal phase of CuPp–TiO2 particles and the effect of CuPp on the crystal structure of TiO2. As shown in Fig. 5, there is almost no difference between CuPp–TiO2 and TiO2. XRD spectra taken in 2θ configuration have exhibited peaks at 25.3°, 38.3°, 48.0°, 54.2°, 63.4°, 69.1° and 75.5°, and they can be indexed to the (1 0 1), (0 0 4), (2 0 0), (1 0 5), (2 1 1), (2 0 4), and (2 1 5) lattice planes reflection of anatase TiO2 (JCPDS card 21-1272),35 which gives higher photocatalytic activity than rutile and brookite.36,37 The CuPp diffraction fringe fails to display owing to the infinitesimal amount of CuPp on the surface of TiO2, indicating that the CuPp loaded on the surface of TiO2 microsphere could scarcely transform the intrinsic crystal of TiO2.3.3 UV-vis-DRS analysis
The UV-visible diffuse-reflectance (UV-vis-DRS) spectra were measured in the range of 200–800 nm in order to explore the optical response of TiO2 and CuPp–TiO2. As shown in Fig. 6, no obvious absorption peaks were observed above 400 nm for TiO2. However, CuPp–TiO2 photocatalyst, which exhibited peaks typical of CuPp, demonstrated that the CuPp might influence the band width of TiO2. This proves that the CuPp unit was perfectly impregnated onto the surface of TiO2. As a result, the CuPp–TiO2 photocatalyst expanded the absorption range for the solar spectrum in comparison with TiO2. It is noticed that the Soret and Q bands of the CuPp–TiO2 are slightly blue-shifted and broadened relative to CuPp in a DMF solution, implying that CuPp in CuPp–TiO2 photocatalyst had adopted a type H stack mode.38 The band gap energies can be determined by extrapolating the absorption edge onto the energy axis (shown in Fig. 6 insertion), wherein the conversion of the reflectance to absorbance data was obtained by the Kubelka–Munk function (K–M).39,40 The band gap energies of TiO2 and CuPp–TiO2 samples are 3.23 and 3.18 eV, respectively.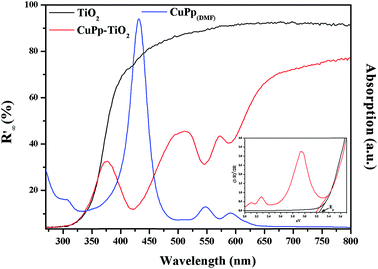 | ||
| Fig. 6 UV-vis-DRS spectra of TiO2 (black line), CuPp–TiO2 (red line) composite and UV-vis spectrum of CuPp (blue line), the insertion is the Kubelka–Munk function. | ||
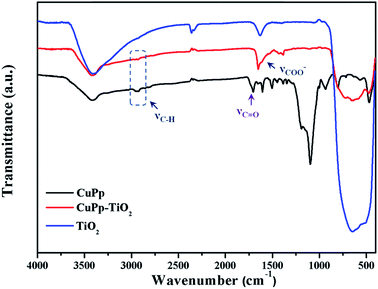
3.4 FT-IR spectra analysis
The FT-IR spectra were used to verify the interaction between CuPp and TiO2. As shown in Fig. 7, there exists a broad characteristic vibration band of Ti–O around 707 cm−1. The disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the carboxyl group in CuPp molecules around 1705 cm−1 and the new band at around 1650 cm−1 and the weak absorptions at around 1406 cm−1 in CuPp–TiO2 might be ascribed to the emergence of asymmetric and symmetric stretch of –COO−. Therefore, this serves as sufficient evidence that CuPp is able to be chemisorbed on the surface of TiO2. Moreover, enhancement of electron transferring between CuPp and Ti orbital manifold of TiO2 can be tuned by O
O stretching vibration of the carboxyl group in CuPp molecules around 1705 cm−1 and the new band at around 1650 cm−1 and the weak absorptions at around 1406 cm−1 in CuPp–TiO2 might be ascribed to the emergence of asymmetric and symmetric stretch of –COO−. Therefore, this serves as sufficient evidence that CuPp is able to be chemisorbed on the surface of TiO2. Moreover, enhancement of electron transferring between CuPp and Ti orbital manifold of TiO2 can be tuned by O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O–Ti bonds.41,42 The appearance of C–O–Ti and C
C–O–Ti bonds.41,42 The appearance of C–O–Ti and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in CuPp–TiO2, which were provided with polarity, resulted in asymmetric electronic structures, contributing to the improvement of photocatalytic activity further.
O groups in CuPp–TiO2, which were provided with polarity, resulted in asymmetric electronic structures, contributing to the improvement of photocatalytic activity further.
3.5 XPS analysis
X-ray Photoelectron Spectroscopy (XPS) analyses were used to investigate the surface chemical states and composition change of TiO2 and CuPp–TiO2. Fig. 8 showed the XPS spectra of both TiO2 and CuPp–TiO2. The survey spectra (Fig. 8a) show that the CuPp–TiO2 composite contains the Cu, Ti, O and C, while TiO2 consists of Ti and O, which was consistent with the corresponding EDX-measured elemental maps (Fig. S2†). The element of C for TiO2 mainly comes from adventitious carbon-based contaminants. There is an enhancement of the C 1s peak intensity in CuPp–TiO2, which may attributed to the massive amounts of adsorbed CuPp molecules. As for Ti 2p spectra (Fig. 8b), the Ti 2p3/2 binding energy of the CuPp–TiO2 (465.0 eV) is higher than that of the TiO2 sample (464.7 eV); this suggested that the Ti atom of the TiO2, as the acceptor, coordinates with the O atom of CuPp as the donor, i.e. Ti atom of the TiO2 adopted the electrons of the O atom in the carbonyl group (O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) from CuPp molecules,43,44 which is in agreement with the FT-IR results. As a result, the electron cloud density of O atom in CuPp decreased and is accompanied with an increased valence for the O atom, and consequently an increased binding energy of O 1s (530.8 eV) (Fig. 8c) compared to that of TiO2 (530.2 eV).45 Moreover, the peaks located at 934.1 and 954.1 eV (ref. 46) also imply the presence of CuPp in CuPp–TiO2 composite, indicating CuPp molecules adsorbed on the TiO2 nanoparticle surface.
O) from CuPp molecules,43,44 which is in agreement with the FT-IR results. As a result, the electron cloud density of O atom in CuPp decreased and is accompanied with an increased valence for the O atom, and consequently an increased binding energy of O 1s (530.8 eV) (Fig. 8c) compared to that of TiO2 (530.2 eV).45 Moreover, the peaks located at 934.1 and 954.1 eV (ref. 46) also imply the presence of CuPp in CuPp–TiO2 composite, indicating CuPp molecules adsorbed on the TiO2 nanoparticle surface.
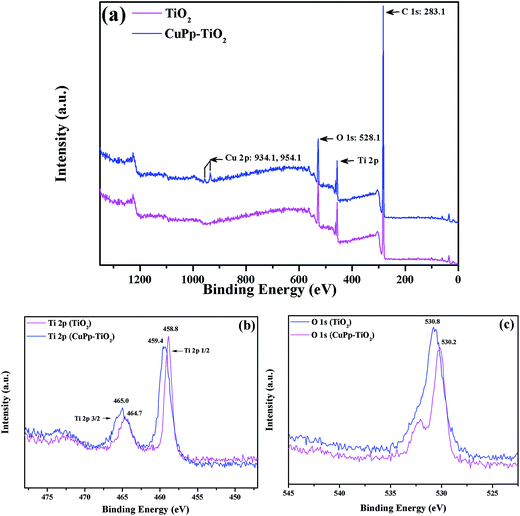 | ||
| Fig. 8 XPS spectra of the TiO2 and CuPp–TiO2 composite photocatalyst, (a) survey spectra, (b) Ti 2p spectra, (c) O 1s spectra. | ||
3.6 BET analysis
N2 adsorption analyses were used to evaluate the porous properties of the samples. Fig. 9 demonstrated the nitrogen adsorption–desorption isotherm and pore size distribution curve of TiO2 and CuPp–TiO2 composite. The pore size distribution is calculated from the desorption branch of a nitrogen isotherm by the Barrett–Joyner–Halenda (BJH) method.47 The samples exhibit a type IV adsorption isotherm with a hysteresis loop according to IUPAC classifications, whose adsorption branch of the isotherm is not consistent with the desorption branch, indicating that both of them are typical characteristics of a mesoporous structure.48 The detailed tested data are summarized in Table 1. The as-prepared TiO2 and CuPp–TiO2 possessed high BET specific surface area of 127.89–140.57 m2 g−1, which are far higher than those of commercial TiO2 (anatase, BET specific surface area 8 m2 g−1).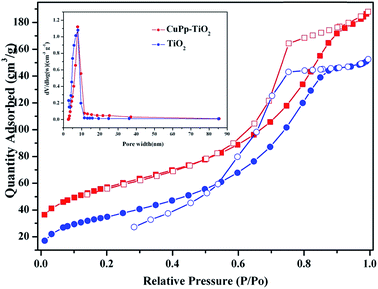 | ||
| Fig. 9 N2 adsorption (solid symbols)–desorption (open symbols) isotherms of TiO2 and CuPp–TiO2 composite. The inset picture was pore size distribution. | ||
| Samples | BET (m2 g−1) | Pore width (nm) | VBJH (cm3 g−1) |
|---|---|---|---|
| TiO2 | 127.89 | 7.21 | 0.32 |
| CuPp–TiO2 | 140.57 | 7.15 | 0.29 |
3.7 Photocatalytic activity analysis
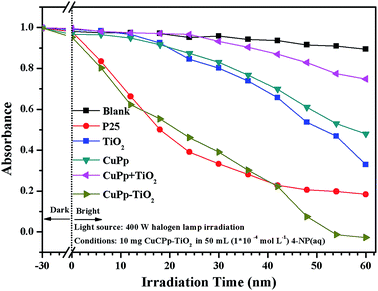
The photocatalytic activities were assessed by the degradation of organic dyes 4-nitrophenol (4-NP) in aqueous solution under white light illumination. P25, the mixture of CuPp and TiO2 serve as references for investigating photocatalytic activities. Fig. 10 shows the photocatalytic efficiency of all samples compared to the blank test, which contained 4-NP(aq) only. As expected, all samples exhibit relatively effective photocatalytic ability in comparison with the blank test. P25 exhibits a favorable performance in photocatalytic activity, whereas the CuPp–TiO2 composite photocatalyst displays a higher removal rate in 4-NP photocatalytic degradation. As such, the CuPp–TiO2 composite photocatalyst manifests inherent superior catalytic activity to the mixture of CuPp and TiO2, which confirmed that it is the heterojunction structures formed by sol–gel progress and solvothermal conditions that improve the efficiency of the photoreactivity process.3.8 The repeatability test
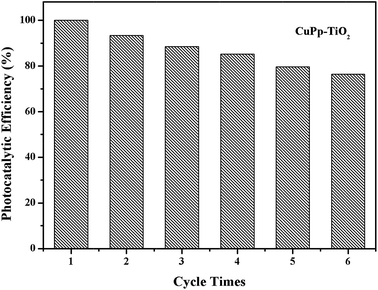
The photostability and reusability are important indicators to measure the practical performance of the catalyst. The study of CuPp–TiO2 reproducibility in photocatalytic degradation of 4-NP was carried out to evaluate stability of CuPp–TiO2 composite. As can be seen in Fig. 11, 100% 4-NP was removed in the first run, and the degradation efficiency decreased gradually with increasing cycle times. The catalytic efficiency of CuPp–TiO2 composite for the next five cycles was reduced to 93.3%, 88.5%, 85.6%, 81.5% and 78.1% respectively, indicating the photocatalytic activity did not obviously decrease within six cycles of reutilization, which exhibited relatively high stability in photodegradation. The stability test confirmed that the CuPp dispersed on the surface of TiO2 exhibited remarkable stability under irradiation, because of the interactions between Cu(II) porphyrin and TiO2 in CuPp–TiO2 composite photocatalyst.3.9 Mechanism explanation
Active species trapping experiment has been carried out to detect the active species in accordance with previous reports.49,50 It has been widely known that the hydroxyl radicals (˙OH), superoxide anion radicals (˙O2−) and photogenerated holes (h+) are the main active species in the photocatalysis process,51,52 which can be detected through the trapping experiment with scavengers isopropyl alcohol (IPA), benzoquinone (BQ) and ammonium oxalate (AO) respectively.53–55 As shown in Fig. 12, the degradation efficiency of 4-NP is changed slightly with the addition of AO as a h+ scavenger, indicating that h+ does not dominate the photodegradation progress. On the contrary, the degradation percentage reduced to a certain extent with the participation of IPA and TBA, which served as ˙OH scavengers. The degradation activity is dramatically inhibited after the addition of BQ, which inferred that ˙O2− is the main active species in 4-NP photocatalytic degradation (Fig. S3†). It can therefore be concluded that 4-NP degradation was inhibited to some degree by the addition of each of the three scavengers, which suggested that ˙OH, h+, and ˙O2− were all involved in the photodegradation process.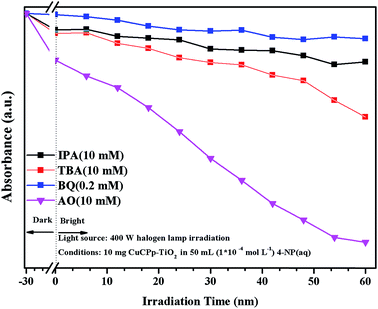 | ||
| Fig. 12 Effects of some selected scavengers on the photocatalytic degradation of 4-NP over CuPp–TiO2 composite photocatalyst under visible light irradiation. | ||
The photocatalytic mechanism of CuPp–TiO2 is explicated in the following description based on the above results. The valence band (VB) and conduction band (CB) energies for CuPp are 1.28 and −0.87 eV respectively,56 which is lower than that of TiO2 (2.91, −0.29 eV). Therefore, the energy level of the excited CuPp and the CB position of TiO2 overlap well. The electrons in VB of TiO2 can be excited and injected into the CB of TiO2 when the light irradiates on the surface of the photocatalyst CuPp–TiO2. Photocatalytic activity is greatly enhanced due to the heterojunction structure in the CuPp–TiO2 composite photocatalyst.51,57 As shown in Fig. 13, the photogenerated electrons generated by the absorption of visible light in the CB of the CuPp migrate to the CB of the TiO2 through the heterojunction structure forming reduction sites. The excited electrons were captured by O2 to produce peroxyl radicals (˙O2−), which were capable of oxidizing 4-NP molecules. Furthermore, the photoinduced holes in the VB of TiO2 flow to the VB of CuPp forming oxidation sites, thus resulting in the reaction with H2O to produce ˙OH and the degradation of 4-NP in the final step. Consequently, photogenerated electrons and holes are spatially isolated with high efficiency, which greatly inhibits their undesirable recombination.38,58,59 Moreover, the mesoporous structure of CuPp–TiO2 with large BET surface area is beneficial to pollutant adsorption and leads to more active interaction with TiO2 and 4-NP.
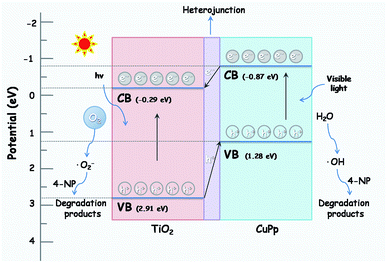 | ||
| Fig. 13 The possible photocatalytic mechanism of CuPp–TiO2 composite photocatalyst under halogen lamp irradiation. | ||
4. Conclusion
A feasible route for the generation of Cu(II) porphyrin based TiO2 composite photocatalyst with a heterogeneous structure has been comprehensively expounded in this study. Spectral techniques and gas sorption experiments have been used to characterize its spectroscopic properties and porous structures. The TiO2 and CuPp–TiO2 obtained by sol–gel process and solvothermal method exhibit anatase phase, as confirmed by XRD, contributing to a high efficiency of the photodegradation of 4-NP in the photocatalytic tests. CuPp–TiO2 composite photocatalyst retained remarkable stability in photocatalysis experiments even after six cycles. It is hoped that this methodology for the porphyrin–TiO2 photocatalyst preparation could serve as a foundation for future applications in photocatalytic degradation.Conflicts of interest
There are no conflicts declare.Acknowledgements
The authors acknowledge the research grant provided by the National Natural Science Foundation of China (No. 21671158) and Top-rated Discipline construction scheme of Shaanxi higher education.References
- A. Fujishima, J. Photochem. Photobiol., C, 2012, 13(3), 169–189 CrossRef.
- R. Vasilić, S. Stojadinović and N. Radić, et al., Mater. Chem. Phys., 2015, 151, 337–344 CrossRef.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115(46), 13211–13241 CrossRef CAS PubMed.
- Y. And and R. Joseph, J. Phys. Chem. B, 2003, 107(43), 11970–11978 CrossRef.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95(3), 735–758 CrossRef CAS.
- T. H. Tran, A. Y. Nosaka and Y. Nosaka, J. Phys. Chem. B, 2006, 110(50), 25525–25531 CrossRef CAS PubMed.
- Y. Kakuma, A. Y. Nosaka and Y. Nosaka, Phys. Chem. Chem. Phys., 2015, 17(28), 18691–18698 RSC.
- S. S. Lee, H. Bai, Z. Liu and D. D. Sun, Environ. Sci. Technol., 2015, 49, 2541–2548 CrossRef CAS PubMed.
- S. Xu, H. Lu, L. Chen and X. Wang, RSC Adv., 2014, 4(85), 45266–45274 RSC.
- D. Yu, J. Bai, H. Liang, T. Ma and C. Li, Dyes Pigm., 2016, 133, 51–59 CrossRef CAS.
- M. M. Yu, J. Li and W. J. Sun, et al., J. Mater. Sci., 2014, 49(16), 5519–5528 CrossRef CAS.
- S. Bingham and W. A. Daoud, J. Mater. Chem., 2011, 21, 2041–2050 RSC.
- J. Yu, X. Zhao and Q. Zhao, Thin Solid Films, 2000, 379(1–2), 7–14 CrossRef CAS.
- Z. Xiong, L. Zhang and X. S. Zhao, Chem.–Eur. J., 2014, 20(45), 14715–14720 CrossRef CAS PubMed.
- S. Motoyama, R. Makiura and O. Sakata, et al., J. Am. Chem. Soc., 2011, 133(15), 5640–5643 CrossRef CAS PubMed.
- S. Kumar, M. Y. Wani, C. Audia and C. T. Arranja, et al., J. Mater. Chem. A, 2015, 3(39), 19615–19637 CAS.
- Z. Zhang, W. Y. Gao and L. Wojtas, et al., Angew. Chem., 2012, 51(37), 9330–9334 CrossRef CAS PubMed.
- S. Afzal, W. A. Daoud and S. J. Langford, ACS Appl. Mater. Interfaces, 2013, 5(11), 4753–4759 CAS.
- J. Roales, J. M. Pedrosa and M. Cano, et al., RSC Adv., 2013, 4(4), 1974–1981 RSC.
- C. Wang, J. Li and G. Mele, et al., Dyes Pigm., 2010, 84(2), 183–189 CrossRef CAS.
- G. Mele, E. Garcìalòpez and L. Palmisano, et al., Appl. Catal., B, 2007, 38(4), 309–319 CrossRef.
- Y. Luo, J. Li and G. P. Yao, et al., Catal. Sci. Technol., 2012, 2, 841–846 CAS.
- L. Tasseroul, S. Lambert and M. C. Páez, et al., Dev. Brain Res., 2011, 44(2), 314–318 Search PubMed.
- C. Wang, J. Li and G. Mele, et al., Efficient degradation of 4–nitrophenol by using functionalized porphyrin–TiO2, photocatalysts under visible irradiation, Appl. Catal., B, 2007, 76(3–4), 218–226 CrossRef CAS.
- G. Mele, S. R. Del and G. Vasapollo, et al., J. Phys. Chem. B, 2005, 109(25), 12347–12352 CrossRef CAS PubMed.
- S. Sun, M. Pan and X. Hu, et al., Catal. Lett., 2016, 146(6), 1087–1098 CrossRef CAS.
- G. P. Yao, J. Li and Y. Luo, et al., J. Mol. Catal. A: Chem., 2012, 361–362(9), 29–35 CrossRef CAS.
- H. G. Yang, C. H. Sun and S. Z. Qiao, et al., Nature, 2008, 453, 638–641 CrossRef CAS PubMed.
- R. Wang, K. Hashimoto and A. Fujishima, et al., Adv. Mater., 2010, 10(2), 135–138 CrossRef.
- M. H. Yang, P. C. Chen and M. C. Tsai, et al., CrystEngComm, 2013, 15(15), 2966–2971 RSC.
- L. Wang, L. Zang and J. Zhao, et al., Chem. Commun., 2012, 48(96), 11736–11738 RSC.
- M. M. Yu, C. Wang and J. Li, et al., Appl. Surf. Sci., 2015, 342, 47–54 CrossRef CAS.
- X. Zhao, X. Liu and M. M. Yu, et al., Dyes Pigm., 2017, 136, 648–656 CrossRef CAS.
- C. Liu, D. Yang and J. Yang, et al., ACS Appl. Mater. Interfaces, 2013, 5(9), 3824–3832 CAS.
- H. Wang, X. Gao and G. Duan, et al., J. Environ. Chem. Eng., 2015, 3(2), 603–608 CrossRef CAS.
- T. A. Kandiel, L. Robben and A. Alkaim, et al., Photochem. Photobiol. Sci., 2013, 12(4), 602–609 CAS.
- J. Zhang, P. Zhou and J. Liu, et al., Phys. Chem. Chem. Phys., 2014, 16(38), 20377–20381 RSC.
- A. D'Urso, M. E. Fragala and R. Purrello, Chem. Commun., 2012, 43(49), 8165 RSC.
- H. Lin, C. P. Huang and W. Li, et al., Appl. Catal., B, 2006, 68(1–2), 1–11 CrossRef CAS.
- B. K. Das, S. J. Bora, M. Chakrabortty, L. Kalita, R. Chakrabarty and R. Barman, J. Chem. Sci., 2006, 118, 487–494 CrossRef CAS.
- D. Chen, D. Yang, J. Geng, J. Zhu and Z. Jiang, Appl. Surf. Sci., 2008, 255(5), 2879–2884 CrossRef CAS.
- P. Zhou, J. Yu and M. Jaroniec, Adv. Mater., 2014, 26(29), 4920–4935 CrossRef CAS PubMed.
- S. J. Zhang, Ultrason. Sonochem., 2012, 19(4), 767–771 CrossRef CAS PubMed.
- J. Yu, G. Dai and Q. Xiang, et al., J. Mater. Chem., 2011, 21(4), 1049–1057 RSC.
- D. A. H. Hanaor and C. C. Sorrell, J. Mater. Sci., 2011, 46(4), 855–874 CrossRef CAS.
- H. Wang, D. Zhou and S. Shen, et al., RSC Adv., 2014, 4, 28978–28986 RSC.
- A. I. Carrillo, E. Serrano and J. C. Serrano–Ruiz, et al., Appl. Catal., A, 2012, 435–436(17), 1–9 CrossRef CAS.
- W. Xuan, C. Zhu and Y. Liu, et al., Chem. Soc. Rev., 2012, 41(5), 1677–1695 RSC.
- L. L. He, Z. F. Tong and Z. H. Wang, J. Colloid Interface Sci., 2018, 509, 448–456 CrossRef CAS PubMed.
- H. Huang, N. Huang and Z. H. Wang, J. Colloid Interface Sci., 2017, 502, 77–88 CrossRef CAS PubMed.
- J. Shu, Z. Wang, G. Xia and Y. Zheng, et al., Chem. Eng. J., 2014, 252, 374–381 CrossRef CAS.
- N. Serpone, Sol. Energy Mater. Sol. Cells, 1995, 38, 369–379 CrossRef CAS.
- Y. N. Wang, K. J. Deng and L. Z. Zhang, J. Phys. Chem. C, 2011, 115, 14300–14308 CAS.
- L. Q. Ye, K. J. Deng, F. Xu, L. H. Tian, T. Y. Peng and L. Zan, Phys. Chem. Chem. Phys., 2012, 14, 82–85 RSC.
- X. P. Song, Q. Yang, X. H. Jiang, M. Y. Yin and L. M. Zhou, Appl. Catal., B, 2017, 217, 322–330 CrossRef CAS.
- W. J. Sun, J. Li and G. Mele, et al., J. Mol. Catal. A: Chem., 2013, 366(1), 84–91 CrossRef CAS.
- H. Wang, L. Zhang and Z. Chen, et al., Chem. Soc. Rev., 2014, 43(15), 5234–5244 RSC.
- J. Low, J. Yu and M. Jaroniec, et al., Adv. Mater., 2017, 29(20), 1601694–1601714 CrossRef PubMed.
- J. Yu, J. Low, W. Xiao, P. Zhou and M. Jaroniec, J. Am. Chem. Soc., 2014, 136, 8839–8842 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Integral TEM images of (a) TiO2, (b) CuPp–TiO2 photocatalyst (Fig. S1). Corresponding EDX element mapping of CuPp–TiO2 composite photocatalyst (j) for carbon, (k) for oxygen, (l) for titanium, (d) for nitrogen, (e) for copper (Fig. S2). The degradation efficiency of 4-NP in the presence of CuPp–TiO2 composite photocatalyst with scavengers (Fig. S3). See DOI: 10.1039/c7ra09585f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |