Open Access Article
Open Access ArticleC–C coupling reactions using a gold(III) phosphorus complex confined within metal–organic framework fibers in aqueous solution
Seyed Mohsen Sadeghzadeh *ab,
Rahele Zhianiab,
Shokufe Emraniab and
Mahdieh Ghabdianab
*ab,
Rahele Zhianiab,
Shokufe Emraniab and
Mahdieh Ghabdianab
aDepartment of Chemistry, Faculty of Sciences, Neyshabur Branch, Islamic Azad University, Neyshabur, Iran. E-mail: seyedmohsen.sadeghzadeh@gmail.com
bYoung Researchers and Elite Club, Neyshabur Branch, Islamic Azad University, Neyshabur, Iran
First published on 1st November 2017
Abstract
Novel fibrous nano-silica (KCC-1) was functionalized with chlorodiphenylphosphine with phosphine-functionalized nanoparticle groups acting as strong performers, so that gold(III) could form complexes without aggregation on the fibers of KCC-1/PPh2 microspheres (KCC-1/PPh2/Au). Compared to the traditional mesoporous silica materials, KCC-1 remarkably enhances the accessibility of gold(III) due to its three dimensional hierarchical channel structure. HPG@KCC-1/PPh2/Au NPs were used for the first time as a catalyst for the C–C cross-coupling between allylarenes or methyl acrylate and benzoxazole, and they showed excellent catalytic activities under green conditions.
Introduction
Metal complexes have high catalytic activities, and they have been a topic of research due to these properties.1–5 Among the reported metals used in catalysis, gold is the most stable catalyst, and it has been widely studied because of its catalytic properties, which can be leveraged for various uses, such as in the Sonogashira,6–8 Suzuki-Miyaura,9–11 Heck12–14 and Hiyama15–17 reactions, in the Larock heteroannulation reaction,18–21 for the degradation of pollutants,22 for hydrogenation23 and in fuel cells.24 In recent years, it has been proven that the use of complex functional groups, either grafted or smeared onto solid supports, plays an important role in preventing the aggregation of metal catalysts.25–27In view of developing new solid supported catalysts, metal complexes with a support of choice provide a large field for the discovery of new, highly active nanocatalysts for important and challenging reactions, which also offer the additional advantage of recyclability.28–30 Mesoporous silica nanoparticles (MSNs) such as SBA-15, MCM-41 and HMS are widely applied as ideal supports for heterogeneous catalysts due to their high surface area, tunable pore size and excellent stability. However, the poor accessibility of the active sites inside the pores, resulting from the mass transfer of reactants or clogging of the pores, may limit the application of these materials in some reactions.31,32 KCC-1 has a high surface area, but more importantly, this high surface area is due to fibres and not pores, which makes this material one-of-a-kind.33 Additionally, the 3D architectures generated by the hierarchical pore structure with macropores can also improve the mass transfer of the reactant.34,35 This would be an ideal catalyst support for making noble metal-based catalysts that provide a high availability of active sites and excellent catalytic activity.
The C–C cross-coupling reaction between aryl halides and organoboranes is one of the most efficient methods for the synthesis of biaryls, which have significant applications in the synthesis of biologically active materials.36–38 Herein, we report the synthesis of KCC-1, which was functionalized with glycidol to form HPG@KCC-1, which was then phosphine-functionalized to form the ordered mesofiber organosilica material (HPG@KCC-1/PPh2) using chlorodiphenylphosphine. The HPG@KCC-1/PPh2 material was then used as an efficient support for the immobilization of gold(III) (HPG@KCC-1/PPh2/Au) and applied for different C–C cross-coupling reactions. We are pleased to report cross-coupling reactions between allylarenes or methyl acrylate and benzoxazole (Scheme 1).
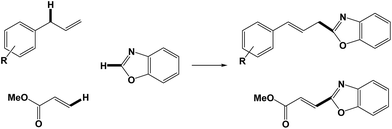 | ||
| Scheme 1 Arylation of benzoxazole with simple allylarenes or methyl acrylate in the presence of HPG@KCC-1/PPh2/Au NPs. | ||
Experimental
Materials and methods
Chemical materials were purchased from Fluka and Merck in high purity. Melting points were determined in open capillaries using an Electrothermal 9100 apparatus and are uncorrected. The particle size and morphology were investigated by SEM using a FESEM-TESCAN MIRA3. The chemical composition of the catalyst on the surface of the MOF was determined by X-ray photoelectron spectroscopy (XPS) using a Kratos Axis Ultra DLD spectrometer with Al Kα as the source. Thermogravimetric analysis (TGA) was carried out on a NETZSCH STA449F3 at a heating rate of 10 °C min−1 under nitrogen. 1H and 13C NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer at 300.13 and 75.46 MHz, and a BRUKER DRX-400 AVANCE spectrometer at 400.22 and 100.63 MHz, respectively. Elemental analyses for C, H and N were performed using a Heraeus CHN–O–Rapid analyzer. Purity determination of the products and reaction monitoring were accomplished by TLC on silica gel polygram SILG/UV 254 plates. Mass spectra were recorded on a Shimadzu GCMS-QP5050 mass spectrometer.General procedure for the preparation of KCC-1
The KCC-1 NP core–shell microspheres were synthesized according to the previously reported method.39 TEOS (2.5 g) was dissolved in a solution of cyclohexane (30 mL) and 1-pentanol (1.5 mL). A stirred solution of cetylpyridinium bromide (CPB, 1 g) and urea (0.6 g) in water (30 mL) was then added. The resulting mixture was continually stirred for 45 min at room temperature then placed in a Teflon-sealed hydrothermal reactor and heated at 120 °C for 5 h. The silica formed was isolated by centrifugation, washed with deionized water and acetone, and dried in a drying oven. This material was then calcined at 550 °C for 5 h in air.General procedure for the preparation of HPG@KCC-1 NPs
2 mmol of KCC-1 NPs was dispersed in a mixture of 80 mL of toluene and 1.0 mmol of potassium methylate (CH3OK), followed by the addition of 10 mL of anhydrous dioxane. 2.0 g of glycidol was added dropwise over a period of 1 h. After vigorous stirring for 2 h, the final suspension was repeatedly washed, filtered several times and dried at 60 °C in air.39General procedure for the preparation of HPG@KCC-1/PPh2 NPs
HPG@KCC-1 (0.6 g) was suspended in 60 mL of a 0.1 M toluene solution of ClPPh2 and the colloidal solution was refluxed for 48 h. The HPG@KCC-1/PPh2 NPs were isolated and purified by repeated washing (firstly in ethanol and then in deionized water) and centrifugation.40General procedure for the preparation of HPG@KCC-1/PPh2/Au NPs
HPG@KCC-1/PPh2/Au nanoparticles were prepared by a simple method from our previous work.40 Sodium tetrachloroaurate (III) hydrate (5 mmol) was dispersed in dry CH2Cl2 (20 mL) and HPG@KCC-1/PPh2 (0.1 g) nanoparticles were added. The mixture was then heated to 60 °C for 12 h under a nitrogen atmosphere. The resulting solid was separated by centrifugation and washed 3 times with CH2Cl2, ethanol and H2O. After drying at room temperature under vacuum, HPG@KCC-1/PPh2/Au NPs were obtained as a powder.General procedure for the arylation of benzoxazole
A mixture of benzoxazole (1 mmol), allylarenes or methyl acrylate (1 mmol), K2CO3 (1 mmol) and HPG@KCC-1/PPh2/Au NPs (1 mg) was stirred with heating under reflux in TFA (3 mL) for 2 hours. K2S2O8 (1 mmol) was then added consecutively. The mixture was stirred at room temperature for 10 min under an N2 atmosphere, then heated under reflux for 6–8 h. The catalyst was separated by filtration. Evaporation of the solvent of the filtrate under reduced pressure gave the crude products. The pure products were isolated by chromatography on silica gel eluted with petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc (3
EtOAc (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Results and discussion
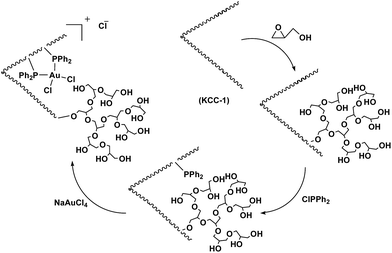
The synthesis of the HPG@KCC-1/PPh2/Au NPs involved several steps. The fibers of KCC-1 have many Si–OH groups on their surfaces, and thus it was expected that KCC-1 could be easily functionalized with chlorodiphenylphosphine, followed by the ring opening polymerization of glycidol to form HPG@KCC-1/PPh2. Furthermore, the phosphine groups on HPG@KCC-1/PPh2 could support gold(III) complexes (Scheme 2).We examined the effect of the solvent for the arylation of benzoxazole with allylarene using HPG@KCC-1/PPh2/Au NPs at room temperature (Table 1). The solvent does affect the catalyst performance. n-Hexane, dioxane, CCl4 and cyclohexane (non-polar solvents) did not give any amount of the desired product (Table 1, entry 11–14). i-PrOH, ethanol, methanol and H2O (protic polar solvents) gave the product in lower yields. CH3CN, THF, CH2Cl2, DMF, toluene, dioxane, CHCl3, EtOAc and DMSO (aprotic solvents) also gave the product in lower yields. In this study, it was found that conventional heating in TFA was more efficient than using organic solvents. We also studied the decisive role of temperature in the arylation of benzoxazole in the presence of HPG@KCC-1/PPh2/Au NPs as a catalyst. The best temperature for this reaction was heating under reflux, which does cause changes in the efficiency of the reaction, showing that the catalytic activity is susceptible to the reaction temperature.
| Entry | Solvent | Temp. (°C) | Yielda (%) |
|---|---|---|---|
| a Isolated yields. | |||
| 1 | Solvent-free | 100 | — |
| 2 | TFA | Reflux | 87 |
| 3 | EtOH | Reflux | 29 |
| 4 | i-PrOH | Reflux | 23 |
| 5 | MeOH | Reflux | 26 |
| 6 | CH3CN | Reflux | 11 |
| 7 | CH2Cl2 | Reflux | 18 |
| 8 | EtOAc | Reflux | 15 |
| 9 | CHCl3 | Reflux | 22 |
| 10 | DMSO | Reflux | 21 |
| 11 | Dioxane | Reflux | — |
| 12 | Cyclohexane | Reflux | — |
| 13 | n-Hexane | Reflux | — |
| 14 | CCl4 | Reflux | — |
The activity of the described catalyst was investigated through the reaction of benzoxazole with allylarene. Initially, we examined the arylation of benzoxazole with allylarene in different bases and oxidants (Table 2). It is well known that bases play a crucial role in coupling reactions, so we attempted the reaction in the presence of various organic and inorganic bases (Table 2, entries 6–15). As a result, we found that the most-used base, K2CO3, was the most efficient base for these reaction conditions. Also, it was found that K2S2O8 is the best oxidant for the reaction. The removal of K2S2O8 from the reaction system completely shuts down the reaction (Table 2, entry 7).
| Entry | Base | Oxidant | Yielda (%) |
|---|---|---|---|
| a Isolated yields. | |||
| 1 | K2CO3 | — | — |
| 2 | K2CO3 | AgOAc | 34 |
| 3 | K2CO3 | Ag2O | 61 |
| 4 | K2CO3 | Cu(OAc)2 | 49 |
| 5 | K2CO3 | (NH4)2S2O8 | 18 |
| 6 | K2CO3 | K2S2O8 | 87 |
| 7 | — | K2S2O8 | — |
| 8 | CsF | K2S2O8 | 68 |
| 9 | Na2CO3 | K2S2O8 | 54 |
| 10 | Et3N | K2S2O8 | 41 |
| 11 | NaOAc | K2S2O8 | 35 |
| 12 | KOH | K2S2O8 | 29 |
| 13 | K3PO4 | K2S2O8 | 42 |
| 14 | Cs2CO3 | K2S2O8 | 55 |
| 15 | tBuOK | K2S2O8 | 14 |
The amount of catalyst is another important factor for the arylation of benzoxazole (Fig. 1). As shown in Fig. 1, in the absence of catalyst, no product was obtained. When 0.2–0.8 mg of catalyst was used in the model reaction, moderate yields of product were obtained. The best result was achieved when the model reaction was carried out in the presence of 1 mg of catalyst. A rise in the catalyst amount gave no yield increase in the model reaction.
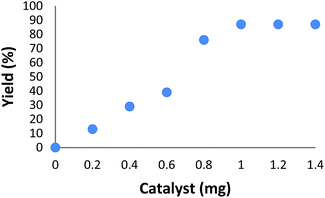 | ||
| Fig. 1 The effect of increasing the amount of HPG@KCC-1/PPh2/Au NPs on the arylation of benzoxazole. | ||
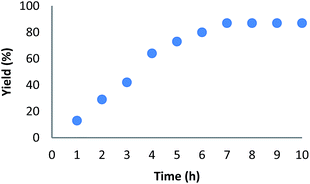
We also investigated the crucial role of time on the arylation of benzoxazole in the presence of HPG@KCC-1/PPh2/Au NPs as a catalyst. The results clearly indicated that the catalytic activity is sensitive to the reaction time. The best time for this reaction was 7 h. A time of more than 7 h did not cause changes in the yield of the reaction (Fig. 2).
To further investigate the efficiency of the catalyst, different control experiments were performed, and the obtained information is shown in Table 3. Initially, a standard reaction was carried out using KCC-1, which showed that no amount of the desired product was formed after 10 h of reaction time (Table 3, entry 1). Also, when KCC-1/PPh2 and HPG@KCC-1/PPh2 were used as the catalyst, a reaction was not observed (Table 3, entries 2 and 3). PPh2 or HPG could not provide the satisfactory catalytic activity under mild reaction conditions. Based on these disappointing results, we continued the studies to improve the yield of the product by adding Au(III). Notably, there was not much difference in the reaction yields when the reaction was carried out using HPG@KCC-1/PPh2/Au NPs and an [Au(bpy)Cl2]Cl catalyst (Table 3, entries 4 and 8), however, [Au(bpy)Cl2]Cl was not recoverable or reusable for the next runs. These observations show that the reaction cycle is mainly catalyzed by Au(III) species complexed on the HPG@KCC-1/PPh2 nanostructure. The nano-sized particles increase the exposed surface area of the active site of the catalyst, thereby enhancing the contact between the reactants and catalyst dramatically, mimicking homogeneous catalysts. As a result, HPG@KCC-1/PPh2/Au was used in the subsequent investigations because of its high reactivity, high selectivity and easy separation. The results illustrate that the amount of catalyst strongly affects the reaction progress, and the best result was obtained in the presence of 0.8 mol% of catalyst (Table 3, entry 5). Also, the activity and selectivity of the nanocatalyst can be manipulated by tailoring the chemical and physical properties, such as the size, shape, composition and morphology. To assess the exact impact of the presence of KCC-1 in the catalyst, the HPG@KCC-1/PPh2/Au NPs were compared to HPG@SiO2/PPh2/Au NPs (Table 3, entries 5 and 7). To check this, we looked at HPG@SiO2/PPh2/Au and HPG@KCC-1/PPh2/Au NPs, which have the same compositions but different structures. When HPG@SiO2/PPh2/Au was used as the catalyst, the yield of the desired product was moderate, but the yield for HPG@KCC-1/PPh2/Au was good (Table 3, entries 5 and 7). The loading amount of Au(III) in the HPG@SiO2/PPh2/Au and HPG@KCC-1/PPh2/Au NPs was determined by inductively coupled plasma mass spectrometry (ICP-MS). The amount of Au(III) in the HPG@KCC-1/PPh2/Au NPs was almost double the amount of Au(III) in the HPG@SiO2/PPh2/Au NPs (Table 4, entries 1 and 3).
| Entry | Catalyst | Catalyst loading | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzoxazole (1 mmol), allylarene (1 mmol), HPG@KCC-1/PPh2/Au NPs (1 mg), K2CO3 (1 mmol) and K2S2O8 (1 mmol) were stirred with heating under reflux in TFA (3 mL) for 10 hours.b Isolated yields. | |||
| 1 | KCC-1 | 1 mg | — |
| 2 | KCC-1/PPh2 | 1 mg | — |
| 3 | HPG@KCC-1/PPh2 | 1 mg | — |
| 4 | HPG@KCC-1/PPh2/Au | 1 mol% | 87 |
| 5 | HPG@KCC-1/PPh2/Au | 0.8 mol% | 87 |
| 6 | HPG@KCC-1/PPh2/Au | 0.6 mol% | 81 |
| 7 | HPG@SiO2/PPh2/Au | 0.8 mol% | 59 |
| 8 | [Au(bpy)Cl2]Cl | 1 mol% | 88 |
| Entry | Catalyst | wt% |
|---|---|---|
| 1 | HPG@SiO2/PPh2/Au | 1.8 |
| 2 | KCC-1/PPh2/Au | 3.5 |
| 3 | HPG@KCC-1/PPh2/Au | 3.7 |
| 4 | KCC-1/PPh2/Au after ten reuses | 1.9 |
| 5 | HPG@KCC-1/PPh2/Au after ten reuses | 3.6 |
Gold(III) leaching was studied by inductively coupled plasma mass spectrometry (ICP-MS) analysis of the catalyst after ten reaction cycles. The loading amount of gold(III) was found to be 3.6 wt%, which shows negligible gold(III) leaching. These results confirm the high recyclability of the gold(III) nanocatalyst (Table 4). Also, the loading amount of gold(III) in the KCC-1/PPh2/Au NPs was determined by ICP-MS. The amount of gold(III) in the HPG@KCC-1/PPh2/Au NPs was almost equal to that in the KCC-1/PPh2/Au NPs. However, it is interesting that this amount of gold(III) was almost the same after the catalyst was reused for ten consecutive cycles of catalysis. The amount of gold(III) in the HPG@KCC-1/PPh2/Au NPs was approximately double that in the KCC-1/PPh2/Au NPs after ten reuses. This remarkable ability of the HPG@KCC-1/PPh2/Au mesostructure may be attributed to the HPG units, which effectively manage the reaction by preventing Au agglomeration and releasing and recapturing gold during the reaction process (Table 4).
To examine the scope of the catalytic properties of the catalyst for the Stille coupling reaction, various types of allylarene were reacted with benzoxazole in the presence of a catalytic amount of HPG@KCC-1/PPh2/Au NPs. It is noteworthy that electron-rich and electron-poor aryl halides react smoothly with phenyltributyltin under similar reaction conditions. The electron-rich allylarenes show moderate to excellent reactivity (81–87% yield) for the formation of the corresponding products in the C–C coupling reactions under similar reaction conditions. The relative reactivity of allylarenes towards the Au metal centre decreases in the order: Me > MeO (Table 5, entry 2 and 4). As shown in Table 5, the C–C coupling reaction of benzoxazole with chloroallyl arene proceeds smoothly under mild reaction conditions, giving the desired product in good yield (Table 5, entry 3). When methyl acrylate was used, the product was obtained under the same conditions (Table 5, entry 5).
| Entry | Allylarene | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: allylarenes or methyl acrylate (1 mmol), benzoxazole (1 mmol), K2CO3 (1 mmol), K2S2O8 (1 mmol) and HPG@KCC-1/PPh2/Au NPs (1 mg) were stirred with heating under reflux in TFA (3 mL) for 6–8 hours.b Isolated yields. | |||
| 1 | 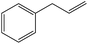 |
7 | 87 |
| 2 | 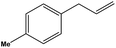 |
8 | 86 |
| 3 | 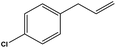 |
7 | 81 |
| 4 | 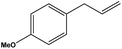 |
7 | 83 |
| 5 | 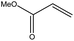 |
6 | 71 |
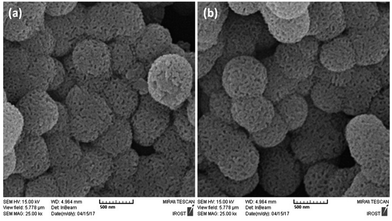
To further understand the underlying reason for the significant difference in recyclability, XPS and SEM were employed to characterize the fresh and reused HPG@KCC-1/PPh2/Au NPs. The XPS spectra are shown in Fig. 3. For the fresh HPG@KCC-1/PPh2/Au NPs, the Au 4f5/2 and Au 4f7/2 binding energies were determined to be 89.4 and 85.7 eV, respectively. After the catalyst was reused ten times, the 89.4 eV (4f5/2) and 85.7 eV (4f7/2) binding energies were fixed, corresponding to the Au(III) binding energy (Fig. 3). The SEM images provided further information about the HPG@KCC-1/PPh2/Au NPs. SEM images for the fresh HPG@KCC-1/PPh2/Au NPs and the HPG@KCC-1/PPh2/Au NPs after ten reuses are displayed in Fig. 4. After being reused ten times, the dandelion-like structure of the catalyst could still be observed, although the dandelion-like structure collapsed to some extent. The similarity between the structures of the fresh HPG@KCC-1/PPh2/Au NPs and the HPG@KCC-1/PPh2/Au NPs after ten reuses accounts for the high level of recyclability.
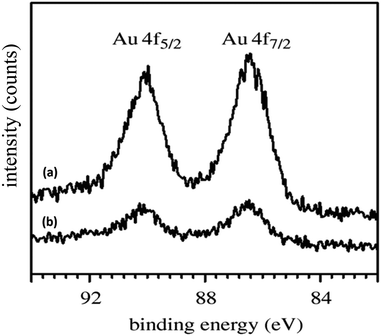 | ||
| Fig. 3 XPS spectra of (a) fresh HPG@KCC-1/PPh2/Au NPs and (b) HPG@KCC-1/PPh2/Au NPs after ten reuses. | ||
In an effort to make the synthesis more applicable, the reusability of the catalyst was also examined for the standard reaction of allylarenes or methyl acrylate with benzoxazole. After completion of the reaction, the mixture was filtered and the catalyst was washed with distilled water and methanol, then dried under reduced pressure. The HPG@KCC-1/PPh2/Au NPs were found to be effective for reuse in up to ten consecutive cycles whilst maintaining a high level of activity and selectivity. The catalytic activity was not reduced extremely after ten catalytic cycles, showing that the catalyst is stable and can be regenerated for repeated use (Fig. 5).
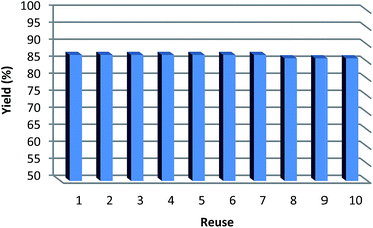 | ||
| Fig. 5 The reusability of the catalyst for cross-coupling reactions between allylarenes and benzoxazole. | ||
In order to study the catalytic activity of various HPG@KCC-1/PPh2 complexes of metal ions, we examined their efficiency for the reaction of allylarenes and benzoxazole (Table 6). Nine separate reactions were examined in the presence of Au(III), Cu(II), Cr(III), Cd(II), Hg(II), Mn(II), Ni(II), Zn(II) and Co(II) complexes. The results indicate that the catalytic efficiency of Au(III) immobilized onto HPG@KCC-1/PPh2 was the highest out of all metal ions tested.
Conclusions
In summary, a novel class of short-fiber KCC-1 mesoporous silica nanoparticles, functionalized with phosphorus ligands, was synthesized for the selective capturing of gold(III), and the nanoparticles exhibited excellent catalytic activity for novel one-pot C–C coupling reactions for the convenient arylation of benzoxazole with good yields. In addition, the catalyst was easily recoverable and reusable. Such a rational design for single-site catalysts with the full utilization of each gold(III) active site, as well as having excellent recyclability and negligible metal leaching, could be favourable for use in green chemistry. Thus, this study of HPG@KCC-1/PPh2/Au nanocatalysts may provide a potential platform for the fabrication of other metal complexes with easy accessibility, which could be used as highly efficient catalysts for various metal complex-based catalytic reactions. This process may be used in the future to develop additional nanocatalysts that possess favorable properties, such as efficiency and ease of reuse.Conflicts of interest
There are no conflicts to declare.Notes and references
- M. L. Wang, T. T. Jiang, Y. Lu, H. J. Liu and Y. Chen, J. Mater. Chem. A, 2013, 1, 5923–5933 CAS.
- X. Zhang and Z. Su, Adv. Mater., 2012, 24, 4574–4577 CrossRef CAS PubMed.
- Z. M. de Pedro, E. Diaz, A. F. Mohedano, J. A. Casas and J. J. Rodriguez, Appl. Catal., B, 2011, 103, 128–135 CrossRef CAS.
- Y. M. Liu, H. Tsunoyama, T. Akita, S. H. Xie and T. Tsukuda, ACS Catal., 2011, 1, 2–6 CrossRef CAS.
- X. Yang, C. Huang, Z. Y. Fu, H. Y. Song, S. J. Liao, Y. L. Su, L. Du and X. J. Li, Appl. Catal., B, 2013, 140, 419–425 CrossRef.
- A. S. K. Hashmi, J. P. Weyrauch, M. Rudolph and E. Kurpejovic, Angew. Chem., Int. Ed., 2004, 43, 6545 CrossRef CAS PubMed.
- A. S. K. Hashmi, M. Rudolph, J. P. Weyrauch, M. Wolfle, W. Frey and J. W. Bats, Angew. Chem., Int. Ed., 2005, 44, 2798–2801 CrossRef CAS PubMed.
- N. Debono, M. Iglesias and F. Sanchez, Adv. Synth. Catal., 2007, 349, 2470–2476 CrossRef CAS.
- A. S. K. Hashmi, M. Rudolph, H. U. Siehl, M. Tanaka, J. W. Bats and W. Frey, Chem.–Eur. J., 2008, 14, 3703–3708 CrossRef CAS PubMed.
- N. D. Shapiro, Y. Shi and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 11654–11655 CrossRef CAS PubMed.
- Y. Zhang, B. Feng and C. Zhu, Org. Biomol. Chem., 2012, 10, 9137–9141 CAS.
- J. A. O’Neill, G. M. Rosair and A.-L. Lee, Catal. Sci. Technol., 2012, 2, 1818–1821 Search PubMed.
- V. K. Y. Lo, Y. G. Liu, M. K. Wong and C. M. Che, Org. Lett., 2006, 8, 1529–1532 CrossRef CAS PubMed.
- V. K. Y. Lo, M. K. Wong and C. M. Che, Org. Lett., 2008, 10, 517–519 CrossRef CAS PubMed.
- M. Z. Wang, M. K. Wong and C. M. Che, Chem.–Eur. J., 2008, 14, 8353–8364 CrossRef CAS PubMed.
- W. Henderson, Adv. Organomet. Chem., 2006, 54, 207–265 CrossRef CAS.
- V. K. Y. Lo, K. K. Y. Kung, M. K. Wong and C. M. Che, J. Organomet. Chem., 2009, 694, 583–591 CrossRef CAS.
- K. K. Y. Kung, G. L. Li, L. Zou, H. C. Chong, Y. C. Leung, K. H. Wong, V. K. Y. Lo, C. M. Che and M. K. Wong, Org. Biomol. Chem., 2012, 10, 925–930 CAS.
- K. K. Y. Kung, V. K. Y. Lo, H. M. Ko, G. L. Li, P. Y. Chan, K. C. Leung, Z. Zhou, M. Z. Wang, C. M. Che and M. K. Wong, Adv. Synth. Catal., 2013, 355, 2055 CrossRef CAS.
- A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed.
- D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395–403 CrossRef CAS PubMed.
- Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239–3265 CrossRef CAS PubMed.
- A. Corma, A. Leyva-Perez and M. J. Sabater, Chem. Rev., 2011, 111, 1657–1712 CrossRef CAS PubMed.
- N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994–2009 CrossRef CAS PubMed.
- L. Zhou, C. Gao and W. Xu, Langmuir, 2010, 26, 11217–11225 CrossRef CAS PubMed.
- Z. Chen, L. Zhou, F. Zhang, C. Yu and Z. Wei, Appl. Surf. Sci., 2012, 258, 5291–5298 CrossRef CAS.
- Q. Du, W. Zhang, H. Ma, J. Zheng, B. Zhou and Y. Li, Tetrahedron, 2012, 68, 3577–3584 CrossRef CAS.
- P. Serp and K. Philippot, Nanomaterials in Catalysis, Wiley-VCH, Weinheim, 2013, p. 496 Search PubMed.
- V. Polshettiwar, Angew. Chem., Int. Ed., 2013, 52, 11199 CrossRef CAS.
- V. Polshettiwar and T. Asefa, Nanocatalysis: Synthesis and Applications, 2013 Search PubMed.
- Z. Dong, X. Le, X. Li, W. Zhang, C. Dong and J. Ma, Appl. Catal., B, 2014, 158–159, 129–135 CrossRef CAS.
- Z. S. Qureshi, P. B. Sarawade, M. Albert, V. D’Elia, M. N. Hedhili, K. Köhler and J. M. Basset, ChemCatChem, 2015, 7, 635–642 CrossRef CAS.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652–9656 CrossRef CAS PubMed.
- N. D. Petkovich and A. Stein, Chem. Soc. Rev., 2013, 42, 3721–3739 RSC.
- C. M. Parlett, K. Wilson and A. F. Lee, Chem. Soc. Rev., 2013, 42, 3876–3893 RSC.
- C. Liu, X. Li, Z. Gao, X. Wang and Z. Jin, J. Catal., 2015, 71, 3954–3959 CAS.
- G. Durgun, O. Aksın and L. Artok, J. Mol. Catal. A: Chem., 2007, 278, 189–199 CrossRef CAS.
- S. Paul and J. H. Clark, Green Chem., 2003, 5, 635–638 RSC.
- S. M. Sadeghzadeh, Catal. Sci. Technol., 2016, 6, 1435–1441 CAS.
- S. M. Sadeghzadeh, J. Mater. Chem. A, 2016, 423, 216–223 CAS.
| This journal is © The Royal Society of Chemistry 2017 |